Abstract
Background
There is the ongoing debate over the effect of inspired oxygen fraction (FiO2) during mechanical ventilation on postoperative atelectasis. We aimed to compare the effects of low (30%) and moderate (60%) FiO2 on postoperative atelectasis. The hypothesis of the study was that 30% FiO2 during mechanical ventilation could reduce postoperative atelectasis volume compared with 60% FiO2.
Methods
We performed a randomized controlled trial with 120 patients. Subjects were randomly assigned to receive 30% or 60% FiO2 during mechanical ventilation in a 1:1 ratio. The primary outcome was the percentage of postoperative atelectasis volume in the total lung measured using chest CT within 30 min after extubation. The secondary outcomes included different aeration region volumes, incidence of clinically significant atelectasis, and oxygenation index.
Results
In total, 113 subjects completed the trial, including 55 and 58 subjects in the 30% and 60% FiO2 groups, respectively. The percentage of the postoperative atelectasis volume in the 30% FiO2 group did not differ from that in the 60% FiO2 group. Furthermore, there was no significant difference in the atelectasis volume between the two groups after the missing data were imputed by multiple imputation. Additionally, there were no significant differences in the volumes of the over-aeration, normal-aeration, and poor-aeration regions between the groups. No significant differences in the incidence of clinically significant atelectasis or oxygenation index at the end of surgery were observed between the groups.
Conclusions
Compared with 60% FiO2, the use of 30% FiO2 during mechanical ventilation does not reduce the postoperative atelectasis volume.
Trial registration
Chinese Clinical Trial Registry (http://www.chictr.org.cn). Identifier: ChiCTR1900021635. Date: 2 March 2019. Principal invetigator: Weidong Gu.
Similar content being viewed by others
Background
Postoperative atelectasis diagnosed by CT occurs in 60–90% of patients with mechanical ventilation under general anesthesia [1,2,3,4]. The postoperative atelectasis is associated with prolonged hospitalization, increased hospital costs, and increased postoperative 90-day mortality [5,6,7]. Therefore, prevention of postoperative atelectasis is important for perioperative management, especially in patients undergoing major surgery.
Mechanical ventilation provides the necessary oxygen supply for patients under general anesthesia during surgery, however, the optimal inspired oxygen fraction (FiO2) during mechanical ventilation remains controversial. The World Health Organization (WHO) recommends high FiO2 to reduce the risk of postoperative surgical site infections in patients undergoing general anesthesia [8]. However, this recommendation has sparked debate on the benefits and harms of hyperoxia [9, 10]. High oxygen concentrations have been reported to be associated with postoperative pulmonary complications (PPCs), especially atelectasis [11]. Kim et al. found that postoperative atelectasis occurred more frequently with 100% FiO2 than with 40% FiO2 [12]. And a meta-analysis found that the extent of postoperative atelectasis was more severe in the high intraoperative FiO2 group compared with the low FiO2 group [13]. Conversely, two randomised controlled trials have shown no differences in the incidence of PPCs, including atelectasis, between 80% and 30% FiO2 [3, 14].
Previous studies have provided conflicting results; thus the effect of FiO2 on atelectasis requires further investigation. Additionally, several issues in such research should be noted and improved upon. First, most of the previous studies usually compared the effects of extremely high FiO2 (80–100%) and low FiO2 (30–40%) on atelectasis. In clinical practice, a moderate FiO2 of 50–60% is more conventionally used. However, the effect of low (30%) versus moderate (60%) FiO2 on atelectasis remains unclear. Second, positive end-expiratory pressure (PEEP) and recruitment maneuvers have often been used in studies to investigate the effect of FiO2 on atelectasis [15, 16]. As PEEP and recruitment maneuvers could reduce the incidence and extent of atelectasis [17], the independent effect of FiO2 on atelectasis remains to be investigated. Third, although lung ultrasound was used in previous studies to diagnose atelectasis, it cannot measure the volume of atelectasis [16]. Computed tomography (CT) is the current gold standard for diagnosing atelectasis and it can accurately measure the volume of different aeration regions [18].
