Abstract
Introduction
Early identification of patients with sepsis at high risk of death remains a challenge, and whether brain natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) has a prognostic effect on patients with sepsis is controversial. Here, we clarified the prognostic value of BNP and NT-proBNP and sought to establish suitable cutoff values and intervals.
Methods
We searched five databases to identify studies that met the inclusion criteria. The primary outcomes were the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), area under the curve (AUC), and corresponding 95% credible interval (95% CI) of BNP and NT-proBNP. The secondary outcomes were the sensitivity and specificity of BNP or NT-proBNP in subgroup analyses.
Results
Forty-seven studies were included in our meta-analysis. The pooled sensitivity of NT-proBNP (0.77 [0.68, 0.84]) was weaker than that of BNP (0.82 [0.76, 0.87]), the pooled specificity of NT-proBNP (0.70 [0.60, 0.77]) was less than that of BNP (0.77 [0.71, 0.82]), and the AUC of BNP (0.87 [0.83–0.89]) was greater than that of NT-proBNP (0.80 (0.76–0.83]). The results of the subgroup analysis showed that the cutoff range of 400–800 pg/mL for BNP had high sensitivity (0.86 [0.74–0.98]) and specificity (0.87 [0.81–0.93]) and was probably the most appropriate cutoff range.
Conclusions
Elevated levels of BNP and NT-proBNP were significantly related to the mortality of patients with sepsis and had a moderate prognostic value in predicting the mortality of patients with sepsis. In addition, our meta-analysis preliminarily established appropriate cutoff values for BNP and NT-proBNP.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Over the years, great progress has been made in understanding the complex pathophysiology of sepsis; however, it remains the main cause of morbidity and mortality worldwide. Indeed, it is estimated that more than 30 million people worldwide are diagnosed with sepsis annually, resulting in approximately 6 million deaths [2].
Early identification of patients with sepsis at high risk of death remains a challenge. Several severity scoring systems have been developed, including Acute Physiology and Chronic Health Assessment (APACHE) and its revisions (APACHE II, III, and IV), Simplified Acute Physiology Score (SAPS) and SAP II, and Mortality Probability Model (MPM). In addition, the third international consensus definition of sepsis and septic shock (Sepsis-3) suggests using the SOFA score to predict the in-hospital mortality of patients with sepsis [3]. However, these scoring systems are often complicated and contain too many evaluation parameters, which leads to an untimely evaluation. Therefore, it is essential to identify reliable biomarkers as a valuable tool to predict the prognosis of patients with sepsis in a timely manner. Biomarkers can also assist with monitoring the progress of the disease and identify patients with an increased risk of complications, thereby representing important prognostic indicators for patients with sepsis [4]. At present, C-reactive protein (CRP), calcitonin (PCT), and other inflammatory markers (e.g., white blood cells) are widely used to aid in the diagnosis of sepsis and predict its progress. However, although these markers have certain clinical diagnostic value for sepsis, their prognostic ability is relatively limited [4, 5]. Recently, brain natriuretic peptide (BNP), a cardiac neurohormone synthesized by ventricular myocytes, has been suggested as a more useful laboratory parameter in aiding in the prognosis of sepsis. The N-terminal pro-brain natriuretic peptide precursor (NT-pro-BNP) is an inactive polypeptide of pro-hormone BNP [6]; both are synthesized in myocardial cells to respond to hemodynamic pressure or inflammatory state [7], and as prognostic markers of inflammatory state in critical patients [8,9,10], they have diagnostic value for patients with heart failure [11]. Two meta-analyses have been conducted on the efficacy of BNP and NT-proBNP in the prognosis of sepsis [12, 13]. However, only a few studies were included, and no potential confounding factors that might affect the prognostic value of BNP were investigated, limiting the universality of the results. In addition, neither of the two published meta-analyses conducted subgroup analysis of BNP and NT-proBNP according to the severity of sepsis, but simply considered the utility of these biomarkers in the whole spectrum of sepsis. Therefore, the efficacy of BNP and NT-proBNP in predicting mortality may be different in patients with sepsis with different severities. In addition, the best cutoff values of two biomarkers have not yet been proposed.
Given that several related studies have been published recently, we aim to provide an updated meta-analysis to further understand the predictive value of BNP and NT-proBNP in sepsis-related mortality.
Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol for this meta-analysis is available in PROSPERO (CRD42022357140).
Search for trials
We searched PubMed, Web of Science, Cochrane Library, Embase and China National Knowledge Infrastructure (up to 1 January 2024) using the keywords “NT-proBNP,” “BNP,” “Septic Shock”, and “Sepsis” to identify studies that met the inclusion criteria. There were no restrictions on language. The detailed search strategy is presented in Supplement file 1.
Selection criteria
Two authors (JLS and LQQ) independently determined the eligibility of all studies identified in the initial research. The inclusion criteria were as follows: (1) adult patients with sepsis; (2) outcome, the association between NTproBNP or BNP and risk of mortality, and the prognostic value of NT-proBNP or BNP in mortality; and (3) studies with odds ratio (ORs) data;
Data extraction
Two researchers (JLS and BF) independently extracted the following information from each study: author, region, optimal timing, tested method, outcome, design, sepsis criteria, population, sample size (n); cutoff; and outcome data (sensitivity, specificity, nonsurvivors; survivors; ). The OR data were also extracted. In cases where values from multivariate analyses were unavailable, those from univariate analysis were used. Discrepancies were resolved by consensus.
