Abstract
Background
We aimed to compare the hemodynamic effect of two ratios of propofol and ketamine (ketofol), namely 1:1 and 1:3 ratios, in rapid-sequence induction of anesthesia for emergency laparotomy.
Methods
This randomized controlled study included adult patients undergoing emergency laparotomy under general anesthesia. The patients were randomized to receive either ketofol ratio of 1:1 (n = 37) or ketofol ratio of 1:3 (n = 37). Hypotension (mean arterial pressure < 70 mmHg) was managed by 5-mcg norepinephrine. The primary outcome was total norepinephrine requirements during the postinduction period. Secondary outcomes included the incidence of postinduction hypotension, and the intubation condition (excellent, good, or poor).
Results
Thirty-seven patients in the ketofol-1:1 and 35 patients in the ketofol 1:3 group were analyzed. The total norepinephrine requirement was less in the ketofol-1:1 group than in the ketofol-1:3 group, P-values: 0.043. The incidence of postinduction hypotension was less in the ketofol-1:1 group (4 [12%]) than in ketofol-1:3 group (12 [35%]), P-value 0.022. All the included patients had excellent intubation condition.
Conclusion
In patients undergoing emergency laparotomy, the use of ketofol in 1:1 ratio for rapid-sequence induction of anesthesia was associated with less incidence of postinduction hypotension and vasopressor consumption in comparison to the 1:3 ratio with comparable intubation conditions.
Clinical trial registration
NCT05166330. URL: https://clinicaltrials.gov/ct2/show/NCT05166330.
Similar content being viewed by others
Introduction
Anesthesia-induced hypotension is associated with serious organ failure and death [1]. The postinduction period constitute about one-third of intraoperative hypotensive episodes [2, 3]. Post-induction hypotension has several contributing factors; however, it is closely related to the anesthetic drugs [4]. Therefore, manipulation of induction agents makes post-induction hypotension likely preventable.
Patients undergoing emergency laparotomy are usually hemodynamically compromised and prone to post-induction hypotension; furthermore, these patients are usually at high risk of aspiration of gastric contents and require rapid-sequence induction of anesthesia and optimum intubating conditions.
Thus, induction of anesthesia for emergency laparotomy requires meticulous balance between achieving adequate hypnosis and maintenance of stable hemodynamics. Propofol is the commonest hypnotic agent worldwide. However, it is usually associated with hypotension especially in compromised patients. Ketamine produces dissociative anesthesia and sympathetic stimulation which provides more stable hemodynamic profile; however, ketamine is not widely used as a routine hypnotic [5].
Nevertheless, ketamine still has a role in induction of anesthesia in patients with shock and during procedural sedation [6, 7]. Ketamine is also used as analgesic adjuvant during general anesthesia [8].
Propofol/ketamine admixture (ketofol) was introduced in anesthetic practice aiming to counterbalance the side effects of the two drugs and to provide, consequently, the desired balance between adequate hypnosis and hemodynamic stability [9]. Ketofol is currently used with a diversity in the ratio between the two drugs which ranges between 1:1 and 1:10 [10,11,12]. Despite its frequent use in sedation and complete anesthesia, most of the available literature for comparisons of different ketofol mixtures was restricted to procedural sedation whose results are not applicable in induction of anesthesia due to the different desirable level of hypnosis and recovery. Therefore, the best combination of the two components of ketofol for induction of anesthesia is unknown.
The aim of this study is to compare two ratios of propofol and ketamine, namely 1:1 and 1:3 ratios, in rapid-sequence induction of anesthesia for emergency laparotomy regarding the vasopressor consumption, hemodynamic profile, adequacy of hypnosis and intubation conditions.
Materials and methods
Study design and order
This randomized controlled trial was conducted in Cairo University Hospital, emergency surgical theatre, from January to May 2022, after institutional Research Ethics Committee approval (October 10, 2021, No: MS-450-2021) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at clinicaltrials.gov (NCT05166330, Date of registration: 21/12/2021). All methods were carried out in accordance with the principles set forth in Helsinki.