Therefore, we conducted a randomised controlled study to investigate the effects of 30% versus 60% FiO2 without PEEP and recruitment maneuvers on postoperative atelectasis volume measured by CT scans. We tested the hypothesis that 30% FiO2 during mechanical ventilation could reduce postoperative atelectasis volume compared with 60% FiO2.
Methods
Ethics
This prospective, randomized study was conducted from April 2019 to September 2020 at the Huadong Hospital affiliated to Fudan University, Shanghai, China. The study was appoved on 6 March 2019 by the Ethics Commission of Huadong Hospital affiliated to Fudan University under the approval number 20,190,030. All patients were informed about the research purposes along with the practical aspects and gave written informed consent prior to inclusion. The trial was registered prior to patient enrollment at Chinese Clinical Trial Registry (http://www.chictr.org.cn; Registration date: 02/03/2019; Identifier: ChiCTR1900021635).
Study population
Patients were included if they met all the following criteria: (1) scheduled to undergo neurosurgery with an expected duration ≥ 2 h (the reason for choosing neurosurgery is that postoperative chest CT scans could be performed at the same time as routine brain CT scans in patients undergoing neurosurgery); (2) supine position during surgery; (3) age ≥ 18-years-old; (4) American Society of Anesthesiologists (ASA) of I-III; (5) oxygen saturation (SpO2) ≥ 94% when breathing room air; (6) body mass index (BMI) < 35 kg/m2. The exclusion criteria were as follows: (1) chronic obstructive pulmonary disease; (2) pre-existing atelectasis or pulmonary infection on chest CT scans; (3) obstructive sleep apnea syndrome; (4) heart failure; (5) anticipated difficult intubation; (6) chemotherapy within 3 months; (7) general anesthesia surgery within 1 month. The drop-out criteria included inability to maintain SpO2 ≥ 94% during the surgery, operating duration ≤ 2 h, and inability to be extubated. This manuscript adheres to the applicable guidelines of the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Randomization and blinding
A stratified block randomization method was conducted, dividing patients into 30% and 60% FiO2 groups. As age is an independent risk factor for postoperative atelectasis [19], the trial was stratified by age (< 60 and ≥ 60-years-old). Within each stratum, the participants were randomised at a 1:1 ratio in parallel groups by block randomization with a fixed size of 4. Computer-generated random numbers were implemented by an independent statistician, and allocation with intervention details was sealed in an opaque envelope by an individual not involved in the study.
An anesthesiologist, who was not involved in recruiting patients or collecting outcome data, opened the sealed envelope before the start of anesthesia and provided the designated FiO2 setting during mechanical ventilation based on the group assignment. A nurse who was not involved in the study recorded the patient’s vital signs and medication management during the operation. Chest CT was performed by a blinded technician within 30 min after extubation. Postoperative data were collected by a blinded anesthesiologist at 1–3 days after surgery. Throughout the study, the anesthesiologist and nurse in the operating room were aware of group allocation. Patients, clinical researchers, radiologists, technicians, statisticians, and surgical teams were blinded to the allocation information.
Anesthesia
The participants in the trial followed the standard anesthesia protocol. An arterial catheter was placed into the dorsal artery of the foot under local anesthesia for repeated blood gas sampling and continuous blood pressure monitoring. Propofol, sufentanil, and rocuronium were used for induction of general anesthesia. After tracheal intubation, both groups were ventilated in volume-control mode with a tidal volume of 6–8 ml·kg− 1 (predicted body weight) [20], a ventilation rate adjusted to maintain end-tidal CO2 between 35 and 45 mmHg, an inspiratory/expiratory ratio of 1:2, and no PEEP or recruitment maneuvers. Anesthesia was maintained with intravenous infusion of propofol and remifentanil.
Neuromuscular blockage was reversed before emergence using neostigmine/anticholinergic agent based on train-of-four ratio stimulation (TOF) monitoring [21]. The patients were extubated after full recovery from the neuromuscular block (TOF ratio ≥ 0.9). After extubation, the patients were transferred to the post-anesthesia care unit (PACU) and supplied with an oxygen face mask with a reservoir.
