Abstract
Background
Few interventions are known to reduce the incidence of respiratory failure that occurs following elective surgery (postoperative respiratory failure; PRF). We previously reported risk factors associated with PRF that occurs within the first 5 days after elective surgery (early PRF; E-PRF); however, PRF that occurs six or more days after elective surgery (late PRF; L-PRF) likely represents a different entity. We hypothesized that L-PRF would be associated with worse outcomes and different risk factors than E-PRF.
Methods
This was a retrospective matched case-control study of 59,073 consecutive adult patients admitted for elective non-cardiac and non-pulmonary surgical procedures at one of five University of California academic medical centers between October 2012 and September 2015. We identified patients with L-PRF, confirmed by surgeon and intensivist subject matter expert review, and matched them 1:1 to patients who did not develop PRF (No-PRF) based on hospital, age, and surgical procedure. We then analyzed risk factors and outcomes associated with L-PRF compared to E-PRF and No-PRF.
Results
Among 95 patients with L-PRF, 50.5% were female, 71.6% white, 27.4% Hispanic, and 53.7% Medicare recipients; the median age was 63 years (IQR 56, 70). Compared to 95 matched patients with No-PRF and 319 patients who developed E-PRF, L-PRF was associated with higher morbidity and mortality, longer hospital and intensive care unit length of stay, and increased costs. Compared to No-PRF, factors associated with L-PRF included: preexisiting neurologic disease (OR 4.36, 95% CI 1.81–10.46), anesthesia duration per hour (OR 1.22, 95% CI 1.04–1.44), and maximum intraoperative peak inspiratory pressure per cm H20 (OR 1.14, 95% CI 1.06–1.22).
Conclusions
We identified that pre-existing neurologic disease, longer duration of anesthesia, and greater maximum intraoperative peak inspiratory pressures were associated with respiratory failure that developed six or more days after elective surgery in adult patients (L-PRF). Interventions targeting these factors may be worthy of future evaluation.
Similar content being viewed by others
Background
Postoperative respiratory failure (PRF) is a significant source of increased hospital length of stay, in-hospital and post-discharge morbidity, and in-hospital and long-term mortality; translating into markedly increased costs [1,2,3,4,5]. However, it is thought to be a potentially preventable adverse event [6] that is amenable to appropriate care [7]. With the volume of surgical procedures increasing annually, [8] there is an urgent and unmet need to reduce the incidence of PRF [9] by elucidating vulnerable PRF subpopulations by phenotypic presentation to identify: 1) which patients are most at risk, 2) which pathways contribute to increased morbidity and mortality, and 3) which patients are most likely to benefit from targeted preventive and therapeutic interventions [10].
The disparate burden of PRF by phenotype in surgical patients remains understudied. Also underreported, are differences between patients who develop PRF early versus late in their postoperative course. While there is no universal consensus on when respiratory failure is related to the surgical procedure, most clinicians, including our co-authors who are practicing anesthesiologists, surgeons, and critical care intensivists in the largest healthcare system in the state of California, describe the acute postoperative phase as lasting two to 3 days, and state that 5 days is a very reasonable cutoff that is clinically relevant when studying early postoperative complications. This five-day cutoff falls within the 0–7-day range described in four publications [11,12,13,14]. Among these publications is a study in which the authors used data from 4366 patients, 113 of whom developed early postoperative acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), defined as occurring during postoperative day (POD) 0–5, to develop the Surgical Lung Injury Prediction Model [14]. The Surgical Lung Injury Prediction Model uses readily available preoperative risk factors to predict risk of early postoperative ALI or ARDS with high accuracy. The three additional studies used POD 0–3 [13], POD 0–5 [12], and POD 0–7 [11] to define the outcome of PRF, ALI, or ARDS. The dynamic nature of the postoperative timeframe is affected by many pre-, intra-, and post-operative factors acting synergistically and it is challenging to determine when PRF is a direct complication of the surgical events or a later consequence of additional events that occur during hospitalization [15]. Yet, it is precisely this lack of consensus that has hindered efforts to identify phenotypes and clinical trajectories to determine modifiable risk factors and reduce the incidence of PRF. There is also little convergence of research on the risk factors associated with PRF. Different patient populations, combined with the low incidence of this rare event, have led to risk models with varying factors [15,16,17,18].
The University of California Critical Care Research Collaborative (UC3RC) previously reported on patient- and procedure-related risk factors associated with PRF that developed on or before postoperative day five (early PRF; E-PRF) for 319 patients [19]; however, cases that occur later likely represent a different population of patients. In this study we compare patient outcomes and patient- and procedure-related risk factors associated with PRF that developed on or after postoperative day six (late PRF; L-PRF) to patients who did not develop PRF (No-PRF). We also separately compared outcomes of the L-PRF patients to cases of E-PRF. We hypothesize that patients who develop L-PRF will have worse outcomes and a unique set of modifiable patient- and procedure-related risk factors.
Methods
This multicenter study by the UC3RC was approved by the Institutional Review Boards at the University of California, Davis (lead site) and Irvine, Los Angeles, San Diego, and San Francisco (collaborating sites), all of whom waived the requirement for informed consent for participation in the study. We previously described our case-control study methods, [19] including the validity of case ascertainment [20]. This manuscript adheres to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [21].