Quality of evidence
The quality of evidence for the included studies was assessed independently by the two researchers based on the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2).
Statistical analysis
Threshold effects were calculated by testing the Spearman correlation using STATA 14.0 software, with values > 0.05 indicating no significant threshold effects. If there was no evidence of a threshold effect, then pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), area under the curve (AUC), and corresponding 95% credible interval (CI) were calculated using a bivariate regression model. I2 and a bivariate boxplot were used to measure the heterogeneity caused by non-threshold effects. If the I2 value was ≥ 50% and the P-value was ≤ 0.05, then meta-regression analysis was performed to identify the sources of heterogeneity. For meta-regression models, covariates were manipulated as mean-centered continuous or as dichotomous (yes = 1, no = 0) fixed effects. The effect of each covariate on sensitivity was estimated separately from that on specificity. Deek’s funnel plot was used to detect publication bias, with P < 0.05 indicating publication bias. The following guidelines have been suggested for interpretation of intermediate AUROC values: low (0.5 ≥ AUC ≤ 0.7), moderate (0.7 ≥ AUC ≤ 0.9), or high (0.9 ≥ AUC ≤ 1) accuracy.
Results
Retrieved studies and their characteristics
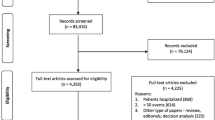
The database search identified 645 records that potentially qualified for inclusion. The titles and abstracts of these records were then filtered, and after screening the abstracts, 320 articles were excluded because they were irrelevant to the current meta-analysis.
Full texts of 125 records were screened, and 47 met the inclusion criteria. Eventually, 47 studies were included in the meta-analysis (Fig. 1), of which 22 studies [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] reported NT-proBNP, 24 reported BNP [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58], and 1 [59] reported both BNP and NT-proBNP. A total of 36 studies could construct a 2 × 2 table of results, while the remaining 11 studies only reported ORs.
Additional file 2: Table S1 lists the main characteristics of the 47 studies included in the meta-analysis. In terms of region, 30 (63.9%) trials recruited patients from Asia, 10 (21.3%) from Europe, 5 (10.6%) from North America, and 2 (4.2%) from Oceania. In terms of the subject population, 11 included patients with sepsis and 36 with severe sepsis or septic shock. In terms of trial design, 35 studies were prospective cohort studies, and 12 were retrospective cohort studies.
Quality of evidence
Figure 2 present the findings of the risk of bias assessment. Among the 47 studies analyzed in our meta-analysis, 14 studies demonstrated low bias in patient selection. Furthermore, 41 studies were deemed to have low bias in the administration of index tests, while all 47 studies were identified as having low bias in terms of reference standards. Additionally, 14 studies were found to have low bias in flow and timing. Regarding applicability concerns, 17 studies exhibited low bias in patient selection, 30 studies were rated as having low bias in relation to index tests, and all 47 studies were considered to have low bias in relation to reference standards.
Association between NT-proBNP, BNP, and mortality
For NT-proNP, pooled analysis showed that an elevated NT-proBNP level was significantly associated with patient mortality (OR [95% CI]: 10.28 [3.30, 32.04], P = 0.003, I2 = 72.8%) (adjusted OR [95% CI]: 1.36 [1.20, 1.54], P < 0.001, I2 = 92.9%) (Figs. 3A and 4A). For BNP, pooled analysis showed that an elevated NT-proBNP level was significantly associated with patient mortality (OR [95% CI]: 8.58 [3.39, 21.71], P < 0.001, I2 = 86.8%) (adjusted OR [95% CI]: 1.0088 [1.0004, 1.0174], P < 0.001, I2 = 89.2%) (Figs. 3B and 4B).
Forest plot of the association between NT-proBNP or BNP and mortality in patients with sepsis adjusted for multivariate factors; A: The association between NT-proBNP and mortality in patients with sepsis adjusted for multivariate factors; B:The association between BNP and mortality in patients with sepsis adjusted for multivariate factors
Threshold effect and heterogeneity
The Spearman correlation coefficient and P-value for NT-proBNP and BNP were 0.07 and 0.08, respectively, which indicated that there was no significant threshold effect. We used I2 and a bivariate boxplot to measure the heterogeneity caused by non-threshold effects (Fig. 5). For NT-proBNP and BNP, the I2 values were 97% and 96%, respectively.
Forest plot and area under the summary ROC (SROC) curve
Forest plots of sensitivity and specificity are shown in Fig. 6. The pooled sensitivity, specificity, PLR, NLR, DOR, AUC, and corresponding 95% CI (95% CI) of NT-proBNP and BNP were 0.77 (0.68, 0.84), 0.82 (0.76, 0.87); 0.70 (0.60, 0.77), 0.77 (0.71, 0.82); 2.5 (1.9, 3.3); 3.6 (2.7, 4.6); 0.33 (0.24, 0.47), 0.23 (0.17, 0.32); 8 (4, 13), 15 (9, 26); and 0.80 (0.76–0.83), 0.87 (0.83–0.89), respectively. Supplement Fig. 1 shows the SROC curve for the prognosis of sepsis.
