Abstract
Background
This meta-analysis aimed to explore the correlation between the different doses of remifentanil-based anaesthesia and postoperative pain in randomised trials.
Methods
The electronic databases including PubMed, Cochrane, clinical trial registries, and Google Scholar were searched up to November 2022 for randomised controlled trials (RCTs) that assessed the dose dependent efficacy of remifentanil for postoperative pain intensity and hyperalgesia.
Results
31 studies involving 2019 patients were included for analysis. Compared with the high remifentanil dose administration, patients in low doses showed less postoperative pain intensity at 1-2 h (weighted mean differences (WMD): 0.60, 95% CI, 0.05 to 1.15), 3-8 h (WMD: 0.38, 95% CI, 0.00 to 0.75), 24 h (WMD: 0.26, 95% CI, 0.04 to 0.48) and 48 h (WMD: 0.32, 95% CI, 0.09 to 0.55). Remifentanil-free regimen failed to decrease the pain score at 24 h (WMD: 0.10, 95% CI, -0.10 to 0.30) and 48 h (WMD: 0.15, 95% CI, -0.22 to 0.52) in comparison with remifentanil-based anaesthesia. After excluding trials with high heterogeneity, the dose of the remifentanil regimen was closely correlated with the postoperative pain score (P=0.03). In addition, the dose of the remifentanil regimen was not associated with the incidence of postoperative nausea and vomiting (PONV) (P=0.37).
Conclusions
Our meta-analysis reveals that the low dose of remifentanil infusion is recommendable for general anaesthesia maintenance. No evidence suggests that remifentanil-free regimen has superiority in reducing postoperative pain. Moreover, remifentanil doesn’t have a dose dependent effect in initiating PONV.
Trial registration
The protocol of present study was registered with PROSPERO (CRD42022378360).
Similar content being viewed by others
Introduction
Opioids are commonly used to alleviate perioperative pain during surgery. However, opioid, especially remifentanil use, can cause opioid tolerance and induce paradoxical pain [1]. Remifentanil was associated with primary and secondary hyperalgesia and can lead to opioid addiction.
In recent years, opioid-free general anaesthesia has been introduced to avoid unexpected pain. Opioid-free anaesthesia using dexmedetomidine or propofol [2,3,4] has been associated with less postoperative pain, resulting in less postoperative opioid consumption. However, the absence of remifentanil or other opioids during the surgery increases the amount of sedative infusion and results in delayed recovery [5].
It was found that remifentanil had a dose-dependent correlation with postoperative pain threshold [6, 7]. However, these findings are contrary to those of other studies that did not show an effect of the intraoperative opioid dose on postoperative pain intensity and rescue morphine consumption [8]. There is still a pending question about whether remifentanil infusion should be abandoned. There are a limited number of studies that have evaluated different doses of remifentanil and their relationship to postoperative pain intensity.
This meta-analysis aimed to evaluate dose dependent effect of remifentanil on the postoperative analgesic effect, secondary hyperalgesia, and side effects after general anaesthesia.
Materials and methods
This meta-analysis of randomised, controlled trials (RCTs) was performed in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Additional file 1). The protocol was registered with PROSPERO (CRD42022378360).
Eligibility criteria
Inclusion Criteria: randomised controlled trials were based on remifentanil anaesthesia or remifentanil free anaesthesia and focused on postoperative pain intensity and hyperalgesia in adults.
In order to exclude the impact of dexmedetomidine, the trials with dexmedetomidine only used in the remifentanil-free group were not included in the meta-analysis. So the exclusion criteria were as follows: dexmedetomidine was only applied in remifentanil-free group, general anaesthesia with epidural analgesia or nerve block, observational studies, non-randomised controlled trials, studies published as abstracts, duplicate articles, populations with chronic opioid use, and articles reporting no indispensable data.
Search strategy
PubMed, Cochrane, clinical trial registries, and Google Scholar were searched to retrieve studies published up to November 2022 without language restrictions (by XH and JS). The following search string was used ("remifentanil" OR "remifentanyl" OR "opioid" OR "opiate") AND ("hyperalgesia" OR "hyperalgesia" OR "hyperalgesias" OR "hyperanalgesia" OR "nociception" OR "nociceptive" OR "pronociception" OR "pronociceptive" OR "allodynia" OR "tolerance") (Full links are given in Additional file 2). The searches were limited to human trials. A manual search of the references listed in the reports and reviews was performed. We reviewed the trial registries when available. In the case of secondary publications, the original papers were reviewed.