Population
Inclusion criteria included American society of anesthesiologist (ASA) physical status I-III patients aged 18–65 years old, scheduled for emergency laparotomy under general anesthesia.
Patients with a history of difficult intubation, abnormal airway examination, cardiac morbidities (impaired contractility with ejection fraction < 50%, heart block, arrhythmias, tight valvular lesions), patients on angiotensin converting enzyme inhibitors and angiotensin receptor blockers medications, patients with uncontrolled hypertension, patient with allergy of any of the study drugs were excluded from the study. Patients on vasopressor infusion, patients with high shock index (heart rate / systolic blood pressure > 1), body mass index > 35 kg/m2, increased intracranial tension and pregnant women were also excluded.
Study protocol
Randomization was achieved by computer-generated sequence in a 1:1 ratio. Opaque sequentially numbered envelopes were prepared containing group assignment and drugs preparation instruction. The opening of the envelopes and drug preparation were done by an independent researcher with no further involvement in the study. The attending anesthetist and data collector were blinded to the study group.
Drug preparation
Ketofol-1:1 group: 10 mL propofol (Propofol 1%, 10 mg/1 ml, FRESENIUS KABI DEUTSCHLAND GmbH, Deutschland) was mixed with 2 mL ketamine (Ketam 50 mg/mL, EPICO, Cairo, Egypt) and then diluted to a total volume of 20 mL to have a final concentration of 5 mg/mL propofol and 5 mg/mL ketamine.
Ketofol-1:3 group: 15 mL propofol (150 mg) was mixed with 1 mL ketamine (50 mg) and then diluted to a total volume of 20 mL to have a final concentration of 7.5 mg/mL propofol and 2.5 mg/mL ketamine.
Preoperatively, a trained anesthetist assessed the patients regarding the fasting hours, medical history, medications, laboratory investigation, as well as the patient’s airway.
In the operating room, electrocardiogram, pulse oximetry, and non-invasive blood pressure monitor were applied. After obtaining vascular access, slow intravenous 4 mg dexamethasone (DEXAMETHASONE 4 mg/mL – MUP, Medical Union Pharmaceutical, Cairo, Egypt) was given for prophylaxis against postoperative nausea and vomiting and for its analgesic property. In the supine position, baseline blood pressure was recorded as the average of three consecutive readings with difference < 10% in the systolic blood pressure.
Before induction of anesthesia for all study patients, volume assessment was done by measuring the baseline pulse pressure then giving a fluid challenge of 4ml/kg over 10 min. If the pulse pressure increased by > 15% of baseline, the patient was considered to be fluid responder, and the fluid challenge was repeated untill the increase in the pulse pressure was < 15% of baseline.
After 3-minutes preoxygenation, patients in the two groups received 1 mg/kg lidocaine in a separate syringe (Lidocaine Hydrochloride 2%, Sunny Pharmaceutical, Cairo, Egypt) plus 0.15–0.20 mL/kg of the prepared admixture until achieving clinical loss of consciousness (defined as no response to auditory command and the disappearance of a patient’s eyelash reflex).
After loss of consciousness, succinylcholine 1 mg/kg (Succinylcholine Chloride Injection 500 mg/5mL, Misr Co. for Pharm. Ind. S.A.E.) was administered over 5 s, and tracheal intubation was done through direct laryngoscopy after 60 s.
The intubation conditions were graded by the same anesthetist who performed intubation. The assessment included 1- ease of laryngoscopy (easy: jaw relaxed, no resistance to blade insertion; fair: jaw not fully relaxed, slight resistance to blade insertion; difficult: poor jaw relaxation, active resistance of the patient to laryngoscopy), 2- vocal cord position (easy: abducted; fair: intermediate/moving; difficult: closed), and 3- reaction to insertion of the tracheal tube and cuff inflation (Diaphragmatic movement/coughing) (easy: none; fair: one to two weak contractions or movement for less than 5 s; difficult: more than two contractions and/or movement for longer than 5 s).