Likelihood ratio scattergram
For NT-proBNP and BNP, the summary LRP and LRN for index testing were on the right lower quadrant (RLQ), indicating that NT-proBNP or BNP was unable to identify patients with sepsis at high risk of dying (Supplement Fig. 2).
Publication bias
Supplementary Fig. 3 shows the assessment of publication bias. Based on the P-values of NT-proBNP and BNP (0.55 and 0.08, respectively) and the corresponding Deek’s funnel plot, no significant publication bias was observed.
Pair-wise comparisons
Additional file 2: Table S2 shows the results of pairwise comparisons between statistical indicators of sensitivity, specificity, and AUC. The pooled sensitivity of NT-proBNP (0.77 [0.68, 0.84]) was weaker than that of BNP (0.82 [0.76, 0.87]); the pooled specificity of NT-proBNP (0.70 [0.60, 0.77]) was less than that of BNP (0.77 [0.71, 0.82]); and the AUC of BNP (0.87 [0.83–0.89]) was greater than that of NT-proBNP (0.80 [0.76–0.83]).
Meta-regression analysis
Meta-regression analysis of sensitivity, specificity, and joint models was performed to identify potential sources of heterogeneity (Additional file 2: Tables S3–S4 and Supplementary Fig. 4). According to the results of meta-regression analysis, we specified subgroups based on population, study design, outcome, region, method, test time, and cutoff value.
Subgroup analysis
The results of the subgroup analysis are shown in Additional file 2: Tables S5, S6 and S7.
For NT-proBNP, the specificity of NT-proBNP in Europe (0.82 [0.71–0.92]) was significantly higher than that in Asia (0.65 [0.56–0.75]). In terms of study design, the sensitivity of retrospective cohort studies (0.81 [0.70–0.92]) was significantly higher than that of prospective cohort studies (0.74 [0.64–0.84]). For the cutoff, the sensitivity of NT-proBNP obtained at a cutoff interval of 3000–6000 pg/mL (0.78 [0.67–0.88]) was higher than its sensitivity at a cutoff interval of > 6000 pg/mL (0.70 [0.60–0.81]). For sepsis criteria, the specificity of Sepsis-1.0 criteria (0.78 [0.68–0.88]) was significantly higher than the Sepsis-2.0 criteria (0.70 [0.60–0.79]) and Sepsis-3.0 criteria (0.59 [0.43–0.75]). For the subject population, NT-proBNP had high sensitivity (0.85 [0.69–1.00]) and specificity (0.87 [0.81–0.94]) in patients with severe sepsis. In addition, there were no statistically significant differences in the sensitivity and specificity of outcomes, cutoff values, test time, and method.
In terms of regions, BNP had high sensitivity (0.84 [0.79–0.90]) and specificity (0.81 [0.76–0.87]) in Asia than in Europe (0.71 [0.54–0.88] and 0.61 [0.43–0.79]) and North America (0.73 [0.56–0.90] and 0.64 [0.46–0.83]). Regarding the method, the immunoradiometric assay was significantly more sensitive and specific (0.84 [0.79–0.90]) and (0.84 [0.79–0.90]) than the immunofluorescence assay (0.79 [0.73–0.85] and 0.73 [0.67–0.79]). In terms of study design, the sensitivity of retrospective cohort studies (0.85 [0.77–0.91]) was significantly higher than that of prospective cohort studies (0.73 [0.67–0.80]). For BNP, a cutoff interval of 400–800 pg/L had high sensitivity (0.90 [0.83–0.94]) and specificity (0.87 (0.82–0.91]). Regarding the outcome, the sensitivity of BNP in 28-day mortality (0.83 [0.78–0.88]) was significantly higher than that of in-hospital mortality (0.57 [0.31–0.83]). For Sepsis criteria, the specificity of the Sepsis-3.0 criteria (0.83 [0.73–0.93]) was significantly higher than that of Sepsis-1.0 criteria (0.71 [0.59–0.82]). In addition, there were no statistically significant differences in the sensitivity and specificity of test time and population.
Multiple subgroup analyses
The results of multiple subgroup analyses are shown in Additional file 2: Tables S5, S6 and S7.
In terms of method and region, for NT-proBNP, the sensitivity of ECLI in Asia (0.82 [0.69–0.92]) was significantly higher than that in Europe (0.65 [0.45–0.85]). For BNP, the immunofluorescence assay had high sensitivity (0.82 [0.75–0.88]) and specificity (0.78 [0.71–0.85]) in Asia than in Europe (0.71 [0.53–0.90) and 0.59 [0.37–0.80], respectively) and North America (0.73 [0.58–0.88] and 0.64 [0.45–0.82], respectively).
In terms of cutoff and region, in Asia, the sensitivity of NT-proBNP obtained at a cutoff interval of < 3000 pg/mL (0.90 [0.81–0.99]) was higher than its sensitivity at a cutoff interval of 3000–6000 pg/mL (0.78 [0.65–0.91]) and > 6000 pg/mL (0.73 [0.67–0.80]). For BNP, the cutoff range of 400–800 pg/mL had high sensitivity (0.86 [0.74–0.98) and specificity (0.87 [0.81–0.93]) and was probably the most appropriate cutoff range in Asia. Moreover, the sensitivity of BNP obtained at a cutoff interval of 400–800 pg/mL (0.88 [0.82–0.93]) was higher than its sensitivity at a cutoff value < 400pg/mL (0.83 [0.78–0.88]) and > 800 pg/mL (0.76 [0.61–0.90]), while its specificity obtained at a cutoff interval of 400–800 pg/mL (0.87 [0.82–0.92]) was higher than its specificity at a cutoff value of < 400 pg/mL (0.71 [0.62–0.80]).