Selection of included studies
Three reviewers (XH, JC, and ZL) independently screened the titles and abstracts obtained by the literature search. The remaining full texts were independently retrieved and evaluated by the authors to determine whether the retrieved trials met the inclusion criteria. Disagreements were discussed among the investigators to reach a consensus.
Data extraction
The following data were extracted from the included studies: participant demographics, type of surgery, anaesthetic selection, intraoperative remifentanil regimens, pain scores at all reported times, postoperative allodynia, time to the first analgesic request, and opioid-related side effects. Pain scores on different scales were converted to a standardized 0-10 analogue scale. Any differences resulting from discrepant assessments during data extraction and analysis were resolved through discussion among the study authors. Data reported in the form of a graph were extracted with the assistance of graphics processing software (Web plot Digitalise, HTML5 Software, University of Notre Dame, USA).
Postoperative outcomes
Primary outcome: Pain score at 1-2, 3-8, 24, and 48 h postoperatively.
Secondary outcomes: Periincisional wound allodynia and forearm allodynia, time to first postoperative analgesic requirement, postoperative consumption of rescue analgesics in milligrams of morphine equivalence, postoperative nausea and vomiting (PONV), and postoperative shivering.
Assessment of Methodological Quality and Risk of Bias:
The risk of bias was independently assessed using the Cochrane Collaboration tool [9]. Studies with a dropout rate of less than 20% were considered “low-risk” of attrition bias; otherwise, they were assessed as “high risk of bias”. “Other potential sources of bias” were assessed as high-risk in studies that had fewer than 15 participants per arm. However, there is currently no consensus on the trial size in this setting.
Data synthesis and analysis
Meta-analyses were performed with the assistance of Review Manager 5.4 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark), Comprehensive Meta-analysis version 2.2.034 (Biostat, USA), Trial Sequential Analysis Viewer version 0.9.5.5 Beta (Copenhagen Trial Unit, 2016) and STATA 15.0 (STATA CORP, Texas, USA).
For trials that did not report the results in the form of mean ± standard deviation (SD), the corresponding authors were contacted thrice by mail to supply the missing data. If no response was obtained, the sample size (n), median (m), minimum value (a), first quartile (q1), third quartile (q3), maximum value (b), were converted to mean ± SD by the specific formula [10, 11]. Note that the data may not always be given in full. The three frequently encountered scenarios are: C1 = {a, m, b; n}, C2 = {a, q1, m, q3, b; n}, C3 = {q1, m, q3; n}. The skew data can be diagnosed and transformed automatically based on the formular link: https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html.
We estimated the weighted mean differences (WMD) or standardised mean differences (SMD) with 95%CI for continuous data and the odds ratio (OR) for categorical data among the groups, with an overall estimate of the pooled effect. Forest plots were used to present the results graphically. Statistical heterogeneity across trials was assessed using the I2 value. A value of I2>50% or P<0.1 was considered as high heterogeneity. A random-effects model was applied in the case of high heterogeneity; otherwise, a fixed-effects model was adopted. For the primary outcome (pain score at postoperative 1-2, 3-8, 24 and 48 h), a priori sensitivity analysis was performed by removing the studies with a high risk of bias.
Mixed meta-regression was used to explore any potential dose-related interaction between the intraoperative remifentanil dose and postoperative pain intensity / PONV. In volunteers, remifentanil infusion at a rate of 0.10 μg/kg/min was reported [12] to provoke hyperalgesia, while opioid infusion at a rate of 0.05 μg/kg/min failed to induce RIH after discontinuation. The infusion rate at 0.1 µg/kg/min was proved to achieve a stable plasma concentration ranging between 2.7 and 2.9 ng/ml [13]. In addition, remifentanil plasma concentrations of 1.6 and 3.2 ng/ml correspond to steady-state concentrations achieved when infusing remifentanil at a constant rate of about 0.065 and 0.13 µg/kg/min. The result partially proved the linear correlation between the plasma concentration and constant infusion rate [14]. According to the linear formula, the trial which used a dose with 0.05 µg/kg/min was equally with the remifentanil concentrations of 1.2 ng/ml. Therefore, studies with remifentanil infusion less than 0.05 μg/kg/min or 1.2 ng/ml were allocated to the control group when performing meta-regression analysis. Based on the outcome of the mixed meta-regression analysis, piecewise linear regression was performed to define a cutoff value of the remifentanil dose to induce postoperative pain intensity.