Study design and setting
This was a retrospective matched case-control study of all eligible adult elective surgery discharges between October 1, 2012, and September 30, 2015, at the five University of California academic medical centers. The outcome of interest was the diagnosis of L-PRF. We selected all predictor variables based on literature review and clinical expertise. L-PRF cases, which developed six or more days after surgery, were first compared to the No-PRF cohort and then to the E-PRF cohort.
Study population
We used hospital administrative data submitted to the University Healthsystem Consortium (UHC), now Vizient Inc., based on the Agency for Healthcare Research and Quality (AHRQ) algorithm for Patient Safety Indicator 11 (PSI 11), Postoperative Respiratory Failure Rate, [22] to identify at-risk patients and potential cases of L-PRF among all adult patients discharged following an elective surgical procedure from all five sites during the study period. Because we were interested in potentially preventable cases of PRF, we excluded patients who required an open thoracic procedure (e.g., esophageal resection, lung cancer resection, open heart surgery).
Ascertainment of cases
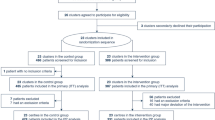
Among 59,073 consecutive adult patients admitted for elective non-cardiac and non-pulmonary surgical procedures, 437 possible cases of PRF were identified based on administrative data. Each of these possible cases was then reviewed to confirm presence of at least one of the following criteria: [20, 23].
-
1)
arterial oxygen partial pressure (PaO2) < 60 mmHg on room air; a ratio of arterial oxygen partial pressure (PaO2) to the fractional inspired oxygen (FiO2) < 300 [24]; or
-
2)
physician documentation of PRF (due to hypoxemia or hypercarbia) or acute respiratory distress syndrome (ARDS); or
-
3)
physician documentation of one of the following procedures because of respiratory compromise, insufficiency, or failure: [22]
-
a.
unplanned postoperative endotracheal re-intubation; or
-
b.
continuous mechanical ventilation (MV) for > 48 hours.
We omitted 23 false positive cases and confirmed 319 cases of E-PRF and 95 cases of L-PRF (Fig. 1).
Matching and verification of no-PRF
We matched L-PRF cases to No-PRF in a 1:1 ratio, randomly selecting the control(s) within strata based on age (by decade) (Additional file 1, Table S1), hospital (Additional file 2, Table S2), and principal ICD-9-CM procedure code (grouped by anatomic region and open versus minimally invasive approach using the Healthcare Cost and Utilization Project Clinical Classification Tools and Software [25]; (Additional files 3 and 4, Table S3 and Table S4). Once matched, each of the flagged No-PRF cases was reviewed to confirm the absence of true clinical PRF using the criteria defined above. This control group is referred to as No-PRF.
Sample size and power analysis
A priori, using methods described by Dupont and Plummer, [26,27,28] we determined the odds ratio we would be able to detect for a sample size of 95 cases matched 1:1 to No-PRF. We calculated we would be able to detect true odds ratios more extreme than 0.25 or 2.81 in exposed relative to unexposed subjects with power of 80% at an α level of 0.05, assuming a probability of exposure among No-PRF of 20% and a correlation coefficient for exposure between matched cases and No-PRF of 0.2.
Instrument development
We modified the abstraction instrument from a prior UHC PRF benchmarking project [29] for use in this study via the REDCap™ electronic platform. We previously published our study instrument as an online supplement [20]. A condensed table of definitions of comorbidities, risk factors, and outcome variables can be found in the online supplement (Additional file 5, Table S5). The instrument contains information on demographics, pre-existing comorbidities, preoperative laboratory and radiographic test results, procedures and diagnoses, length of stay, intra- and perioperative care (e.g., ventilator settings, fluid intake and output, and medication administration), and discharge disposition. We used methodology developed by Vizient, Inc., to determine total costs of care for each encounter, inclusive of direct and indirect costs, from charge data [30].
Data collection
The entire health record for each sampled hospitalization was reviewed, and data were manually extracted and entered into a REDCap™ database. Five abstractors participated in data collection and the first author validated the data abstraction of 100% of the records. Interrater reliability was not explicitly measured, but disagreements after the initial training period were rare.
Statistical analysis
We performed conditional logistic regression to assess potential risk factors for L-PRF. We calculated unadjusted odds ratios (ORs) and 95% confidence intervals (CIs). We assessed all predictors supported by prior studies and predictors with p < 0.20 for collinearity and excluded all variables with a variance inflation factor ≥ 2.5 from consideration for multivariable analysis [31].
We developed multivariable conditional logistic regression models using purposeful variable selection [32] and a 10% change-in-estimate procedure [33] to determine if the potential for confounding was present and warranted adjustment. For final model selection, we relied on Akaike’s Information Criteria (AIC) and Bayesian Information Criteria (BIC) to identify best model fit [34]. We calculated adjusted odds ratios and 95% confidence intervals, using Stata MP® version 15.1 for all analyses. We then performed Poisson regression to analyze differences in hospital and intensive care unit length of stay between L-PRF cases and non-cases and between E-PRF cases and L-PRF cases. We used linear regression to analyze differences in total cost.