In terms of the Sepsis criteria and population, for Sepsis-3.0 criteria, the sensitivity of NT-proBNP in patients with sepsis (0.84 [0.71–0.96]) was significantly higher than that in patients with sepsis and septic shock (0.58 [0.41–0.75]). In the Sepsis-1.0/2.0 criteria, the specificity of NT-proBNP in patients with severe sepsis (0.87 [0.81–0.93]) was significantly higher than that in patients with septic shock (0.68 [0.57–0.79]). For the Sepsis-3.0 criteria, the specificity of NT-proBNP (0.59 [0.43–0.76]) in predicting mortality in patients with all subtypes of sepsis was significantly lower than that of BNP (0.83 [0.71–0.95]), but no significant difference between these two markers was found in the Sepsis-1.0/2.0 criteria. The specificity of NT-proBNP (0.53 [0.33–0.72]) in predicting mortality in patients with sepsis was significantly lower than that of BNP (0.77 [0.62–0.91]), but there was no statistical difference between them in predicting mortality in patients with septic shock and severe sepsis. The above results show that the ability of BNP in predicting the mortality of all subtypes of sepsis in the Sepsis-3.0 criteria was higher than that of NT-proBNP, but this was only reflected in predicting ordinary sepsis; for patients with severe sepsis and septic shock, there was no statistical difference between the two markers in predicting the mortality of patients.
Sensitivity analysis
Sensitivity analysis was performed by sequential exclusion of each study. Additional file 1: Tables S8 and S9 show the combined DOR and 95% CI calculated after deleting each study. The combined DOR after removal did not change significantly, suggesting that the results were robust.
Discussion
The meta-analysis showed that elevated levels of BNP and NT-proBNP were significantly related to the mortality of patients with sepsis. In addition, SROC curve analysis showed that NT-proBNP and BNP had moderate prognostic value in predicting the mortality of patients with sepsis. Finally, our meta-analysis preliminarily established appropriate cutoff values and intervals of BNP and NT-proBNP. The most appropriate cutoff range of BNP and NT-proBNP was 400–800 and < 3000 pg/mL, respectively. However, the heterogeneity of our results limits the strength of these conclusions.
Although the concept of sepsis is widely used, the standard definition of this common disease has not yet been established, which leads to differences in the criteria for diagnosis. Therefore, the term sepsis probably includes diseases and severities that differ among the studies included in this meta-analysis, which may also explain the high levels of heterogeneity observed in our analysis. In addition, we performed meta-regression analysis based on population, study design, outcome, region, method, test time, and cutoff value to identify potential sources of heterogeneity. The results of subgroup analysis showed significant differences in the sensitivity and specificity of NT-proBNP in terms of cutoff value, study design, regions, Sepsis criteria, and population. For BNP, there were significant differences in outcome, region, study design, cutoff value, detection method, and Sepsis criteria.
It has been shown that BNP values are significantly different between patients with sepsis and septic shock [60, 61] and between patients with severe sepsis and septic shock [43, 62]; however, no clear cutoff value has been proposed for this purpose to distinguish patients in these stages in clinical practice. The results of subgroup analysis showed that the sensitivity and specificity of NT-proBNP in predicting the mortality of severe sepsis was higher than that of septic shock and sepsis. For BNP and NT-proBNP, the specificity of NT-proBNP in predicting mortality in patients with sepsis was significantly lower than that of BNP, but there was no statistical difference between them in predicting mortality in patients with septic shock and severe sepsis. However, because the studies included in this meta-analysis did not provide cutoff value information for different stages of sepsis syndrome, we could not further evaluate the ability of markers to distinguish septic shock from sepsis or severe sepsis.
In addition to BNP and NT-proBNP, which were identified in this study as predictors of mortality in sepsis patients, emerging biomarkers such as cell free DNA, Pentraxin-3, and Neutrophil-to-lymphocyte ratio have demonstrated potential prognostic value in predicting mortality in sepsis [63,64,65]. Recent meta-analysis findings suggest that the AUC values of these novel biomarkers ranged from 0.73 to 0.80, slightly lower than those of BNP and NT-proBNP in the current study [63,64,65]. However, there is a lack of comparative studies evaluating BNP and NT-proBNP against these biomarkers for prognostic purposes in sepsis, thus hindering the assessment of their relative effectiveness. Moreover, the clinical utility of these novel biomarkers is limited by various factors, including infrequent measurement in routine clinical settings, small sample sizes in studies, and incomplete understanding of the causal relationship between these biomarkers and sepsis outcomes. Consequently, current guidelines recommend against the use of biomarkers for prognostic evaluation in sepsis [66]. Although previous studies showed that BNP and NT-proBNP were more sensitive than SOFA [39, 45, 49], our meta-analysis shows that the use of BNP or NT-proBNP alone cannot predict the mortality of patients with sepsis. Considering the low sensitivity and high specificity of the clinical severity score, future studies should investigate whether single or multiple biomarkers, when combined with the Clinical Severity Score, can provide a more accurate assessment of the prognosis of sepsis.