Trial sequential analysis. In order to estimate the number of patients needed to allow for reliable statistical inference, we performed a sample size calculation to ensure that a sufficient number of patients were included in the meta-analysis. The random effect model using DerSimonian-Laird method was selected for the Trial sequential analysis program to integrate effective sizes. The required information size and the adjusted significance threshold for the postoperative pain score were calculated, with an anticipated 20% reduction of mean difference in pain score (mean difference=0.4) and variance of 0.4 with a 5% risk of type 1 error (β=0.8), and model variance-based heterogeneity correction.
Assessment of publication bias
The risk of potential publication bias was evaluated using the Egger’s regression test.
Results
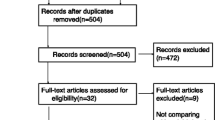
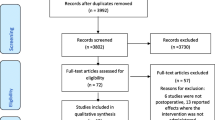
The literature search yielded 7633 results. 31 studies with a total of 2019 patients between 2000 and 2020 met our inclusion criteria, and were included in the meta-analysis [5,6,7,8, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Fig. 1). No unpublished data were identified from clinical register or major annual meetings of anaesthesiology.
Study characteristics
4 studies were at a high risk of attrition bias [23, 32, 39, 40]. The other studies were all at low risk or unclear (Fig. 2). The characteristics of the included studies [5,6,7,8, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] are shown in Table 1. The quality of the included studies is shown in Additional file 3, Additional file 4 and Table 2.
Remifentanil intervention was used in a wide range of surgical procedures. The doses of remifentanil administration ranged from 0.03 to 1.2 μg/kg/min. 22 trials compared high dose group with low-dose groups [5,6,7,8, 15, 17, 19,20,21,22, 28, 29, 32,33,34,35,36, 38,39,40,41]. 12 trials compared remifentanil with remifentanil-free groups [5, 16, 18, 23,24,25,26,27, 30, 31, 34, 37].
Postoperative pain intensity
High vs. low dose of remifentanil administration
Compared with high-dose remifentanil administration, the postoperative pain score after low dose was significantly lower, including 1-2 h (909 participants in 13 studies, P=0.03) (Fig. 2A), 24 h (1269 participants in 18 studies, P=0.02) (Fig. 2C), and 48 h (467 participants in 8 studies, P=0.006) (Fig. 2D). There was no difference at 3-8 h (1122 participants in 16 studies, P=0.05) (Fig. 2B) between high- and low-dose group. Trial sequential analysis revealed that the pooled estimate on the primary endpoint exceeded the conventional and monitoring boundaries. We can conclude with sufficient statistical force that further studies will not modify the profile obtained with the meta-analysis on the primary endpoint. (Additional file 5). Low doses of remifentanil significantly inhibited postoperative pain and secondary hyperalgesia, characterised by a higher pain threshold for periincisional wound allodynia (441 participants in 7 studies, P<0.00001, I2: 61%, SMD: -1.14, 95% CI, -1.47 to -0.80) and forearm allodynia (174 participants in 3 studies, P=0.01, I2: 28%, SMD: -0.46, 95% CI, -0.82 to -0.10) (Fig. 3).
Forest plots for the periincisional wound and forearm allodynia. Data were pooled using a random-effects model to calculate the SMD and 95% CI for each outcome. Intervention refers to the high dose remifentanil, and control refers to the low dose remifentanil group. CI indicates confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; SMD, standardized mean differences
The time to the first postoperative analgesic requirement was prolonged in the low-dose remifentanil group compared to that in the high-dose group (521 participants in 9 studies, P<0.00001, WMD: -7.53, 95% CI, -10.31 to -4.75) (Table 3).