Results
Matched case-control sample (L-PRF and no-PRF)
There were 95 confirmed cases of L-PRF among 59,073 eligible discharges from the five sites for an overall rate of 1.61 per 1000 discharges. After 1:1 matching of L-PRF to No-PRF, our total sample (n = 190) was majority female, white, non-Hispanic, and covered by a third-party payer (Table 1). Most patients (59%) were 60 years of age or older. The baseline acuity of most patients was high: 72.1% had an American Society of Anesthesiologists (ASA) class of III or greater; 63.2% had two or more pre-existing comorbid conditions on admission.
Among the matched cohort, most patients had general anesthesia (n = 186, 97.9%) and received a neuromuscular blocking agent (n = 178, 93.7%) (Table 2). The most common neuromuscular blocking agent used for induction was rocuronium (n = 134, 70.9%); the next most common was vecuronium (n = 17, 9.0%). Surgery most often involved open procedures of the abdomen or pelvis (n = 122, 64.6%), followed by open procedures of the head and neck (n = 21, 11.1%). Most patients received a benzodiazepine (n = 133, 70.0%) and almost all patients (n = 181, 95.3%) received an opioid. Ventilator tidal volume ranged from a median low of 6.8 cc/kg for predicted body weight (IQR 6.1, 7.7) to a median high of 8.8 cc/kg for predicted body weight (IQR 7.9, 9.9). Most patients had a positive fluid balance at the end of the operating room procedure (n = 183, 96.3%) and 24 hours after the operating room procedure (n = 167, 87.9%).
The etiology of L-PRF was primarily of pulmonary origin (e.g., aspiration pneumonitis, pneumonia) in 58 cases (61.0%) and of extrapulmonary in origin (e.g., sepsis, postoperative hemorrhage) in 37 cases (39.0%) (Additional file 6, Table S6). The most common etiologies were infectious in nature (n = 38, 40.0%) (e.g., sepsis, pneumonia).
Outcomes: L-PRF versus no-PRF
Cases of L-PRF had higher unadjusted in-hospital mortality, hospital and intensive care unit (ICU) length of stay, and total costs (Table 3). Compared to cases of No-PRF, and adjusting for age, hospital, procedure, ASA class, and total number of comorbidities, the average hospital length of stay for cases of L-PRF was 5.03 (95% CI 4.01–6.31) times as long, the average ICU length of stay was 21.99 (95% CI 11.76–41.11) times as long, and the average total cost was $166 K greater (95% CI $131 K - $202 K). Compared to No-PRF, cases of L-PRF were less often discharged home able to care for themselves and more often discharged to a long-term care facility or a skilled nursing facility.
Outcomes: E-PRF versus L-PRF
The median postoperative day of diagnosis for E-PRF was 1 (IQR 0, 2; Range 0–5) and for L-PRF was 10 (IQR 7, 15; Range 6–57). Compared to cases of E-PRF, cases of L-PRF had higher unadjusted in-hospital mortality, hospital length of stay, ICU length of stay, and total costs (Table 3). Compared to E-PRF, and adjusting for age, hospital, procedure, ASA class, and total number of comorbidities, the average hospital length of stay of cases of L-PRF was 2.30 (95% CI 1.89–2.79) times as long, the average ICU length of stay was 2.28 (95% CI 1.81–2.88) times as long, and the average total cost was $99,952 greater (95% CI $61,532 - $138,372). Compared to E-PRF, cases of L-PRF were less often discharged home able to care for themselves and more often discharged to a long-term care facility or a skilled nursing facility.
Relative to E-PRF, L-PRF was associated with increased odds (OR 2.2, 95% CI 1.66–2.97) of having another adverse, non-mortality patient safety event. L-PRF was associated with increased odds of perioperative hemorrhage or hematoma (OR 4.05, 95% CI 1.66–9.85) and postoperative acute kidney injury requiring dialysis (OR 6.55, 95% CI 2.97–14.43). The concept of “cascade iatrogenesis”, the sequential development of additional medical complications following a seemingly harmless first event, such as analgesia for postoperative pain, has been described in terms of nursing care for older adults who develop PRF [35,36,37] and may be applicable here as well. We did not find an association with any other patient safety events or hospital acquired infections (e.g., perioperative sepsis, surgical site infection).
Risk factors: L-PRF versus no PRF
Among patient-related factors, ASA class of III or greater, pre-existing cardiac disease, pre-existing neurologic disease, higher number of pre-existing comorbidities, and low albumin were associated with increased unadjusted odds of L-PRF (Table 1). Procedure-related factors associated with increased unadjusted odds of L-PRF included: longer duration of anesthesia and surgery; higher peak inspiratory pressure; higher intra-operative volume of infused colloid; receiving blood in the operating room; increased intravenous fluid intake in the operating room and at 24 hours postop; and 24-hour net positive fluid balance (Table 2).
Multivariable analysis
In the final multivariable conditional logistic regression model, after matching cases with L-PRF 1:1 to cases with No-PRF based on age, hospital, and surgical procedure, factors associated with L-PRF included: the presence of neurologic disease at admission, anesthesia duration, and maximum peak inspiratory pressure (Table 4).