FiO2 setting
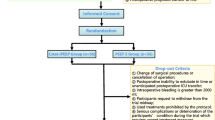
All patients received standard FiO2 setting following a detailed protocol. All investigators participating in the study were personally instructed by the principal investigator. During preoxygenation and induction, FiO2 was set at 100% in all patients to ensure sufficient oxygen reserves and improve safety when a potentially long period of apnea occurs because of difficulties in airway management. After intubation, the maintenance FiO2 was adjusted to 30% or 60% throughout the procedure based on group allocation. After extubation, patients in the 30% or 60% FiO2 groups received oxygen at a flow rate of 1 or 6 L/min via an oxygen mask with a reservoir. The FiO2 management during the perioperative period is shown in Fig. 1. It should be noted that if the patient’s SpO2 < 94% during the operation and in the PACU, the anesthesiologist should increase FiO2 or conduct recruitment maneuvers to raise the SpO2 to 94–98% [22], proceeding to the withdrawal of the patient.
Primary outcome
Within 30 min of extubation, the chest CT was performed by a trained and experienced technician who was unaware of the group assignment. All CT images were assessed by an experienced radiologist. The primary outcome was the postoperative atelectasis volume, expressed as a percentage of the total lung volume. The calculation of the percentage of atelectasis volume consisted of three steps. The first step was to measure the total lung area by accurately depicting the contour of the lung image on each CT image with a thickness of 5 mm. The pulmonary hilus vessels were manually excluded from the lung region of interest. The second step was to delineate the volume of the atelectasis region in each CT image. When drawing the atelectasis area, it should be outlined as close to the pleura as possible, and vascular structures with diameters larger than 3 mm should be manually excluded. Lastly, we used the histogram functional view using ITK-SNAP software (version 3.6.0) to identify the atelectasis region (Fig. 2), which was defined as -100 to 100 Hounsfield units [2, 18]. The calculated area was expressed as a percentage of the total lung area in the basal image.
Secondary outcomes
The percentages of different aeration volumes were considered as secondary outcomes. Areas of different aeration were measured using a workstation software (Sinvo.gia, Siemens Healthcare GmbH) by setting the histogram parameters between − 1,000 and − 901, − 900 and − 501, and − 500 and − 101 Hounsfield Units for over-aeration, normal-aeration, and poor-aeration, respectively [18, 23]. The incidence of clinically important atelectasis, which is defined as a volume of atelectasis of more than 1% lung volume [3], was considered as another secondary outcome. The oxygenation index (PaO2/FiO2 ratio) before anesthesia and at the end of surgery were considered as secondary outcomes.
Sample size
Twenty patients were randomly assigned to the 30% or 60% FiO2 groups in a pilot study. According to the results of the pilot study, the percentage of postoperative atelectasis volume was 3.56 ± 1.72 in the 30% FiO2 group and 4.70 ± 2.44 in the 60% FiO2 group. Using the PASS software (version 15.0), setting parameters to α = 0.05 and β = 0.2, the sample size of each group was 55 cases. Further setting the loss to follow-up rate to 10%, the sample size of each group was 60 cases, and the sample size of the two groups combined was 120 cases.
Statistical analysis
According to the distribution of the data evaluated using the Kolmogorov-Smirnov test, continuous variables were analyzed using the two-sample t-test or Mann-Whitney U test and presented as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Categorical variables were analyzed using the Chi-square test or Fisher’s exact test and reported as numbers and percentages. The primary outcome (the percentage of postoperative atelectasis volume) was normalized using the square root transformation and then analyzed using the two-sample t-test. Moreover, the Mann-Whitney U test was performed to assess the differences in the unnormalized primary outcome data between the two groups. The differences in the oxygenation index at the end of surgery between the two groups were compared, with the oxygenation index before anesthesia as a covariate. A two-sided P-value < 0.05 was considered significant for all statistical tests.
We handled missing normalized primary outcome data using multiple imputation by chained equations (MICE), and the iterations were set to 5 [24]. Age, sex, BMI, history of smoking, FiO2, and anesthetic duration were used as covariates to impute missing data for multiple imputation. In addition, we performed K-nearest neighbor (KNN) imputation (k = 10, Euclidean distance), regression imputation, and mean imputation as sensitivity analysis to assess the robustness of the primary findings.