Our meta-analysis has several limitations. First, there is a high degree of heterogeneity among the studies, although we conducted regression analysis and subgroup analysis on the factors in the team. Secondly, renal failure and ventricular dysfunction resulting from sepsis, along with interventions like catecholamine administration and volume resuscitation, contribute to an increase in BNP/NT-proBNP levels [67]. Conversely, certain sepsis treatments such as positive inotropic agents (levosimendan and dobutamine), intra-aortic balloon pump (IABP) insertion, and continuous renal replacement therapy (CRRT) have been shown to lower BNP/NT-proBNP levels [68,69,70]. In evaluating the prognostic value of BNP in sepsis, the abnormal renal function of patients with sepsis is still a major confounding factor, because the included studies exclude pre-existing chronic kidney diseases to varying degrees, and the adjustment of acute kidney injury is inconsistent in the analysis. In patients with sepsis, we showed contradictory results regarding the correlation between BNP and serum creatinin [29, 50]. Therefore, further research is needed to develop the clinically relevant BNP critical value and stratify patients with sepsis according to their renal function to determine the BNP range of these patients more effectively. Moreover, most studies exclude patients with pre-existing heart disease and do not systematically evaluate cardiac dysfunction. As these studies did not provide the aforementioned details (e.g., fluid balance, cardiac dysfunction, renal function, positive inotropic agents, IABP and CRRT), these factors were not systematically assessed in the individual studies included in our analyses, which limits the generalizability of our findings.
However, despite the above limitations, our results showed that elevated levels of BNP and NT-proBNP were significantly related to the mortality of patients with sepsis and had moderate prognostic value in predicting the mortality of patients with sepsis. In addition, our meta-analysis preliminarily established appropriate cutoff values for BNP and NT-proBNP.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BNP:
-
Brain Natriuretic Peptide
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- APACHE:
-
Acute Physiology and Chronic Health Assessment
- SAPS:
-
Simplified Acute Physiology Score
- CRP:
-
C-Reactive Protein
- PLR:
-
Positive Likelihood Ratio
- NLR:
-
Negative Likelihood Ratio
- DOR:
-
Diagnostic Odds Ratio
- AUC:
-
Area Under the Curve
- SOFA:
-
Sequential Organ Failure Assessment
- CI:
-
Credible Interval
- LVEF:
-
Left Ventricular Ejection Fraction
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Gyawali B, Ramakrishna K, Dhamoon AS, Sepsis. The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:2050312119835043.
Arabi Y, Al Shirawi N, Memish Z, Venkatesh S, Al-Shimemeri A. Assessment of six mortality prediction models in patients admitted with severe sepsis and septic shock to the intensive care unit: a prospective cohort study. Crit Care. 2003;7(5):R116–22.
Huang M, Cai S, Su J. The pathogenesis of Sepsis and potential therapeutic targets. Int J Mol Sci 2019;20(21).
Suárez-Santamaría M, Santolaria F, Pérez-Ramírez A, Alemán-Valls MR, Martínez-Riera A, González-Reimers E, et al. Prognostic value of inflammatory markers (notably cytokines and procalcitonin), nutritional assessment, and organ function in patients with sepsis. Eur Cytokine Netw. 2010;21(1):19–26.
Mauritz GJ, Rizopoulos D, Groepenhoff H, Tiede H, Felix J, Eilers P, et al. Usefulness of serial N-terminal pro-B-type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2011;108(11):1645–50.
Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, et al. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol. 2007;50(3):205–14.
Rodseth RN, Biccard BM, Le Manach Y, Sessler DI, Lurati Buse GA, Thabane L, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol. 2014;63(2):170–80.
Schellings DA, Adiyaman A, Dambrink JE, Gosselink AM, Kedhi E, Roolvink V, et al. Predictive value of NT-proBNP for 30-day mortality in patients with non-ST-elevation acute coronary syndromes: a comparison with the GRACE and TIMI risk scores. Vasc Health Risk Manag. 2016;12:471–6.
Galvani M, Ottani F, Oltrona L, Ardissino D, Gensini GF, Maggioni AP, et al. N-terminal pro-brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation. 2004;110(2):128–34.
Januzzi JL, Morss A, Tung R, Pino R, Fifer MA, Thompson BT, et al. Natriuretic peptide testing for the evaluation of critically ill patients with shock in the intensive care unit: a prospective cohort study. Crit Care. 2006;10(1):R37.
Wang F, Wu Y, Tang L, Zhu W, Chen F, Xu T, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care. 2012;16(3):R74.
Bai YL, Hu BL, Wen HC, Zhang YL, Zhu JJ. Prognostic value of plasma brain natriuretic peptide value for patientswith sepsis: a meta-analysis. J Crit Care. 2018;48:145–52.
Balcan B, Olgun Ş, Torlak F, Sağmen SB, Eryüksel E, Karakurt S. Determination of factors affecting mortality of patients with sepsis in a tertiary intensive care unit. Turk Thorac J. 2015;16(3):128–32.
Biswas S, Soneja M, Makkar N, Farooqui FA, Roy A, Kumar A, et al. N-terminal pro-brain natriuretic peptide is an independent predictor of mortality in patients with sepsis. J Investig Med. 2022;70(2):369–75.