The postoperative analgesic requirement in the low-dose remifentanil group was less than that in the high-dose group at all time points, including the consumption at 0-8 h (675 participants in 9 studies, P=0.004, SMD: 1.28, 95% CI, 0.41 to 2.16), 0-12 h (557 participants in 6 studies, P=0.0002, SMD: 2.50, 95% CI, 1.17 to 3.83) and 24-48 h after surgery (1041 participants in 16 studies, P=0.0007, SMD: 0.94, 95% CI, 0.40 to 1.49) (Table 3).
Remifentanil-free vs. remifentanil regimen
The postoperative pain score in the remifentanil-free protocol showed superiority over the remifentanil regimen only at 1-2 h (426 participants in 8 studies, P=0.01) (Fig. 2A) and 3-8 h (326 participants in 6 studies, P<0.0001) (Fig. 2B). The study failed to detect the benefit of pain relief at 24 h (516 participants in 9 studies, P=0.33) (Fig. 2C) and 48 h (247 participants in 4 studies, P=0.43) (Fig. 2D). Correspondingly, postoperative analgesic requirements did not decrease in comparison with the remifentanil regimen during 24-48 h (446 participants in 8 studies, P=0.68, SMD: 0.08, 95% CI, -0.31 to 0.47) (Table 3).
The remifentanil-free protocol prolonged the time to the first postoperative analgesic requirement in comparison with the remifentanil regimen (200 participants in 3 studies, P<0.00001, WMD: -25.27, 95% CI, -32.09 to -18.46) (Table 3).
Bias of publication
The Egger linear regression test indicated no evidence of publication bias for postoperative pain intensity (at 1-2 h: P=0.078; 3-8 h: P=0.058; 24 h: P=0.633; 48 h: P=0.612) (Additional file 6).
Sensitivity analysis: After exclusion of four studies [23, 32, 39, 40] with a high risk of bias, the remaining 27 studies were robust to post hoc sensitivity analysis. The pain score in the low-dose remifentanil regimen still showed superiority over the high-dose group at all time points, including at 1-2 h (P=0.03, I2: 94%, WMD: 0.60, 95% CI, 0.05 to 1.15), 3-8 h (P=0.05, I2: 85%, WMD: 0.38, 95% CI, 0.00 to 0.75), 24 h (P=0.04, I2: 79%, WMD: 0.26, 95% CI, 0.01 to 0.51), and 48 h (P=0.01, I2: 34%, WMD: 0.40, 95% CI, 0.09 to 0.72). The remifentanil-free protocol still failed to detect the benefit of pain relief at 24 h (P=0.30, I2: 2%, WMD: 0.08, 95% CI, -0.07 to 0.24).
After exclusion of studies with a high risk of bias, meta-regression analysis found that there was no association between remifentanil dose and the pain intensity at 24 h after the surgery (1264 participants in 17 studies, Tau2=0.12, slope of regression line: 0.39; P=0.57; 95% CI, -0.96 to 1.74) (Additional file 7). However, after excluding two trials [17, 18] with extreme data that generated high heterogeneity, the intensity of the pain score at 24 h was closely correlated with the dose of the intraoperative remifentanil infusion. (1085 participants in 15 studies, Tau2=0.09, slope of the regression line: 1.96; 95% CI, 0.19 to 3.72; P=0.03) (Fig. 4A). After piecewise linear regression analysis, the cutoff dose of the remifentanil to initiate postoperative pain was 0.1 μg/kg/min.
Subgroup analysis: The subgroup analysis is performed according to the surgery with high/low pain threshold: thoracotomy-laparotomy and non thoracotomy-laparotomy surgery. The pain score in the low-dose remifentanil regimen still showed superiority over the high-dose group at 24 h (thoracotomy-laparotomy: P=0.004, I2: 0%, WMD: 0.17, 95% CI, 0.05 to 0.28; non thoracotomy-laparotomy: P=0.02, I2: 82%, WMD: 0.35, 95% CI, 0.06 to 0.65). The remifentanil-free protocol still failed to detect the benefit of pain relief at 24 h (thoracotomy-laparotomy: P=0.75, I2: 30%, WMD: 0.05, 95% CI, -0.25 to 0.35; non thoracotomy-laparotomy: P=0.26, I2: 34%, WMD: 0.17, 95% CI, -0.13 to 0.47).