Results
Subject characteristics
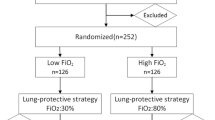
In this study, 120 patients were randomly allocated to either the 30% FiO2 group or 60% FiO2 groups (Fig. 3). Due to accidental violations of the trial protocol, 7 patients were excluded. Among them, three patients were excluded because of operation time < 2 h (two patients in the 30% FiO2 group and one patient in the 60% FiO2 group), and three patients were excluded because the did not undergo chest CT scans (two patients in the 30% FiO2 group and one patient in the 60% FiO2 group). One patient in the 30% FiO2 group was also excluded because of failure to be extubated after surgery. No significant differences in patient characteristics and intraoperative data were detected between the two groups (Table 1).
Primary outcome
There was no significant difference in the percentage of postoperative atelectasis volume between the 30% FiO2 group [median (IQR), 3.26 (1.61 to 4.47), n = 55] and the 60% FiO2 group [median (IQR), 4.29 (1.83 to 7.27), n = 58, P = 0.121] using the Mann-Whitney U test. Moreover, we used square root transformation to normalize the primary outcome, and we also did not find any significant difference in the normalized primary outcome between the 30% FiO2 group (mean ± SD, 1.76 ± 0.76, n = 55) and the 60% FiO2 group (mean ± SD, 2.02 ± 1.02, n = 58, P = 0.124) using a two-sample t-test.
The primary outcome was missing in seven patients among the patients in the randomization, thus we performed multiple imputation to handle missing normalized primary outcome data. Consistent with the results of the original data, none of the five imputations showed significant differences in the percentage of postoperative atelectasis volume between the two groups (Table 2). We further integrated the five imputations datasets and found that there was still no significant difference in the percentage of postoperative atelectasis volume between the two groups (Table 3; Fig. 4). The multiple imputation pattern is shown in Fig. 5.
Sensitivity analysis
KNN imputation, regression imputation, and mean imputation were performed as sensitivity analysis to handle missing normalized primary outcome data (Table 3). No differences in the percentage of postoperative atelectasis volume were observed between the two groups by KNN, regression, or mean imputation, confirming the robustness of our results.
Secondary outcomes
There were no significant differences in the percentages of over-aeration, normal-aeration, or poor-aeration volumes between the two groups. The overall incidence of clinically significant atelectasis was 83.2%, but again, there was no significant intergroup difference. Additionally, after adjusting for blood gas indicators before anesthesia, the oxygenation index at the end of surgery in the 30% FiO2 group did not differ from that in the 60% FiO2 group (Table 4).
Discussion
In this randomised study, we found no significant differences in the percentage of postoperative atelectasis volume in patients ventilated with 30% FiO2 and 60% FiO2. In addition, there were also no significant differences in the secondary outcomes, including the different aeration region volumes, incidence of clinically significant atelectasis, and oxygenation index at the end of surgery between the two groups. Taken together, these results suggest that 30% FiO2 during mechanical ventilation does not improve postoperative atelectasis compared to 60% FiO2.
Recently, a large number of clinical trials have compared the effects of high FiO2 (80-100%) versus low FiO2 (30-40%) on postoperative atelectasis in patients with mechanical ventilation under general anesthesia, observing a higher incidence of atelectasis in the high FiO2 group [3, 12, 25, 26]. However, it is noteworthy that a moderate FiO2 of 50–60% is more conventionally used in clinical practice, while only a few trials have compared moderate FiO2 with low FiO2. In 2021, Park et al. reported a higher incidence of postoperative atelectasis (39%) in the 60% FiO2 group compared with the incidence of atelectasis (20%) in the 35% FiO2 group [16]. Whereas in our study, no statistically significant differences in postoperative atelectasis volume were observed between patients applying 30% and 60% FiO2. There are several reasons for the contradictory results. First, the FiO2 during the postoperative period in the PACU was different between the two studies. Park et al. increased FiO2 when patients arrived at the PACU. It has been demonstrated that the use of a high FiO2 during the immediate postoperative period in the PACU may carry the potential risk of developing atelectasis [27]. Therefore, we maintained the same FiO2 in the PACU as that administered during the intraoperative period. Second, differences in the inclusion population may also have contributed to the differences in the atelectasis between the two groups. The trial by Park et al. included patients undergoing abdominal surgery, most of whom underwent open procedures and were more vulnerable to PPCs. Abdominal surgery may induce elevation of the diaphragm, which may affect pulmonary aeration postoperatively [28,29,30]. The neurosurgery included in this study could exclude the effect of surgical operation on atelectasis. Third and most importantly, the results may be affected by different detection methods of atelectasis. In the study by Park et al., using lung ultrasound, atelectasis after surgery was detected in 29.7% of the patients. In the present study, we used chest CT to detect atelectasis because it can accurately measure the volume of atelectasis. We found that atelectasis with more than 1% lung volume, which is considered as clinically significant, was detected in 83.2% of patients after surgery. The results were consistent with those of the study by Akca et al. [3] They found that 70% of patients, who received mechanical ventilation during general anesthesia, had postoperative atelectasis with more than 1% lung volume.