The intubation condition was graded as excellent if all criteria are excellent, good if all criteria are either excellent or good, or poor if there was any criterion graded as poor [13].
When the trachea was intubated, mechanical ventilation was applied to obtain peripheral oxygen saturation > 95% and end-tidal CO2 between 30 and 40 mmHg and anesthesia were maintained by isoflurane in air/oxygen admixture (with target end tidal isoflurane 1%). Atracurium was administered after patient recovery from succinylcholine at a dose of 0.5 mg/Kg.
Any episode of hypotension (mean arterial pressure < 70 mmHg) was managed by a 5-mcg norepinephrine bolus (Norepinephrine 4 mg/4mL, Sunny Pharmaceutical, Cairo, Egypt), which was repeated if hypotension persists for 2 min).
Hypertension and tachycardia were defined as mean arterial pressure or heart rate > 120% of baseline, respectively. Persistent hypertension (blood pressure increasing after one measurement) was managed by intravenous 0.25 mg/kg propofol. Bradycardia (heart rate < 50 bpm) was managed by 0.5 mg of intravenous atropine.
After skin incision, hemodynamic and anesthetic management was according to the attending anesthetist discretion.
The primary outcome was total norepinephrine requirements during the period from induction of anesthesia until 16-minutes after intubation.
Secondary outcomes were incidence of post-induction hypotension, severe post-induction hypotension (mean arterial pressure < 60 mmHg), hypertension, bradycardia, and tachycardia during the period from induction of anesthesia until 16-minutes after intubation. Mean arterial pressure, heart rate was recorded at baseline, immediately after induction, after intubation, then every 2-minutes for 16-minutes after intubation. The Number of hypotensive episodes per patients, intubation condition (the number of patients with excellent, good, and poor intubation conditions), intubation time (time from insertion of the laryngoscope into the mouth until its removal after tracheal intubation), total ketofol volume, and total propofol and ketamine dose per weight.
Age, sex, weight, body mass index, American society of anesthesiologists-physical status, shock index, and preoperative fluid volume were also recorded.
Statistical analysis
Sample size was calculated using the MedCalc Software version 14 (MedCalc Software bvba, Ostend, Belgium). In a pilot study on 14 patients (7 in each group), the mean norepinephrine dose in patients receiving ketofol 1:3 was 3.9 ± 5.2 mcg; and in patients receiving ketofol 1:1, norepinephrine dose was 0.7 ± 1.7 mcg. At alpha error of 0.05, we calculated that 68 patients would give 80% power to detect significant difference in the norepinephrine dose between the two groups. The number of prepared envelopes was 74 (37 envelopes per group) to compensate for possible dropouts.
Statistical package for social science (SPSS) software, version 26 for Microsoft Windows (Armonk, NY: IBM Corp) was used for data analysis. Categorical data were presented as frequency (%) and were analyzed by the Chi squared test. Continuous data were checked for normality using the Shapiro-Wilk test and were presented as mean ± standard deviation or median (quartiles) as appropriate. Continuous data were analyzed using the unpaired t test or the Mann Whitney test according to normality of the data. Repeated measured data were analyzed using the analysis of variance for repeated measures with post-hoc pairwise comparisons using the Boneferroni tests. A P-value less than 0.05 was considered statistically significant.