Brueckmann M, Huhle G, Lang S, Haase KK, Bertsch T, Weiss C, et al. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation. 2005;112(4):527–34.
Cheng H, Fan WZ, Wang SC, Liu ZH, Zang HL, Wang LZ, et al. N-terminal pro-brain natriuretic peptide and cardiac troponin I for the prognostic utility in elderly patients with severe sepsis or septic shock in intensive care unit: a retrospective study. J Crit Care. 2015;30(3):e6549–14.
Martín-Rodríguez F, Melero-Guijarro L, Ortega GJ, de la Sanz-García A, Manzanares J et al. Combination of Prehospital NT-proBNP with qSOFA and NEWS to Predict Sepsis and Sepsis-Related Mortality. Dis Markers. 2022;2022:5351137.
Guaricci AI, Santoro F, Paoletti Perini A, Ioffredo L, Trivedi C, Pontone G, et al. Correlations between NT-proBNP, outcome and haemodynamics in patients with septic shock. Acta Cardiol. 2015;70(5):545–52.
Li H, Shan-Shan Z, Jian-Qiang K, Ling Y, Fang L. Predictive value of C-reactive protein and NT-pro-BNP levels in sepsis patients older than 75 years: a prospective, observational study. Aging Clin Exp Res. 2020;32(3):389–97.
Alataby H, Nfonoyim J, Diaz K, Al-Tkrit A, Akhter S, David S, et al. The levels of Lactate, Troponin, and N-Terminal Pro-B-Type natriuretic peptide are predictors of mortality in patients with Sepsis and septic shock: a retrospective cohort study. Med Sci Monit Basic Res. 2021;27:e927834.
Ju MJ, Zhu DM, Tu GW, He YZ, Xue ZG, Luo Z, et al. Predictive value of N-terminal pro-brain natriuretic peptide in combination with the sequential organ failure assessment score in sepsis. Chin Med J (Engl). 2012;125(11):1893–8.
Li Z, Wang H, Liu J, Chen B, Li G. Serum soluble triggering receptor expressed on myeloid cells-1 and procalcitonin can reflect sepsis severity and predict prognosis: a prospective cohort study. Mediators Inflamm. 2014;2014:641039.
Lu NF, Jiang L, Zhu B, Yang DG, Zheng RQ, Shao J, et al. Elevated plasma histone H4 level predicts increased risk of mortality in patients with sepsis. Ann Palliat Med. 2020;9(3):1084–91.
Lu Y, Sheng HL, Cui XL, Liu XX, Chen XL, Wang YP. Predictive value of C-reactive protein and NT-pro BNP on death and multiple organ failure in elderly patients with sepsis. Chin J Gerontol. 2021;41(18):4011–4. [In Chinese].
Mokart D, Sannini A, Brun JP, Faucher M, Blaise D, Blache JL, et al. N-terminal pro-brain natriuretic peptide as an early prognostic factor in cancer patients developing septic shock. Crit Care. 2007;11(2):R37.
Park BH, Park MS, Kim YS, Kim SK, Kang YA, Jung JY, et al. Prognostic utility of changes in N-terminal pro-brain natriuretic peptide combined with sequential organ failure assessment scores in patients with acute lung injury/acute respiratory distress syndrome concomitant with septic shock. Shock. 2011;36(2):109–14.
Phua J, Koay ES, Lee KH. Lactate, procalcitonin, and amino-terminal pro-B-type natriuretic peptide versus cytokine measurements and clinical severity scores for prognostication in septic shock. Shock. 2008;29(3):328–33.
Roch A, Allardet-Servent J, Michelet P, Oddoze C, Forel JM, Barrau K, et al. NH2 terminal pro-brain natriuretic peptide plasma level as an early marker of prognosis and cardiac dysfunction in septic shock patients. Crit Care Med. 2005;33(5):1001–7.
Sekino M, Funaoka H, Sato S, Okada K, Inoue H, Yano R, et al. Intestinal fatty acid-binding protein level as a predictor of 28-day mortality and bowel ischemia in patients with septic shock: a preliminary study. J Crit Care. 2017;42:92–100.
Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettilä V. Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35(5):1277–83.
García Villalba E, Bernal Morell E, Egea MP, Marín I, Alcaraz Garcia A, Muñoz A, et al. The N-terminal pro brain natriuretic peptide is the best predictor of mortality during hospitalization in patients with low risk of sepsis-related organ failure. Med Clin (Barc). 2017;149(5):189–95.
Zang X, Chen W, Sheng B, Zhao L, Gu X, Zhen J, et al. Predictive value of early phrase echocardiography and cardiac biological markers in patients with severe sepsis: a five-year single-center retrospective study. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30(4):332–6. [In Chinese].
Zhang XH, Dong Y, Chen YD, Zhou P, Wang JD, Wen FQ. Serum N-terminal pro-brain natriuretic peptide level is a significant prognostic factor in patients with severe sepsis among Southwest Chinese population. Eur Rev Med Pharmacol Sci. 2013;17(4):517–21.
Balcan B, Olgun Ś, Akbaś T, Eryüksel E, Karakurt S. Level of adrenomedullin in cases with adrenal defficiency and its relation to mortality in patients with sepsis. Tuberk Toraks. 2016;64(3):191-7.
Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S, et al. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32(3):660–5.