In the present study, the areas of different aeration were automatically measured using a workstation software. It was found that there were no significant differences between the two groups regarding the percentages of over-aeration, normal-aeration, and poor-aeration volumes. These results were consistent with the volume of atelectasis. In addition, there was no significant difference in the oxygenation index at the end of surgery between the two groups. The oxygenation index is an index to evaluate the gas exchange in the lung, and it is correlated with atelectasis [31]. Altogether, the results of different aeration volumes and oxygenation index further support the primary findings of the study.
It should be noted that the study is not without limitations. Firstly, the effect of recovery of spontaneous breathing after extubation on atelectasis could not be completely excluded. Secondly, the generalizability of the study is slightly weakened by the mode of mechanical ventilation. In order to explore the effect of intraoperative FiO2 on postoperative atelectasis, use of no PEEP/recruitment maneuvers were conducted in the present study, avoiding the interference of other confounding factors during mechanical ventilation. However, the mode of mechanical ventilation (absence of PEEP and recruitment maneuvers) is potentially a promoter of atelectasis [32, 33], which may affect the clinical feasibility of the conclusions of this study. In the future study, we will improve this issue and further explore the comprehensive effects of FiO2 and PEEP/recruitment maneuvers on postoperative atelectasis, so as to obatain more meaningful conclusions for clinical practice.
Conclusions
These results suggest that 30% FiO2 does not reduce the volume of postoperative atelectasis compared to 60% FiO2 in patients with mechanical ventilation under general anesthesia.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- CONSORT:
-
Consolidated Standards of Reporting Trials
- CT:
-
Computed tomography
- FiO2 :
-
Fraction of inspired oxygen
- IQR:
-
Interquartile range
- KNN:
-
K-nearest neighbor
- MICE:
-
Multiple imputation by chained equations
- PACU:
-
Post-anesthesia care unit
- PEEP:
-
Positive end-expiratory pressure
- PPCs:
-
Postoperative pulmonary complications
- SD:
-
Standard deviation
- SpO2 :
-
Oxygen saturation
- TOF:
-
Train-of-four stimulation
- TV:
-
Tidal volume
- WHO:
-
World Health Organization
References
Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102(4):838–54. https://doi.org/10.1097/00000542-200504000-00021
Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol. 1995;36(6):626–32.
Akça O, Podolsky A, Eisenhuber E, Panzer O, Hetz H, Lampl K, et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% oxygen during and 2 hours after colon resection. Anesthesiology. 1999;91(4):991–8. https://doi.org/10.1097/00000542-199910000-00019
Ferrando C, Romero C, Tusman G, Suarez-Sipmann F, Canet J, Dosdá R, et al. The accuracy of postoperative, non-invasive air-test to diagnose atelectasis in healthy patients after surgery: a prospective, diagnostic pilot study. BMJ Open. 2017;7(5):e015560. https://doi.org/10.1136/bmjopen-2016-015560
Park S, Oh EJ, Han S, Shin B, Shin SH, Im Y, et al. Intraoperative Anesthetic Management of patients with chronic obstructive pulmonary disease to decrease the risk of postoperative pulmonary complications after abdominal surgery. J Clin Med. 2020;9(1). https://doi.org/10.3390/jcm9010150
Yan T, Liang XQ, Wang GJ, Wang T, Li WO, Liu Y, et al. Prophylactic penehyclidine inhalation for Prevention of Postoperative Pulmonary Complications in high-risk patients: a double-blind Randomized Trial. Anesthesiology. 2022;136(4):551–66. https://doi.org/10.1097/aln.0000000000004159
Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med. 2011;39(9):2163–72. https://doi.org/10.1097/CCM.0b013e31821f0522
WHO Guidelines Approved by the Guidelines Review Committee. Global guidelines for the Prevention of Surgical Site infection. World Health Organization. Geneva; 2016.