Results
Seventy-seven patients were screened for eligibility, 3 patients were excluded for not fulfilling the inclusion criteria and 74 patients were equally randomized in to one of the study groups. Two patients in the ketofol 1:3 group did not receive the assigned intervention. Thirty-seven patients in the ketofol 1:1group and 35 patients in the ketofol 1:3 group were included and were available for the final analysis. (Fig. 1)
Patients’ demographic data and baseline hemodynamic data were comparable between the two groups. (Table 1)
The total volume of ketofol was comparable between the two groups. All the included patients had excellent intubation condition and the intubation time was comparable between the two groups. (Table 1)
The total norepinephrine requirement was less in the ketofol-1:1 group than in the ketofol-1:3 group, P-values: 0.043. The incidence of postinduction hypotension was less in the ketofol-1:1 group than in ketofol-1:3 group (6 [16%] and 13 [37%], respectively, P-value 0.044). Furthermore, the number of hypotensive episodes per patient were likely to be less in the ketofol-1:1 group than in the ketofol-1:3 group. (Table 2)
The incidence of hypertension and tachycardia were comparable between the two groups and none of the included patients had severe hypotension nor bradycardia. (Table 2)
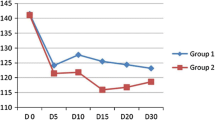
The mean arterial pressure and heart rate were comparable between the two groups. (Figures 2 and 3)
The mean arterial pressure decreased in relation to the baseline value starting 12-minute postintubation in ketofol-1:1 group and 6-minute postintubation in ketofol-1:3 group. (Fig. 2)
The heart rate increased in both groups following the intubation. The heart rate became comparable to the baseline reading 8-minute and 6-minute postintubation in ketofol-1:1 and ketofol-1:3 group, respectively. (Fig. 3)
Discussion
We compared two combinations of propofol and ketamine (1:1 and 1:3 ratio) for rapid-sequence induction of anesthesia in patients undergoing emergency laparotomy and found that the former dose (1:1) produced less incidence of hypotension compared to the 1:3 dose. The additive hypnotic action of the two drugs is well established [9]. Propofol is the most widely used hypnotic for induction of anesthesia and sedation due to its many favorable characteristics such as: rapid onset and offset without residual hang-over; antiemetic effect; and amnesia. However, its main disadvantage is the negative cardiovascular effect leading hypotension which is sometimes severe and serious [14]. Ketamine is another hypnotic agent which produces dissociative anesthesia and analgesia. Ketamine is characterized by a sympathomimetic effect which compensates the hemodynamic depressant effect of propofol [14, 15]. Thus, the combination of the two drugs provides a balance between the advantages and disadvantages of either drug alone [14].
Ketofol had been previously investigated in procedural sedation in the emergency department [16] and showed lower respiratory complication but conflicting results regarding its hemodynamic effect in comparison to propofol. Different ratios of ketofol were compared during procedural sedation and showed comparable hemodynamic effect [11]. When used for induction of anesthesia, ketofol showed better hemodynamic stability compared to propofol alone [17]. However, the hemodynamic effect of different ratios of ketofol for induction of anesthesia has not been yet explored. We hypothesized that at the induction dose, different ketofol ratios would have different hemodynamic effect. Our study is the first to compare different ratios of ketofol for induction of anesthesia in adult patients undergoing emergency laparotomy and showed superiority of the 1:1 ratio over 1:3 ratio. This finding differed from previous data in adults during procedural sedation; and pediatric population under total intravenous anesthesia [11, 12] that failed to find superiority for any ratio of the two drugs over the other. Our study included adult patients scheduled for emergency laparotomy which is usually associated with more hypotension than sedation due to the higher doses of hypnotic drugs as well as the effect of positive pressure ventilation; this might explain the of superiority of the 1:1 ratio in our results. Furthermore, our main objective was the hemodynamic profile of the two drugs while the objective of procedural sedation studies was the frequency of airway events.
In the current study, hypertension and tachycardia occurred in nearly 30% of patients in both groups. This could be due to either a hyperdynamic response to tracheal intubation or reactive hypertension due norepinephrine administration for hypotension treatment. We believe that Inadequate depth of anesthesia is unlikely to be the cause of this observation since the assigned drug was carefully titrated until reaching adequate hypnosis. We used clinical loss of consciousness as the hypnotic endpoint which is supported by the current evidence [18, 19] and all patients had excellent overall intubation condition. Using bispectral index in guiding induction of anesthesia is not feasible with the use of ketamine and lidocaine [20, 21]. During the maintenance period, appropriate depth of anesthesia was achieved by maintaining the end tidal isoflurane concentration at 1% [22]. In addition, the hyperdynamic response to tracheal intubation can occur despite the use of anesthetic doses of hypnotic drugs [23, 24].