Discussion
We compared outcomes for patients who developed L-PRF to patients who did not develop PRF (No-PRF) and to patients who developed E-PRF. L-PRF was associated with increased morbidity, mortality, hospital and ICU length of stay, and total costs when compared to both No-PRF and E-PRF. Cases of L-PRF were often of infectious etiology (e.g., sepsis, pneumonia), with a smaller proportion due to surgical or hospital complications (e.g., acute vascular insufficiency of the intestine, postoperative hemorrhage). These findings align with those described by Moore et al. [38], who found age older than 60 years and presence of any comorbidity to be major risk factors for sepsis, and Chughati et al. [39], who found hospital-acquired and ventilator-associated pneumonia continue to be common complications after surgery, despite recent emphasis on ICU prevention bundles, with non-modifiable risk factors inclusive of age and preoperative functional ability. The incidence of infectious complications emphasizes the need for early recognition of at-risk patients.
While the impact of PRF (e.g., length of stay and cost) have been detailed in two large, multisite studies set in Academic Medical Centers [9] and the Veteran’s Administration, [40] we have quantified, using newer data, the significantly worse outcomes associated with L-PRF when compared to E-PRF and No-PRF. We also assessed for risk factors associated with L-PRF, when compared to a matched cohort of patients with No-PRF, and identified pre-existing neurologic disease, increased anesthesia duration, and increased maximum peak inspiratory pressure as significant and potentially modifiable risk factors. These findings add to a growing body of critical care and surgical literature examining factors associated with PRF.
Intraoperative peak inspiratory pressure
A key finding from our study is that a higher maximum intraoperative peak inspiratory pressure (PIP) was associated with increased odds of developing L-PRF. It has long been accepted that changes in the respiratory system occur as soon as general anesthesia and MV are initiated [41, 42]; it has more recently been postulated that these changes can last several days [43] to several weeks [44,45,46,47]. Less clear is which components of MV are most harmful or protective in existing ARDS as well as in healthy lungs when the intent is to reduce the risk of ventilator induced lung injury.
In a 29-center study of 2466 moderate and severe ARDS patients, [48] there was substantial center-to-center variability in early adherence to lung protective ventilation (LPV) (0–65%) and mortality (16.7–73.3%). Center standardized mortality rates (SMRs), which calculated the ratio between observed and expected mortality, ranged from 0.33–1.98 and, of the treatment-level factors explored, only early LPV was associated with lower SMR. The authors concluded that early adherence to LPV was associated with lower center mortality and postulated LPV to be a surrogate for overall quality of care processes. The authors defined LPV as tidal volume < 6.5 ml/kg of predicted body weight (PBW) and plateau pressure and/or peak inspiratory pressure (PIP) < 30 cmH2O. In a case-control study of 50,367 surgical hospitalizations, with 93 (0.2%) cases of postoperative ARDS, the authors found higher median PIP in ARDS patients (27 versus 21, p < 0.001) and stated their data suggest intraoperative exposure to elevated PIP with a lack of positive end expiratory pressure (PEEP) was associated with the development of postoperative ARDS, possibly due to barotrauma and atelectrauma produced by these ventilator settings [11]. While they recommended further investigation, the authors concluded their findings potentially offered clinicians opportunities to reduce postoperative ARDS. These two studies by Qadir et al. and Blum et al. are relevant to our work as the authors deemed low PIP to be an acceptable surrogate of LPV in the absence of information about plateau pressures, which they found to be measured only 49.6% of the time on day one in the ICU [48] and not collected at all in the intraoperative environment [11]. While we would have liked to evaluate plateau pressures, these data were not documented in the operating suite for adults undergoing elective surgical procedures during the timeframe for our study. Because, in many intraoperative situations, LPV is not always verified with a plateau pressure, we felt our use of PIP was a reasonable surrogate measure of adherence to an LPV approach.
While plateau or driving pressures may best correlate with lung injury, evidence of an association between PIP and hospital mortality from ARDS and acute hypoxic respiratory failure is also provided by the international, multi-site LUNG SAFE study [49]. In 2377 patients enrolled in LUNG SAFE, potentially modifiable factors associated with increased hospital mortality on multivariable analyses included lower PEEP; higher PIP, plateau pressure, and driving pressure; and increased respiratory rate. In an invited editorial of the LUNG SAFE study, the authors further reinforced the conclusion that PIP was higher in non-survivors [50]. They concluded that PIP is a potential target for improvement of outcomes in ARDS patients.
The Qadir et al. findings of low adherence to LPV in known ARDS patients is interesting considering decades of evidence that LPV improves survival in ARDS, [51] but perhaps makes more sense when we consider the evolution of LPV from an initial focus on lower tidal volume and higher PEEP with or without lung recruitment measures, [52,53,54] to the addition of lower plateau pressures, [55] to a more recent focus on lower driving pressure (the difference between plateau pressure and PEEP) [56]. More recently, literature has emerged on dynamic, rather than static, indicators of energy load (e.g., flow amplitude and the clinician-selected flow waveform), [57,58,59] Future research to evaluate applicability in the operating suite ventilator management of adult elective surgery patients and possible associations between these modifiable determinants of ventilator induced lung injury (VILI) and PRF may be warranted.