de Jonge S, Egger M, Latif A, Loke YK, Berenholtz S, Boermeester M, et al. Effectiveness of 80% vs 30–35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta-analysis. Br J Anaesth. 2019;122(3):325–34. https://doi.org/10.1016/j.bja.2018.11.024
Mattishent K, Thavarajah M, Sinha A, Peel A, Egger M, Solomkin J, et al. Safety of 80% vs 30–35% fraction of inspired oxygen in patients undergoing surgery: a systematic review and meta-analysis. Br J Anaesth. 2019;122(3):311–24. https://doi.org/10.1016/j.bja.2018.11.026
Lim CH, Han JY, Cha SH, Kim YH, Yoo KY, Kim HJ. Effects of high versus low inspiratory oxygen fraction on postoperative clinical outcomes in patients undergoing surgery under general anesthesia: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth. 2021;75:110461. https://doi.org/10.1016/j.jclinane.2021.110461
Kim BR, Lee S, Bae H, Lee M, Bahk JH, Yoon S. Lung ultrasound score to determine the effect of fraction inspired oxygen during alveolar recruitment on absorption atelectasis in laparoscopic surgery: a randomized controlled trial. BMC Anesthesiol. 2020;20(1):173. https://doi.org/10.1186/s12871-020-01090-y
Koo CH, Park EY, Lee SY, Ryu JH. The Effects of intraoperative inspired oxygen fraction on postoperative pulmonary parameters in patients with General Anesthesia: a systemic review and Meta-analysis. J Clin Med. 2019;8(5). https://doi.org/10.3390/jcm8050583
Ferrando C, Aldecoa C, Unzueta C, Belda FJ, Librero J, Tusman G, et al. Effects of oxygen on post-surgical infections during an individualised perioperative open-lung ventilatory strategy: a randomised controlled trial. Br J Anaesth. 2020;124(1):110–20. https://doi.org/10.1016/j.bja.2019.10.009
Li XF, Jiang D, Jiang YL, Yu H, Zhang MQ, Jiang JL, et al. Comparison of low and high inspiratory oxygen fraction added to lung-protective ventilation on postoperative pulmonary complications after abdominal surgery: a randomized controlled trial. J Clin Anesth. 2020;67:110009. https://doi.org/10.1016/j.jclinane.2020.110009
Park M, Jung K, Sim WS, Kim DK, Chung IS, Choi JW, et al. Perioperative high inspired oxygen fraction induces atelectasis in patients undergoing abdominal surgery: a randomized controlled trial. J Clin Anesth. 2021;72:110285. https://doi.org/10.1016/j.jclinane.2021.110285
Pereira SM, Tucci MR, Morais CCA, Simões CM, Tonelotto BFF, Pompeo MS, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology. 2018;129(6):1070–81. https://doi.org/10.1097/aln.0000000000002435
Östberg E, Thorisson A, Enlund M, Zetterström H, Hedenstierna G, Edmark L. Positive end-expiratory pressure alone minimizes atelectasis formation in nonabdominal surgery: a Randomized Controlled Trial. Anesthesiology. 2018;128(6):1117–24. https://doi.org/10.1097/aln.0000000000002134
Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, de la Brutel A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296(15):1851–7. https://doi.org/10.1001/jama.296.15.1851
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8. https://doi.org/10.1056/nejm200005043421801
Calef A, Castelgrande R, Crawley K, Dorris S, Durham J, Lee K, et al. Reversing neuromuscular blockade without nerve stimulator Guidance in a postsurgical ICU-An observational study. J Clin Med. 2023;12(9). https://doi.org/10.3390/jcm12093253
O’Driscoll BR, Howard LS, Earis J, Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1–ii90. https://doi.org/10.1136/thoraxjnl-2016-209729
Vieira SR, Puybasset L, Richecoeur J, Lu Q, Cluzel P, Gusman PB, et al. A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1571–7. https://doi.org/10.1164/ajrccm.158.5.9802101
Wang W, He Q, Wang M, Kang Y, Ji P, Zhu S, et al. Associations of Fentanyl, Sufentanil, and Remifentanil with length of Stay and Mortality among mechanically ventilated patients: a Registry-Based Cohort Study. Front Pharmacol. 2022;13:858531. https://doi.org/10.3389/fphar.2022.858531
Benoît Z, Wicky S, Fischer JF, Frascarolo P, Chapuis C, Spahn DR, et al. The effect of increased FIO(2) before tracheal extubation on postoperative atelectasis. Anesth Analg. 2002;95(6):1777–81. https://doi.org/10.1097/00000539-200212000-00058. table of contents.