The ketofol doses used in this study were within the range of what previously reported during induction of anesthesia [9, 10, 25]. We used lidocaine as an adjuvant which has an anesthetic-sparing effects and this helped in reaching adequate hypnosis using the current doses of ketamine and propofol [6, 25, 26].
Emergency gastrointestinal surgery is a high-risk surgery and is usually performed to control a life-threatening pathology [27]. Therefore, emergency laparotomy is commonly associated with major perioperative complications in 50% of the patients [28] and high mortality rates [29].
Hypotension is recognized as a major risk factor for perioperative morbidity and mortality [30] with the postinduction period being recognized as the most critical with a substantial proportion of the total hypotensive episodes during surgery [2]. Postinduction and pre-incision hypotension is associated with impaired cerebral perfusion [31] and postoperative kidney injury [2]. The most recognized threshold for perioperative hypotension that is related to postoperative morbidity and mortality was MAP 60–70 mmHg [32]. In this study we had the advantage of choosing a conservative threshold of MAP 70 mmHg as we used non-invasive blood pressure monitor which in turn tend to overestimate the low blood pressure values [33]. We used the absolute MAP value to define hypotension instead of relative reduction for several reasons; both absolute and relative hypotension threshold had similar postoperative morbidity risk [34]; the use of absolute MAP values is easier to the clinician; and the preoperative blood pressure does not reflect the patient’s ambulatory blood pressure [35].
Another advantage is the use of an opioid-free protocol for induction of anesthesia. Previous data showed that opioid-based protocol for induction of anesthesia increases the risk of postinduction hypotension and that lidocaine-based protocol provided stable postinduction hemodynamic with similar intubating condition in comparison to opioid-based protocol [25]. In addition, ketamine has good analgesic properties which could compensate for the absence of opioids.
We also had the advantage of including a special vulnerable group of (emergency surgery patients). Emergency surgery represents an independent risk factor for postinduction hypotension [3]; therefore, it is desirable to find the optimum anesthetic technique during these procedures to improve patient outcomes.
In this study, the period of assessment of postinduction hypotension was 16 min which is within the range of earlier studies assessing the postinduction hypotension (10–30 min) [3, 36,37,38]. Furthermore, longer assessment period would lead to unnecessary delay of an emergency surgery and shorter assessment period would not allow for proper hemodynamic assessment.
According to our findings, we suggest the use of ketofol 1:1 ratio would provide less incidence of hypotension during induction of anesthesia for emergency laparotomy, our study has some limitations such as being performed in a single center, excluding patients with major cardiac morbidities (e.g., stenotic valvular lesions and poor cardiac contractility) and including low number of patients with high ASA classification. Future studies will be needed to confirm our findings in other surgeries and other groups of patients. In this study, the blood pressure was monitored noninvasively as all our patients were hemodynamically stable and invasive blood pressure monitoring is not routine in such patients during induction of anesthesia. We did not record the postoperative course of the participants and their final outcomes since our main objective was the postinduction hypotension; therefore, future studies are needed to evaluate the effect of anesthetic choice on postoperative outcomes.
In patients undergoing emergency laparotomy, the use of ketofol in 1:1 ratio for rapid-sequence induction of anesthesia was associated with less incidence of postinduction hypotension and vasopressor consumption in comparison to the 1:3 ratio with comparable intubation conditions.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping STJ, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–19.
Maheshwari K, Turan A, Mao G, Yang D, Niazi AK, Agarwal D, et al. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–8.
Südfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57–64.
Saugel B, Bebert EJ, Briesenick L, Hoppe P, Greiwe G, Yang D et al. Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput. 2021.