Secondary outcomes
PONV
Neither remifentanil-free nor low-dose remifentanil exposure showed superiority in inhibiting the incidence of PONV (Table 3). There was no association between the dose of remifentanil infusion and the incidence of PONV (1142 participants in 14 studies, Tau2=0.09, slope of regression line: -1.06; 95% CI, -3.39 to 1.28; P=0.37) (Fig. 4B).
Shivering
Compared with the high-dose group, the low-dose group effectively suppressed the incidence of shivering (572 participants in 6 studies, P<0.00001, I2: 0%, OR: 3.98, 95% CI, 2.59 to 6.13).
Discussion
In this study, low dose of remifentanil was correlated with lower pain score and less allodynia. Compared with the remifentanil regimen, the remifentanil-free group showed no benefit in inhibiting pain at 24 and 48 h. The meta-regression analysis found that the intensity of postoperative pain at 24 h was correlated with the dose of remifentanil infusion.
In a previous animal study [42], remifentanil ranged between 0.66 and 3.33 μg/kg/min has been reported to induce a dose-dependent pronociceptive effect. However, the drug concentrations were far exceeded the clinical demand. In the present study, the maximum remifentanil dose was 1.2 μg/kg/min. A previous meta-analysis [43, 44] with low certainty of evidence has shown that high doses of remifentanil are associated with acute pain after surgery. The use of opioid-free anaesthesia was reported to be associated with a reduction in PONV [45]. Moreover, neither of the papers [43,44,45] explored the dose-dependent association between remifentanil exposure and the incidence of postoperative pain or PONV. In the current meta-analysis that included more studies, quantitative and meta-regression analyses were introduced to conclude that the incidence of PONV was not correlated with the dose of remifentanil regimen, which was in contrast to a previous report [45].
A possible explanation for the higher pain score after remifentanil infusion is remifentanil-induced acute tolerance and hyperalgesia. The exact mechanism underlying opioid-induced hyperalgesia remains unclear. N-methyl-d-aspartate (NMDA) receptors have been shown to play a key role in opioid-induced hyperalgesia [46]. The reason for the inadequate postoperative pain control in the remifentanil protocol was attributed to NMDA activation. Sevoflurane, which is widely used in remifentanil-free groups, was reported to prevent central sensitisation through NMDA receptor antagonistic properties [46].
Opioid-induced thermal hyperalgesia can last for 2-7 days in rats [47]. Several studies have demonstrated that RIH occurred at 2 h and reached maximal at 24-48 h [47, 48]. In the present study, the higher pain scores in the high-dose remifentanil regimen lasted for 2 days after surgery.
Owing to the absence of opioid exposure, remifentanil-free anaesthesia can theoretically provide better resistance against the pathogenesis of hyperalgesia. Nevertheless, we found that there was no superiority over the remifentanil group in terms of postoperative pain scores at 24 and 48 h. Correspondingly, no improvement was detected in the rescue analgesic consumption at 24-48 h in the remifentanil-free group. Caution should be observed when using a remifentanil-free protocol in clinics.
The low-dose group inhibited postoperative pain during the first 48 h after surgery. Postoperative analgesic consumption, pain intensity and secondary hyperalgesia in the low-dose group were less evident than those in high-dose group. Notably, the degree of hyperalgesia was closely correlated with the amount of remifentanil infused. Compared with the remifentanil-free group, a small dose of remifentanil regimen seems recommendable.
The perioperative application of opioids is considered as a major factor in inducing PONV. The risk of PONV increases in direct proportion with the perioperative amount of opioid consumption [49]. Moreover, opioid-induced hyperalgesia requires more rescue opioids, which in turn aggravates PONV. A retrospective observational study [50] reported a dose-dependent association between the dose of intraoperative remifentanil and an increase in the risk of PONV. However, this meta-analysis upends our basic assumption. Regardless of remifentanil-free group or low-dose remifentanil regimen, the incidence of PONV did not decrease in comparison with the high-dose group. In other words, the incidence of PONV was not correlated with the amount of remifentanil infusion.