Chen Y, Li C. Prognostic significance of brain natriuretic peptide obtained in the ED in patients with SIRS or sepsis. Am J Emerg Med. 2009;27(6):701–6.
Chen FC, Xu YC, Zhang ZC. Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J Zhejiang Univ Sci B. 2020;21(7):537–48. [In Chinese].
Hu BC, Wang YJ, Ge WD, Li FZ, Sun RH. [Combination of B-type brain natriuretic peptide with left ventricular diastolic dysfunction for prediction of mortality in the patients with septic shock]. Zhonghua Yi Xue Za Zhi. 2016;96(29):2295–300. [In Chinese].
Khoury J, Arow M, Elias A, Makhoul BF, Berger G, Kaplan M, et al. The prognostic value of brain natriuretic peptide (BNP) in non-cardiac patients with sepsis, ultra-long follow-up. J Crit Care. 2017;42:117–22.
Yue L, Deng X, Yang M, Li X. Elevated B-type natriuretic peptide (BNP) and soluble thrombomodulin (sTM) indicates severity and poor prognosis of sepsis. Ann Palliat Med. 2021;10(5):5561–7.
Li SW, Sun FH, Tao ZX, Song J, Zhao YF. Distribution of pathogens isolated from patients with acute abdomen patients complicated with septic shock and value of brain natriuretic peptide in assessment of prognosis. Chin J Nosocomiology. 2017;27(16):3707–10.
McLean AS, Huang SJ, Hyams S, Poh G, Nalos M, Pandit R, et al. Prognostic values of B-type natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35(4):1019–26.
Papanikolaou J, Makris D, Mpaka M, Palli E, Zygoulis P, Zakynthinos E. New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients. Crit Care. 2014;18(3):R94.
Perman SM, Chang AM, Hollander JE, Gaieski DF, Trzeciak S, Birkhahn R, et al. Relationship between B-type natriuretic peptide and adverse outcome in patients with clinical evidence of sepsis presenting to the emergency department. Acad Emerg Med. 2011;18(2):219–22.
Post F, Weilemann LS, Messow CM, Sinning C, Münzel T. B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med. 2008;36(11):3030–7.
Rivers EP, McCord J, Otero R, Jacobsen G, Loomba M. Clinical utility of B-type natriuretic peptide in early severe sepsis and septic shock. J Intensive Care Med. 2007;22(6):363–73.
Salim MB, Elaasr H, El Damarawy M, Wadee A, Ashour A, Nasr FM. Atrial ejection force and brain natriuretic peptide as markers for mortality in sepsis. Egypt J Crit Care Med. 2015;3(1):29–35.
Singh H, Ramai D, Patel H, Iskandir M, Sachdev S, Rai R, et al. B-Type natriuretic peptide: a predictor for mortality, intensive care unit length of Stay, and hospital length of stay in patients with resolving Sepsis. Cardiol Res. 2017;8(6):271–5.
Suo S, Luo L, Song Y, Huang H, Chen X, Liu C. Early diagnosis and prediction of death risk in patients with Sepsis by combined detection of serum PCT, BNP, Lactic Acid, and Apache II score. Contrast Media Mol Imaging. 2022;2022:8522842.
Ueda S, Nishio K, Akai Y, Fukushima H, Ueyama T, Kawai Y, et al. Prognostic value of increased plasma levels of brain natriuretic peptide in patients with septic shock. Shock. 2006;26(2):134–9.
Wang B, Chen G, Li J, Zeng Y, Wu Y, Yan X. Neutrophil gelatinase-associated lipocalin predicts myocardial dysfunction and mortality in severe sepsis and septic shock. Int J Cardiol. 2017;227:589–94.
Xing B, Wang XZ, Zeng Y, Tan SF, Huang S. Prognostic value of plasma B-type natriuretic peptide in patients with septic shock. China J Mod Med. 2013;23(32):75–9. [In Chinese].
Yang Y, Wang XP, Chen XJ, Ren H. Prognostic value of brain natriuretic peptides in patients with septic shock. Guangdong Med J. 2016;37(17):2631–3. [In Chinese].
Yang Y, Leng J, Chen R, Wang H, Hao C. Correlation between early brain natriuretic peptide level and mortality in cancer patients with septic shock. Ann Palliat Med. 2021;10(4):4214–9.
Yang Y, Leng J, Tian X, Wang H, Hao C. Brain natriuretic peptide and cardiac troponin I for prediction of the prognosis in cancer patients with sepsis. BMC Anesthesiol. 2021;21(1):159.
Yucel T, Memiş D, Karamanlioglu B, Süt N, Yuksel M. The prognostic value of atrial and brain natriuretic peptides, troponin I and C-reactive protein in patients with sepsis. Exp Clin Cardiol. 2008;13(4):183–8.
Zhang Z, Zhang Z, Xue Y, Xu X, Ni H. Prognostic value of B-type natriuretic peptide (BNP) and its potential role in guiding fluid therapy in critically ill septic patients. Scand J Trauma Resusc Emerg Med. 2012;20:86.
Zhao HY, An YZ, Liu F. [Prognostic values of B-type natriuretic peptide in severe sepsis and septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21(5):293–5. [In Chinese].
Sturgess DJ, Marwick TH, Joyce C, Jenkins C, Jones M, Masci P, et al. Prediction of hospital outcome in septic shock: a prospective comparison of tissue doppler and cardiac biomarkers. Crit Care. 2010;14(2):R44.