Our finding of an association between increased intraoperative PIP and L-PRF suggests more research is needed to assess the long-term effects of anesthesia and intraoperative LPV on PRF. Specifically, future research to assess possible associations between measures of mechanical power delivered to healthy lungs and development of PRF are needed. Several publications describe the physiologic changes to the respiratory system (e.g., alteration in respiratory muscle function, modification in respiratory mechanics, and reduction in lung volumes, factors which lead to increased atelectasis, decreased functional residual capacity, and decreased vital capacity) that occur upon induction of general anesthesia and initiation of mechanical ventilation [41, 42] and that can persist for several days [43] to several weeks [44,45,46,47]. These physiologic changes, combined with the published theory of cascade iatrogenesis in adverse events, [36] to include postoperative respiratory failure, [37] and the known injurious effects of barotrauma and atelectrauma, further highlight the need for future research to explore the association of intraoperative LPV, to include PIP, and the development of L-PRF.
Anesthesia duration
Our findings align with prior studies regarding increased risk of L-PRF with increased duration of anesthesia and surgery [60]. While one study identified risk factors for six common procedures (pancreatectomy, hepatectomy, esophagectomy, abdominal aortic aneurysm repair, open aortoiliac repair, and lung resection) and found the risk factors varied by procedure type, the one risk factor that was consistent across all procedures was prolonged procedure time [61]. Aside from consideration of less aggressive operative approaches and optimization of perioperative supplies, team staffing and expertise, there are limited options available to reduce total surgery and anesthesia duration. While we did not find an association between type of anesthesia and L-PRF, very few patients in our cohort received conscious sedation, also known as monitored anesthesia care. In abdominal aortic aneurysm and aortoiliac repair, an endovascular approach using monitored anesthesia care was associated with lower risk of PPCs (OR 0.48, 95% CI 0.24–0.92) [62].
Pre-existing neurologic comorbidities
We also found an association between pre-existing neurologic comorbidities and increased odds of L-PRF. While pre-existing neurologic comorbidities are largely non-modifiable, [7] we believe that—given the mortality, morbidity, and costs associated with PRF—more research is needed to determine if some neurologic disorders might be responsive to preoperative optimization. Examples of potential interventions include protocolized swallow evaluation and swallow training to reduce aspiration risk, incentive spirometry to reduce atelectasis, and exercise programs to improve strength in anticipation of early postoperative mobilization. Some studies on colorectal and cardiac surgery patients have demonstrated positive benefits of multimodal “prehabilitation” to optimize such factors as nutrition, exercise, and smoking cessation [63,64,65]. Delay of elective procedures to better prehabilitate the patient might also be considered in high-risk neurological patient populations. In addition, a better understanding and awareness of which neurologic diseases are at highest risk would allow providers to target these patients for prevention in the perioperative period.
Limitations
Our results should be interpreted with some caution. These findings are based on retrospective analysis of a rare event in five academic medical centers within one health system, for the years 2012–2015, a timeframe during which the UC systems were in the process of implementing perioperative bundles such as Enhanced Recovery After Surgery (ERAS) [66]. These interventions might account for the limited variability we saw between groups with some variables of interest, such as mechanical ventilation tidal volume. We were unable to analyze some variables of interest, such as operative plateau pressure and minute-to-minute documentation of all ventilator settings due to missing documentation and limited resources for manual data abstraction. Lung protective lung ventilation involves multiple ventilator parameters, their interaction with the patient’s physiology, and surgical factors (e.g., laparoscopic surgery with pneumoperitoneum, Trendelenburg position, body habitus) [67]. The rare nature of PRF also makes analysis complex. While the OR and CIs for preexisting neurologic conditions were both high and wide, respectively, due to the low incidence (n = 27 in the L-PRF group and n = 12 in the No-PRF group), the ORs and CIs for anesthesia duration and PIP were reasonable and plausible. We were also unable to analyze the effect of preoperative frailty [68], which has been shown to be associated with significant morbidity and mortality, [69] and other comorbidities of interest due to the incidence of PRF as a rare event. The ability to collect these data is crucial for future studies. These study limitations are similar to those described by Blum et al., in their case-control study of 93 surgical patients who developed postoperative ARDS [11] and Chen et al., in their case-control study of 36 patients who required unplanned reintubation following general anesthesia [70]. Despite the limitations associated with studying rare events, early and novel studies on a topic, such as our study of L-PRF, are often hypothesis generating and serve as a good launching point to design higher powered future studies.
Late PRF is associated with significant morbidity, mortality, and increased hospital and intensive care unit length of stay and healthcare costs, making it of interest to health care clinicians, administrators, and consumers/patients. A strength of our study is our database, which included 414 confirmed cases of PRF, 319 E-PRF and 95 L-PRF, among nearly 60,000 elective surgical admissions over 4 years from five academic medical centers. We believe our analysis is one of the first to describe the different risk factors and outcomes associated with E-PRF versus L-PRF.