Postoperative shivering increases oxygen consumption, leading to an increased incidence of cardiovascular and neurological complications. It has been proposed that shivering results from rapid opioid withdrawal [51]. The present meta-analysis revealed an increased incidence of shivering after high dose of remifentanil.
Limitation
Firstly, the decrease in pain scores (0-10) was on average less than one point if there was a comparison between remifentanil and remifentanil-free regimens or high and low doses of remifentanil regimens. Even if this was statistically significant, it is doubtful that the difference was clinically significant as expected. This will not restrain the use of higher intraoperative remifentanil regimen, especially with the proper use of intraoperative pain monitoring. Secondly, most of the included studies were conducted without using any nociception monitoring. In the absence of a monitoring device, such as the Nociception Level index, the remifentanil dose was not known to be adequate or insufficient during surgery. Furthermore, the low and high doses of opioids overlapped among the trials. Therefore, there is a high heterogeneity for most pain scores comparisons between high and low doses of remifentanil regimens. The meta-regression and sensitivity analysis were performed to exclude the impact of the high heterogeneity and correct selective bias. We believe that this limitation did not affect the validity of our results.
Conclusion
Remifentanil-free anaesthesia has shown insufficient benefits in inhibiting postoperative pain. Patients receiving low dose remifentanil were correlated with lower pain scores, less allodynia and less shivering than those who received high dose remifentanil. In view of the current opioid epidemic, low-dose remifentanil anaesthesia should be recommended. These findings can be broadly generalised to patients across surgical disciplines.
Availability of data and materials
Raw extracted data are available (on request) from the corresponding authors (LC and JS).
Abbreviations
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- NMDA:
-
N-methyl-d-aspartate
- PONV:
-
Postoperative nausea and vomiting
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RIH:
-
Remifentanil-induced hyperalgesia
- SD:
-
Standard deviation
- SMD:
-
Standardised mean differences
- WMD:
-
Weighted mean differences
- 95% CI:
-
95% confidence interval
References
Mercadante S, Arcuri E, Santoni A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs. 2019;33:943–55.
Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 2014;112:906–11.
Mulier JP, Dillemans B. Anaesthetic factors affecting outcome after bariatric surgery, a retrospective levelled regression analysis. Obes Surg. 2019;29:1841–50.
Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74:793–800.
Cho AR, Kim HH, Kim KH, Jung KY, Kim WS, Kwon JY. Effect of remifentanil on postoperative pain in gynaecologic surgery with sevoflurane anaesthesia. Korean J Anesthesiol. 2008;55:182–8.
Song JW, Lee YW, Yoon KB, Park SJ, Shim YH. Magnesium sulphate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg. 2011;113:390–7.
Koo CH, Yoon S, Kim BR, et al. Intraoperative naloxone reduces remifentanil-induced postoperative hyperalgesia but not pain: a randomised controlled trial. Br J Anaesth. 2017;119:1161–8.
Koo CH, Cho YJ, Hong DM, Jeon Y, Kim TK. Influence of high-dose intraoperative remifentanil with intravenous ibuprofen on postoperative morphine consumption in patients undergoing pancreaticoduodenectomy: a randomised trial. J Clin Anesth. 2016;35:47–53.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Koppert W, Angst M, Alsheimer M, et al. Naloxone provokes similar pain facilitation as observed after short-term infusion of remifentanil in humans. Pain. 2003;106:91–9.
Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57.
Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999;89:S7–14.
Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–17.
Cortínez LI, Brandes V, Muñoz HR, Guerrero ME, Mur M. No clinical evidence of acute opioid tolerance after remifentanil-based anaesthesia. Br J Anaesth. 2001;87:866–9.
Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–55.
Lahtinen P, Kokki H, Hynynen M. Remifentanil infusion does not induce opioid tolerance after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:225–9.
Shin SW, Cho AR, Lee HJ, et al. Maintenance anaesthetics during remifentanil-based anaesthesia might affect postoperative pain control after breast cancer surgery. Br J Anaesth. 2010;105:661–7.
Richebé P, Pouquet O, Jelacic S, et al. Target-controlled dosing of remifentanil during cardiac surgery reduces postoperative hyperalgesia. J Cardiothorac Vasc Anesth. 2011;25:917–25.