Groth CM, Acquisto NM, Khadem T. Current practices and safety of medication use during rapid sequence intubation. J Crit Care. 2018;45:65–70.
Fathy S, Hasanin A, Mostafa M, Ramzy E, Sarhan K, Almenesey T et al. The benefit of adding lidocaine to ketamine during rapid sequence endotracheal intubation in patients with septic shock: a randomised controlled trial. Anaesth Crit Care Pain Med. 2020.
Azizkhani R, Bahadori A, Shariati M, Golshani K, Ahmadi O, Masoumi B. Ketamine versus ketamine / magnesium sulfate for Procedural Sedation and Analgesia in the Emergency Department: a Randomized Clinical Trial. Adv Biomed Res. 2018;7:19.
Beloeil H, Garot M, Lebuffe G, Gerbaud A, Bila J, Cuvillon P et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery the postoperative and opioid-free anesthesia (POFA) randomized clinical trial. Anesthesiology. 2021;:541–51.
Hui TW, Short TG, Hong W, Suen T, Gin T, Plummer J, et al. Additive interactions between propofol and ketamine when used for Anesthesia induction in female patients. Anesthesiology. 1995;82:641–8.
Smischney NJ, Nicholson WT, Brown DR, Gallo De Moraes A, Hoskote SS, Pickering B, et al. Ketamine/propofol admixture vs etomidate for intubation in the critically ill: KEEP PACE randomized clinical trial. J Trauma Acute Care Surg. 2019;87:883–91.
Miner JR, Moore JC, Austad EJ, Plummer D, Hubbard L, Gray RO, Randomized. Double-Blinded, clinical trial of Propofol, 1:1 Propofol/Ketamine, and 4:1 Propofol/Ketamine for Deep Procedural Sedation in the Emergency Department. Ann Emerg Med. 2015;65.
Biricik E, Karacaer F, Güleç E, Sürmelioğlu Ö, Ilgınel M, Özcengiz D. Comparison of TIVA with different combinations of ketamine–propofol mixtures in pediatric patients. J Anesth. 2018;32:104–11.
Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789–808.
Alletag MJ, Auerbach MA, Baum CR. Ketamine, propofol, and ketofol use for pediatric sedation. Pediatr Emerg Care. 2012;28:1391–5. quiz 1396–8.
White PF, Way WL, Trevor AJ. Ketamine–its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–36.
Ghojazadeh M, Sanaie S, Paknezhad SP, Faghih SS, Soleimanpour H. Using ketamine and propofol for procedural sedation of adults in the emergency department: a systematic review and meta-analysis. Adv Pharm Bull. 2019;9:5–11.
Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: a randomized, controlled trial. J Trauma Acute Care Surg. 2012;73:94–101.
MacG Palmer J, Pandit J. AAGA during induction of anaesthesia and transfer into theatre. 5th Natl Audit Proj R Coll Anaesth Assoc Anaesth Gt Britain Ireland London Accid Aware Dur Gen Anaesth United Kingdom Irel. 2014;:63–76.
Zetterlund EL, Gréen H, Oscarsson A, Vikingsson S, Vrethem M, Lindholm ML, et al. Determination of loss of consciousness: a comparison of clinical assessment, bispectral index and electroencephalogram: an observational study. Eur J Anaesthesiol. 2016;33:922–8.
Bazin P, Padley J, Ho M, Stevens J, Ben-Menachem E. The effect of intravenous lidocaine infusion on bispectral index during major abdominal surgery. J Clin Monit Comput. 2018;32:533–9.
Hans P, Dewandre P-Y, Brichant JF, Bonhomme V. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–40.
Klein AA, Meek T, Allcock E, Cook TM, Mincher N, Morris C, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021: Guideline from the Association of Anaesthetists. Anaesthesia. 2021;76:1212–23.