Future research
While the importance of studying rare adverse events to better understand modifiable risk factors is paramount to improve patient outcomes, this can only be achieved with increased power. Increasing sample size is the most effective way to improve power but is dependent upon funding for multi-center studies in which each center provides complete data for analysis. Larger, multi-center studies also allow for adjustment for other risk factors, therefore reducing variation and increasing power. Machine learning techniques (e.g., Classification and Regression Tree [CART] and random forest models) can help in identifying risk factors and building strong models. Emerging synthetic health data generation methods may also help accelerate rare event outcomes research. The UC3RC is exploring all of these avenues. We continue to add to our database and work to form collaborative relationships with other leading clinical research institutions to increase sample size and statistical power. We are currently transitioning our data abstraction, curation, and validation methods from manual to automated and mapping the data to standardized taxonomies to better facilitate multi-center collaborations. We also continue to seek additional resources to expand our effort.
Future studies of rare events should consider a composite comorbidity index (e.g., Charlson and/or Elixhauser) rather than a total number of comorbidities. As risk for suboptimal outcomes often changes during the hospitalization, studies should also consider scores like the Sequential Organ Failure Assessment (SOFA) and/or Acute Physiology, Age and Chronic Health Evaluation (APACHE) scores. Analysis of frailty on surgical outcomes is also needed to determine if some elective procedures should be deferred entirely.
While manual data abstraction by trained clinicians has many benefits, it is costly and time-consuming and limits the scope of this type of research. As electronic health records continue to evolve and clinician documentation is increasingly mapped to standardized taxonomies (e.g., SNOMED CT, RxNorm, LOINC), the burden of abstraction will become less prohibitive. While automatic data capture from the electronic health record may prove beneficial for future studies, it must be balanced against burden of validation of the documentation and mapping to the existing standardized taxonomies.
Conclusions
Prevention of PRF is of great importance as PRF is a source of high morbidity and mortality. We have identified, in a consecutive sample of nearly 60,000 elective surgical discharges, three potentially modifiable risk factors for L-PRF: 1) intraoperative peak inspiratory pressure; 2) duration of anesthesia; and 3) pre-existing neurological disease. Identification of these factors should be used to create and investigate targeted interventions aimed at reducing L-PRF. We propose that these interventions might include a reduction of peak inspiratory pressures as a component of a lung protective ventilation, resulting in a personalized anesthesia approach to each patient’s physiologic response. Second, institutional focus on optimization of operating room flow to reduce duration of anesthesia and surgery through enhanced staffing and supplies may be considered. Lastly, identification of sub-populations of patients with pre-existing neurologic disease most apt to benefit from targeted perioperative pre-habilitation. Ongoing research is needed to better understand these risk factors and to develop and validate interventions for prevention of L-PRF.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALI:
-
Acute Lung Injury
- ARDS:
-
Acute Respiratory Distress Syndrome
- AHRQ :
-
Agency for Healthcare Research and Quality
- ASA:
-
American Society of Anesthesiologists
- CI:
-
Confidence Interval
- E-PRF:
-
Early Postoperative Respiratory Failure
- ICU:
-
Intensive Care Unit
- IQR:
-
Interquartile Range
- L-PRF:
-
Late Postoperative Respiratory Failure
- LPV:
-
Lung Protective Ventilation
- MV:
-
Mechanical Ventilation
- No-PRF:
-
No Postoperative Respiratory Failure
- OR:
-
Odds Ratio
- PSI 11:
-
Patient Safety Indicator 11
- PIP:
-
Peak Inspiratory Pressure
- PEEP:
-
Positive End Expiratory Pressure
- PPCs:
-
Postoperative Pulmonary Complications
- PRF:
-
Postoperative Respiratory Failure
- UHC:
-
University Healthsystem Consortium
- UC3RC:
-
University of California Critical Care Research Collaborative
References
Johnson RG, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204(6):1188–98.
Filsoufi F, et al. Logistic risk model predicting postoperative respiratory failure in patients undergoing valve surgery. Eur J Cardiothorac Surg. 2008;34(5):953–9.
Canet J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–50.
Fernandez-Bustamante A, Frendl G, Sprung J, Kor DJ, Subramaniam B, Martinez Ruiz R, Lee JW, Henderson WG, Moss A, Mehdiratta N, Colwell MM, Bartels K, Kolodzie K, Giquel J, Vidal Melo MF. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152(2):157-66. https://doi.org/10.1001/jamasurg.2016.4065.
Serpa Neto A, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014;2(12):1007–15.
Encinosa WE, Hellinger FJ. The impact of medical errors on ninety-day costs and outcomes: an examination of surgical patients. Health Serv Res. 2008;43(6):2067–85.
Zrelak PA, et al. Using the agency for healthcare research and quality patient safety indicators for targeting nursing quality improvement. J Nurs Care Qual. 2012;27(2):99–108.
National Quality Forum, NQF-Endorsed Measures for Surgical Procedures, 2015–2017. Washington, DC: National Quality Forum; 2017.
Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. Jama. 2003;290(14):1868–74.
Filsoufi F, et al. Predictors and early and late outcomes of respiratory failure in contemporary cardiac surgery. Chest. 2008;133(3):713–21.
Blum JM, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118(1):19–29.
Money SR, et al. Risk of respiratory failure after repair of thoracoabdominal aortic aneurysms. Am J Surg. 1994;168(2):152–5.
Brueckmann B, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology. 2013;118(6):1276–85.
Kor DJ, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011;115(1):117–28.
Canet J, Gallart L. Postoperative respiratory failure: pathogenesis, prediction, and prevention. Curr Opin Crit Care. 2014;20(1):56–62.