Edmark L, Auner U, Lindbäck J, Enlund M, Hedenstierna G. Post-operative atelectasis - a randomised trial investigating a ventilatory strategy and low oxygen fraction during recovery. Acta Anaesthesiol Scand. 2014;58(6):681–8. https://doi.org/10.1111/aas.12322
Suzuki S. Oxygen administration for postoperative surgical patients: a narrative review. J Intensive Care. 2020;8:79. https://doi.org/10.1186/s40560-020-00498-5
Jackson CV. Preoperative pulmonary evaluation. Arch Intern Med. 1988;148(10):2120–7.
Ferguson MK, Durkin AE. Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2002;123(4):661–9. https://doi.org/10.1067/mtc.2002.120350
Sameed M, Choi H, Auron M, Mireles-Cabodevila E. Preoperative Pulmonary Risk Assessment Respir Care. 2021;66(7):1150–66. https://doi.org/10.4187/respcare.09154
Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24(2):157–69. https://doi.org/10.1016/j.bpa.2009.12.002
Kim JY, Shin CS, Kim HS, Jung WS, Kwak HJ. Positive end-expiratory pressure in pressure-controlled ventilation improves ventilatory and oxygenation parameters during laparoscopic cholecystectomy. Surg Endosc. 2010;24(5):1099–103. https://doi.org/10.1007/s00464-009-0734-6
Talab HF, Zabani IA, Abdelrahman HS, Bukhari WL, Mamoun I, Ashour MA, et al. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth Analg. 2009;109(5):1511–6. https://doi.org/10.1213/ANE.0b013e3181ba7945
Acknowledgements
The authors would like to acknowledge Zhichao Jin (Department of Health Statistics, Second Military Medical University, Shanghai, China) for his assistance with statistical consultation.
Funding
This work was supported by the project of National Natural Science Foundation of China (82271286), the Science and Technology Commission of Shanghai Municipality (20Y11900200), Shanghai Municipal Health Commission (2020YJZX0119), Huadong Hospital Excellent Project (GZRPY016Y), National Key Research and Development Program of China (2018YFC2002000), Shanghai Municipal Key Clinical Specialty (shslczdzk02801), and High-level Local University Construction Project of Shanghai Medical College (FNDGJ202212).
Author information
Authors and Affiliations
Contributions
ZSJ and SBL: study design, data analysis, data interpretation, and drafting of manuscript; LW: data acquisition, data interpretation and critical revision of manuscript; WLL: data analysis and critical revision of manuscript; CL: data acquisition, data analysis, and critical revision of manuscript; FFL, RXL, YZ, and JJW: data acquisition and critical revision of manuscript; YXC: study design and critical revision of manuscript; WX and ZC: data acquisition and critical revision of manuscript; ZJB: study conception and critical revision of manuscript; ML and WDG: study conception, study design, and critical revision of manuscript. All authors read and approved the final manuscript. All authors agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was appoved on 6 March 2019 by the Ethics Commission of Huadong Hospital affiliated to Fudan University (Chaiperson Prof. Yue Zhu) under the approval number 201900. All patients were informed about the research purposes along with the practical aspects and gave written informed consent prior to inclusion. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Z., Liu, S., Wang, L. et al. Effects of 30% vs. 60% inspired oxygen fraction during mechanical ventilation on postoperative atelectasis: a randomised controlled trial. BMC Anesthesiol 23, 265 (2023). https://doi.org/10.1186/s12871-023-02226-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02226-6