Kandil E, Burack J, Sawas A, Bibawy H, Schwartzman A, Zenilman ME, et al. B-type natriuretic peptide: a biomarker for the diagnosis and risk stratification of patients with septic shock. Arch Surg. 2008;143(3):242–6. discussion 6.
Turner KL, Moore LJ, Todd SR, Sucher JF, Jones SA, McKinley BA, et al. Identification of cardiac dysfunction in sepsis with B-type natriuretic peptide. J Am Coll Surg. 2011;213(1):139–46. discussion 46 – 7.
Pirracchio R, Deye N, Lukaszewicz AC, Mebazaa A, Cholley B, Matéo J, et al. Impaired plasma B-type natriuretic peptide clearance in human septic shock. Crit Care Med. 2008;36(9):2542–6.
Charoensappakit A, Sae-Khow K, Rattanaliam P, Vutthikraivit N, Pecheenbuvan M, Udomkarnjananun S, et al. Cell-free DNA as diagnostic and prognostic biomarkers for adult sepsis: a systematic review and meta-analysis. Sci Rep. 2023;13(1):19624.
Wang G, Jiang C, Fang J, Li Z, Cai H. Pentraxin-3 as a predictive marker of mortality in sepsis: an updated systematic review and meta-analysis. Crit Care. 2022;26(1):167.
Wu H, Cao T, Ji T, Luo Y, Huang J, Ma K. Predictive value of the neutrophil-to-lymphocyte ratio in the prognosis and risk of death for adult sepsis patients: a meta-analysis. Front Immunol. 2024;15:1336456.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49(11):e1063–143.
Pandompatam G, Kashani K, Vallabhajosyula S. The role of natriuretic peptides in the management, outcomes and prognosis of sepsis and septic shock. Rev Bras Ter Intensiva. 2019;31(3):368–78.
Meng JB, Hu MH, Lai ZZ, Ji CL, Xu XJ, Zhang G, et al. Levosimendan Versus Dobutamine in Myocardial Injury patients with septic shock: a Randomized Controlled Trial. Med Sci Monit. 2016;22:1486–96.
Chen W, Sheng B, Zhao L, Lu FP, Wang SZ, Liu L, et al. The clinical application and value of intra-aortic balloon pump in patients with septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012;24(1):46–9. [In Chinese].
Jeong EG, Nam HS, Lee SM, An WS, Kim SE, Son YK. Role of B-type natriuretic peptide as a marker of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Ren Fail. 2013;35(9):1216–22.
Acknowledgements
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Funding
This research was supported by Sichuan Key Clinical Specialty project (2022-16).
Author information
Authors and Affiliations
Contributions
GYC made substantial contributions conception and design of the study; JLS and LQQ searched and screened literature; JLS, BF, QL and BL extracted data from the collected literature and analyzed the data; JLS, LQQ and GYC wrote the manuscript; QL, BF and BL revised the manuscript. All the authors approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article is meta-analysis and does not require ethics committee approval or a consent statement.
Consent for publication
Not applicable. The manuscript does not contain any personal data in any form (including personal details, pictures, or videos).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12871_2024_2661_MOESM1_ESM.docx
Supplement File 1. Summary receiver-operating characteristic (SROC) curves for the prognosis of sepsis. A:SROC of NT-proBNP; B:SROC of BNP AUC = area under the curve
12871_2024_2661_MOESM2_ESM.docx
Supplement File 2. Likelihood ratio scattergrams. A:Scattergrams evaluating the positive likelihood ratios in the prognosis of sepsis for NT-proBNP; or Scattergrams evaluating the positive likelihood ratios in the prognosis of sepsis for BNP.
12871_2024_2661_MOESM3_ESM.docx
Supplement File 3. Deek’s funnel plots. A:Funnel plots evaluating publication bias of NT-proBNP;B:Funnel plots evaluating publication bias of BNP.
12871_2024_2661_MOESM4_ESM.tif
Supplement File 4. Univariable meta-regression analysis of sensitivity and specificity. A:Univariable meta-regression analysis of sensitivity and specificity of NT-proBNP;B:Univariable meta-regression analysis of sensitivity and specificity of BNP.
12871_2024_2661_MOESM6_ESM.tif
Supplement File 6: Supplement tables 1-9 Supplement. table 1: Literature search and characteristics of the included studies, Supplement table 2: Pair-wise comparisons between modalities for sensitivity, Specificity, and AUC. Supplement table 3: The result of meta-regression and subgroup analysis for NT-proBNP. Supplement table 4: The result of meta-regression and subgroup analysis for BNP. Supplement table 5: Subgroup analysis of region and detection method for NT-proBNP and BNP. Supplement table 6: Subgroup analysis of region and cutoff level method for NT-proBNP and BNP. Supplement table 7: Subgroup analysis of sepsis criteria and population for NT-proBNP and BNP. Supplement table 8: Sensitivity analyses of NT-proBNP. Supplement table 9: Sensitivity analyses of BNP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Jl., Fan, B., Qiu, Lq. et al. Brain natriuretic peptide as a predictive marker of mortality in sepsis: an updated systematic review and meta-analysis. BMC Anesthesiol 24, 276 (2024). https://doi.org/10.1186/s12871-024-02661-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02661-z