Gupta H, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140(5):1207–15.
Arozullah AM, et al. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The national veterans administration surgical quality improvement program. Ann Surg. 2000;232(2):242–53.
Attaallah AF, et al. Perioperative risk factors for postoperative respiratory failure. J Perioper Pract. 2019;29(3):49–53.
Stocking JC, et al. Risk factors associated with early postoperative respiratory failure: a matched case-control study. J Surg Res. 2021;261:310–9.
Stocking JC, et al. Postoperative respiratory failure: an update on the validity of the Agency for Healthcare Research and Quality patient safety Indicator 11 in an era of clinical documentation improvement programs. Am J Surg. 2020;220(1):222–8.
von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7.
Agency for healthcare research and quality, Patient Safety Indicator 11 (PSI 11) Postoperative Respiratory Failure Rate. Rockville: Agency for Healthcare Research and Quality; 2017.
Abbott TEF, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120(5):1066–79.
Ferguson ND, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–82.
Elixhauser A, Steiner C, Palmer L. Clinical classifications software (CCS), 2015, U.S. Agency for healthcare research and quality, editor: U.S. Agency for Healthcare Research and Quality, Rockville, MD; 2015. p. 1–54.
Dupont WD. Power calculations for matched case-control studies. Biometrics. 1988;44(4):1157–68.
Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116–28.
Plummer, D. PS: Power and Sample Size Calculation. 2018. Available from: http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize. [cited 2020 Jan 11].
Utter GH, et al. Detection of postoperative respiratory failure: how predictive is the Agency for Healthcare Research and Quality's patient safety Indicator? J Am Coll Surg. 2010;211(3):347–354.e29.
Vizient Inc. Vizient Clinical Data Base user guide. Irving, TX; 2018.
O'Brien R. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–90.
Bursac Z, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340-9. https://doi.org/10.2105/ajph.79.3.340.
Chakrabarti A, Ghosh JK. AIC, BIC and recent advances in model selection. In: Bandyopadhyay PS, Forster MR, editors. Philosophy of statistics. Amsterdam: North-Holland; 2011. p. 583–605.
Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med. 2000;160(18):2717–28.
Thornlow DK, Anderson R, Oddone E. Cascade iatrogenesis: factors leading to the development of adverse events in hospitalized older adults. Int J Nurs Stud. 2009;46(11):1528–35.
Thornlow DK, Oddone E, Anderson R. Cascade iatrogenesis: a case-control study to detect postoperative respiratory failure in hospitalized older adults. Res Gerontol Nurs. 2014;7(2):66–77.
Moore LJ, et al. Sepsis in general surgery: the 2005-2007 national surgical quality improvement program perspective. Arch Surg. 2010;145(7):695–700.
Chughtai M, et al. The epidemiology and risk factors for postoperative pneumonia. J Clin Med Res. 2017;9(6):466–75.
Rivard PE, et al. Using patient safety indicators to estimate the impact of potential adverse events on outcomes. Med Care Res Rev. 2008;65(1):67–87.
Sasaki N, Meyer MJ, Eikermann M. Postoperative respiratory muscle dysfunction: pathophysiology and preventive strategies. Anesthesiology. 2013;118(4):961–78.
Gropper MA. Postoperative respiratory muscle dysfunction: only the strong survive. Anesthesiology. 2013;118(4):783–4.
Warner DO, Weiskopf RB. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000;92(5):1467–72.
Blondonnet R, et al. Complicaciones respiratorias postoperatorias. EMC Anestesia Reanimación. 2021;47:1–19.
Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–34.
Nieuwenhuijs D, et al. Ventilatory responses after major surgery and high dependency care. Br J Anaesth. 2012;108(5):864–71.
Kelkar KV. Post-operative pulmonary complications after non-cardiothoracic surgery. Indian J Anaesth. 2015;59(9):599–605.
Qadir N, et al. Variation in early management practices in moderate-to-severe ARDS in the United States: the severe ARDS: generating evidence study. Chest. 2021;160(4):1304–15.
Laffey JG, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42(12):1865–76.
Guervilly C, Forel JM, Papazian L. Respiratory rate and peak inspiratory pressure, new targets from the LUNG SAFE study analysis or physiopathological artifacts? J Thorac Dis. 2017;9(2):225–7.
Brower RG, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Severgnini P, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118(6):1307–21.
Futier E, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–37.
Güldner A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123(3):692–713.
Ladha K, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. Bmj. 2015;351:h3646.
Neto AS, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4(4):272–80.
Marini JJ, et al. Intracycle power and ventilation mode as potential contributors to ventilator-induced lung injury. Intensive Care Med Exp. 2021;9(1):55.
Tawfik PN, Evans MD, Dries DJ, Marini JJ. Reliable Estimates of Power Delivery During Mechanical Ventilation Utilizing Easily Obtained Bedside Parameters. Respir Care. 2022;67(2):177-83. https://doi.org/10.4187/respcare.09439.
Syed MKH, et al. Elastic power of mechanical ventilation in morbid obesity and severe hypoxemia. Respir Care. 2021;66(4):626–34.
Canet J, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32(7):458–70.
Foster CA, et al. Development and validation of procedure-specific risk score for predicting postoperative pulmonary complication: a NSQIP analysis. J Am Coll Surg. 2019;229(4):355–365.e3.