Terao Y. Intraoperative magnesium sulphate does not suppress remifentanyl-induced acute opioids tolerance and hyperalgesia in surgical patients. Eur Soc Anesthesiol. 2010;27:208.
Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64:301–7.
Hansen EG, Duedahl TH, Rømsing J, Hilsted KL, Dahl JB. Intra-operative remifentanil might influence pain levels in the immediate post-operative period after major abdominal surgery. Acta Anaesthesiol Scand. 2005;49:1464–70.
Sahin A, Canbay O, Cuhadar A, Celebi N, Aypar U. Bolus ketamine does not decrease hyperalgesia after remifentanil infusion. Pain Clinic. 2004;16:407–11.
Yeom JH, Kim KH, Chon MS, Byun J, Cho SY. Remifentanil used as adjuvant in general anaesthesia for spinal fusion does not exhibit acute opioid tolerance. Korean J Anesthesiol. 2012;63:103–7.
Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulphate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anaesthesia. Korean J Anesthesiol. 2011;61:244–50.
Lee C, Song YK, Lee JH, Ha SM. The effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy. Korean J Pain. 2011;24:7–12.
Lee C, Lee HW, Kim JN. Effect of oral pregabalin on opioid-induced hyperalgesia in patients undergoing laparo-endoscopic single-site urologic surgery. Korean J Anesthesiol. 2013;64:19–24.
Agata H, Yumura J, Miki M, Koitabashi T. High dose remifentanil administration during orthognathic surgery is associated with postoperative hyperalgesia. J Jpn Dental Soc Anesthesiol. 2010;38:13–20.
Ryu SH, Lee DW, Kwon JY. The effect of remifentanil with sevoflurane in subtotal gastrectomy patients with patient controlled epidural analgesia. Korean J Anesthesiol. 2007;53:35–41.
Chang SY, Sun RQ, Feng M, et al. The use of remifentanil in critically ill patients undergoing percutaneous dilatational tracheostomy: a prospective randomised-controlled trial. Kaohsiung J Med Sci. 2019;35:111–5.
Florkiewicz P, Musialowicz T, Pitkänen O, Lahtinen P. The effect of two different doses of remifentanil on postoperative pain and opioid consumption after cardiac surgery–a randomised controlled trial. Acta Anaesthesiol Scand. 2015;59:999–1008.
Khidr AM, Khalil MA, Abdulfattah D, El Tahan MR. A Comparison of Different Remifentanil Effect-Site Concentrations to Allow for Early Extubation After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021;35:470–81.
Kim D, Lim HS, Kim MJ, Jeong W, Ko S. High-dose intraoperative remifentanil infusion increases early postoperative analgesic consumption: a prospective, randomized, double-blind controlled study. J Anesth. 2018;32:886–92.
Kim SH, Oh CS, Yoon TG, Cho MJ, Yang JH, Yi HR. Total intravenous anaesthesia with high-dose remifentanil does not aggravate postoperative nausea and vomiting and pain, compared with low-dose remifentanil: a double-blind and randomized trial. ScientificWorldJournal. 2014;2014:724753.
Park JH, Lee YC, Lee J, Kim H, Kim HC. The influence of high-dose intraoperative remifentanil on postoperative sore throat: a prospective randomised study: a CONSORT compliant article. Medicine (Baltimore). 2018;97:e13510.
Polat R, Peker K, Baran I, BuminAydın G, TopçuGülöksüz Ç, Dönmez A. Comparison between dexmedetomidine and remifentanil infusion in emergence agitation during recovery after nasal surgery: A randomised double-blind trial. Anaesthesist. 2015;64:740–6.
Su X, Zhu W, Tian Y, Tan L, Wu H, Wu L. Regulatory effects of propofol on high-dose remifentanil-induced hyperalgesia. Physiol Res. 2020;69:157–64.
Treskatsch S, Klambeck M, Mousa SA, Kopf A, Schäfer M. Influence of high-dose intraoperative remifentanil with or without amantadine on postoperative pain intensity and morphine consumption in major abdominal surgery patients: a randomised trial. Eur J Anaesthesiol. 2014;31:41–9.