Min JH, Chai HS, Kim YH, Chae YK, Choi SS, Lee A, et al. Attenuation of hemodynamic responses to laryngoscopy and tracheal intubation during rapid sequence induction: Remifentanil vs. lidocaine with esmolol. Minerva Anestesiol. 2010;76:188–91.
Lee SY, Min JJ, Kim HJ, Hong DM, Kim HJ, Park HP. Hemodynamic effects of topical lidocaine on the laryngoscope blade and trachea during endotracheal intubation: a prospective, double-blind, randomized study. J Anesth. 2014;28:668–75.
Amin SM, Hasanin A, ElSayed OS, Mostafa M, Khaled D, Arafa AS, et al. Comparison of the hemodynamic effects of opioid-based versus lidocaine-based induction of anesthesia with propofol in older adults: a randomized controlled trial. Anaesth Crit Care Pain Med. 2023;42:101225.
Ben-Shlomo I, Tverskoy M, Fleyshman G, Cherniavsky G. Hypnotic effect of i.v. propofol is enhanced by i.m. administration of either lignocaine or bupivacaine. Br J Anaesth. 1997;78:375–7.
Poulton T, Murray D. Pre-optimisation of patients undergoing emergency laparotomy: a review of best practice. Anaesthesia. 2019;74:100–7.
Vester-Andersen M, Waldau T, Wetterslev J, Møller MH, Rosenberg J, Jørgensen LN, et al. Randomized multicentre feasibility trial of intermediate care versus standard ward care after emergency abdominal surgery (InCare trial). Br J Surg. 2015;102:619–29.
Watt DG, Wilson MSJ, Shapter OC, Patil P. 30-Day and 1-year mortality in emergency general surgery laparotomies: an area of concern and need for improvement? Eur J Trauma Emerg Surg. 2015;41:369–74.
Wijnberge M, Schenk J, Bulle E, Vlaar AP, Maheshwari K, Hollmann MW et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS open. 2021;5.
Chaix I, Manquat E, Liu N, Casadio MC, Ludes P-O, Tantot A, et al. Impact of hypotension on cerebral perfusion during general anesthesia induction: a prospective observational study in adults. Acta Anaesthesiol Scand. 2020;64:592–601.
Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–74.
Lakhal K, Ehrmann S, Boulain T. Noninvasive BP monitoring in the critically ill: time to abandon the arterial catheter? Chest. 2018;153:1023–39.
Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or Absolute Thresholds, and Acute kidney and myocardial Injury after noncardiac surgery. Anesthesiology. 2017;126:47–65.
Saugel B, Sessler DI. Perioperative blood pressure management. Anesthesiology. 2021;134:250–61.
Khan AI, Fischer M, Pedoto AC, Seier K, Tan KS, Dalbagni G, et al. The impact of fluid optimisation before induction of anaesthesia on hypotension after induction. Anaesthesia. 2020;75:634–41.
Kendale S, Kulkarni P, Rosenberg AD, Wang J. Supervised machine-learning Predictive Analytics for Prediction of Postinduction Hypotension. Anesthesiology. 2018;129:675–88.
Hojo T, Kimura Y, Shibuya M, Fujisawa T. Predictors of hypotension during anesthesia induction in patients with hypertension on medication: a retrospective observational study. BMC Anesthesiol. 2022;22:1–8.
Acknowledgements
NA.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Mona Elsherbiny, Ahmed Hasanin, Sahar Kasem, Mohamed Abouzeid, Maha Mostafa, Ahmed Fouad, Yaser Abdelwahab]. The first draft of the manuscript was written by [Ahmed Hasanin, Maha Mostafa] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical approval from Cairo university hospitals research ethics committee was obtained (MS-450-2021). Written informed consents were obtained from participants before inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elsherbiny, M., Hasanin, A., Kasem, S. et al. Comparison of different ratios of propofol-ketamine admixture in rapid-sequence induction of anesthesia for emergency laparotomy: a randomized controlled trial. BMC Anesthesiol 23, 329 (2023). https://doi.org/10.1186/s12871-023-02292-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02292-w