Boulos NM, et al. Monitored anesthesia care is associated with a decrease in morbidity after endovascular angioplasty in Aortoiliac disease. J Cardiothorac Vasc Anesth. 2020;34(9):2440–5.
Dworsky JQ, et al. Gerofit Prehabilitation pilot program: preparing frail older veterans for surgery. J Healthc Qual. 2019;41(2):91–8.
van Rooijen S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98.
Arora RC, et al. "NEW" Prehabilitation: a 3-way approach to improve postoperative survival and health-related quality of life in cardiac surgery patients. Can J Cardiol. 2018;34(7):839–49.
DiGiorgio K, et al. University of California center for health quality and innovation: experiences from a system approach to scaling up effective interventions. Implement Sci. 2015;10(1):A16.
Fogagnolo A, et al. Management of Intraoperative Mechanical Ventilation to prevent postoperative Complications after general anesthesia: a narrative review. J Clin Med. 2021;10(12):2656.
Panayi AC, et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg. 2019;218(2):393–400.
Tov LS, Matot I. Frailty and anesthesia. Curr Opin Anaesthesiol. 2017;30(3):409–17.
Chen S, et al. Risk factors for unplanned reintubation caused by acute airway compromise after general anesthesia: a case-control study. BMC Anesthesiol. 2021;21(1):17.
Acknowledgements
The author(s) wish to thank Esther Lan, Benjamin Mooso, Rebecca Kim, Sabrina Berci, and Anna Aledia for their assistance with data abstraction.
Funding
This work was supported by: 1) The University of California Davis Clinical and Translational Science Center (CTSC) support for the Research Electronic Data Capture (REDCap™) database (National Center for Advancing Translational Sciences [NCATS], National Institutes of Health [NIH], (grant UL1 TR000002); 2) The American Association of Critical Care Nurses (AACN) (Impact Research Grant #20297); 3) sub-award (A21–3192) from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), grant R25HL126140 (Arizona PRIDE-25: translational approaches to health disparities in the lung). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors (JS, CD, JA, MO, AA, RM, LG, MC, MG, PR, CS, CB, IA, GU) comply with the four ICMJE Guidelines for Authorship: 1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND 2) Drafting the work or revising it critically for important intellectual content; AND 3) Final approval of the version to be published; AND 4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRBs) of the University of the University of California, Davis (lead site) and Irvine, Los Angeles, San Diego, and San Francisco (collaborating sites) approved this study (IRB #822917; IRB Reliance #1735). All IRBs waived the requirement for informed consent for participation in the study.
Consent for publication
Not applicable.
Competing interests
Alpesh Amin, MD reported serving as PI or co-I of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Ferring, Seres, Millenium, PeraHealth, HeartRite, Aseptiscope, Sprightly, unrelated to the present study.
Maxime Cannesson, MD has an ownership interest in Sironis, a company developing closed-loop systems and serves as a consultant and receives research support from Edwards Lifesciences (Irvine, CA) and Masimo Corp. (Irvine, CA).
Ivo Abraham, PhD is joint equity owner in Matrix45. By company policy, owners and employees are prohibited from owning equity in client and sponsor organizations (except through mutual funds or other independently administered collective investment instruments), contracting independently with client and sponsor organizations, or receive compensation independently from such organizations. Matrix45 provides similar services to other biopharmaceutical, diagnostics, and medical device companies on a non-exclusivity basis. Of relevance to this paper, Matrix45 has not provided any services to this study, and Ivo Abraham’s participation was as a faculty member at the University of Arizona. Abraham is the Quantitative Methods Editor of JAMA Dermatology, for which he receives compensation. He is Deputy Editor-in-Chief of the Journal of Medical Economics for which he receives no compensation but is given an allotment of submissions free of publication charges.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1 Table S1.
Distribution of Age (by Decade) Used in Matching Process. Distribution of age (by decade) used in matching of case-control pairs.
Additional file 2 Table S2.
Distribution of Hospital Site Used in Matching Process. Distribution of hospital site used in matching of case-control pairs.
Additional file 3 Table S3.
Distribution of Surgical Procedure (by Body Organ or System) Used in Matching Process. Distribution of surgical procedure (by body organ or system) used in matching of case-control pairs.
Additional file 4 Table S4.
Distribution of Surgical Procedure (by Modified Clinical Classification) Used in Matching Process. Distribution of surgical procedure (by modified clinical classification group) used in matching of case-control pairs.
Additional file 5 Table S5.
Definitions of Comorbidities, Risk Factors, and Outcome Variables. Definitions of comorbidities, risk factors, and outcome variables used in analysis of case-control pairs.
Additional file 6 Table S6.
Distribution of Most Likely Etiology of L-PRF. Distribution of most likely etiology of L-PRF, by total cohort, pulmonary subset, and extrapulmonary subset.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stocking, J.C., Drake, C., Aldrich, J.M. et al. Outcomes and risk factors for delayed-onset postoperative respiratory failure: a multi-center case-control study by the University of California Critical Care Research Collaborative (UC3RC). BMC Anesthesiol 22, 146 (2022). https://doi.org/10.1186/s12871-022-01681-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01681-x