Yamashita S, Yokouchi T, Tanaka M. Effects of intraoperative high-dose vs low-dose remifentanil for postoperative epidural analgesia after gynaecological abdominal surgery: a randomized clinical trial. J Clin Anesth. 2016;32:153–8.
Zhang YL, Ou P, Lu XH, Chen YP, Xu JM, Dai RP. Effect of intraoperative high-dose remifentanil on postoperative pain: a prospective, double blind, randomized clinical trial. PLoS One. 2014;9:e91454.
Cabañero D, Campillo A, Célérier E, Romero A, Puig MM. Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: effect of a second surgery. Anesthesiology. 2009;111:1334–45.
Albrecht E, Grape S, Frauenknecht J, Kilchoer L, Kirkham KR. Low- versus high-dose intraoperative opioids: A systematic review with meta-analyses and trial sequential analyses. Acta AnaesthesiolScand. 2020;64:6–22.
Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004.
Salomé A, Harkouk H, Fletcher D, Martinez V. Opioid-Free Anaesthesia Benefit-Risk Balance: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J Clin Med. 2021;10:2069.
Richebé P, Rivalan B, Rivat C, et al. Effects of sevoflurane on carrageenan- and fentanyl-induced pain hypersensitivity in Sprague-Dawley rats. Can J Anaesth. 2009;56:126–35.
Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009;5:76.
Yuan Y, Wang JY, Yuan F, Xie KL, Yu YH, Wang GL. Glycogen synthase kinase-3β contributes to remifentanil-induced postoperative hyperalgesia via regulating N-methyl-D-aspartate receptor trafficking. Anesth Analg. 2013;116:473–81.
Apfel CC, Kranke P, Eberhart LH. Comparison of surgical site and patient’s history with a simplified risk score for the prediction of postoperative nausea and vomiting. Anaesthesia. 2004;59:1078–82.
Hozumi J, Egi M, Sugita S, Sato T. Dose of intraoperative remifentanil administration is independently associated with increase in the risk of postoperative nausea and vomiting in elective mastectomy under general anaesthesia. J Clin Anesth. 2016;34:227–31.
Lewis SR, Nicholson A, Smith AF, Alderson P. Alpha-2 adrenergic agonists for the prevention of shivering following general anaesthesia. Cochrane Database Syst Rev. 2015;8:CD011107.
Acknowledgements
None.
Funding
This work was supported by the Chinese National Natural Science Young Scientists Funding [81701094] and Zhejiang Provincial Natural Science Funding [LY20H090008], Zhejiang Provincial Health Commission Funding [2023RC204 and 2022KY501].
Author information
Authors and Affiliations
Contributions
Conception and design, XH and JS; search strategy and procurement of studies, XH and JS; studies selection, XH, JC, and ZL, with LC as an arbiter; data extraction, XH, JC, and ZL; analyses, XH.; analysis interpretation, XH, QZ, and ZZ; final manuscript drafting, XH and XZ. Both XH, JS, and LC were responsible for updating the final paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA checklist.
Additional file 2.
Search Strategy Based on initial PubMed Search.
Additional file 3.
Risk of bias summary.
Additional file 4.
Risk of bias assessment for included studies.
Additional file 5.
Trial sequential analysis of pain score between the two different remifentanil doses at the 24 h. X-axis: the number of patients randomised; Y-axis: the cumulative Z-score; the blue cumulative Z-curve was constructed using a random-effects model. Red vertical line with diamonds: required information size of a meta-analysis.
Additional file 6.
Publication bias for pain scores at 1-2 h (A; P=0.078), 3-8 h (B; P=0.058), 24 h (C; P=0.633), and 48 h (D; P=0.612) postoperatively.
Additional file 7.
Mixed meta-regression (methods of moment) to assess the interaction between remifentanil dose equivalent and hyperalgesia at 24 h postoperatively before sensitivity analysis (P=0.57). The size of the markers is proportional to the size of the study. Std diff, standardized difference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, X., Cai, J., Lv, Z. et al. Postoperative pain after different doses of remifentanil infusion during anaesthesia: a meta-analysis. BMC Anesthesiol 24, 25 (2024). https://doi.org/10.1186/s12871-023-02388-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02388-3