Abstract
Luronium natans (L.) Raf. is a European endemic species and is becoming increasingly rare and endangered in most countries. This study aimed to compare the community structure and environmental conditions of shallow and deep-water habitats of Luronium, and related anthropogenic influences. A total of 21 Luronium lake habitats were surveyed at Pomerania Lakeland (NW Poland). Luronium occurs mainly with other isoetids, as well as bryophytes, specifically Sphagnum denticulatum. It can also be found in oligotrophic lakes at a depth of 1.0 ± 0.6 m and in water with a large pH range (4.52 – 8.76), as well having a low conductivity (38.3 ± 20.9 µS cm−1; 19.0 – 106.1) and calcium concentration (3.9 ± 2.4 mg dm−3; 1.6 – 11,7).
The largest Luronium cover occurs at a depth of 1.5 m (44.8 ± 35.3%), but occasionally as deep as 3.5 m. In the depth gradient, the structure of underwater vegetation and environmental conditions exhibit obvious changes, which presents a clear distinction between shallow and deep-water habitats of Luronium. The differences mainly pertain to the abundances of Isoëtes lacustris and Elodea canadensis in the community, as well as environmental factors, such as water calcium, nitrogen and phosphorus concentrations, PAR, conductivity, and water color.
Compared to other isoetids, Luronium usually occurs in habitats with intermediate features, which are characterized by values between the typical, but deep-water, Isoëtes and shallow water Lobelia and Littorella. However, Luronium clearly prefers waters with higher temperatures (23.8 ± 2.7 °C), which are thus less oxygenated (96.6 ± 20.0%). In terms of pH, conductivity, and calcium concentration, Luronium occurs in waters having slightly lower values compared to other isoetids. Therefore, Luronium is a species that significantly expands the diversity of habitat number 3110 in the Natura 2000 network. Therefore, it can be considered as an indicator species of lobelia lakes.
An increased anthropogenic pressure primarily results in an increased water conductivity and a decreased water transparency. Consequently, Luronium increasingly inhabits shallower waters that are more oxygenated. Moreover, Luronium abundance is decreasing, while the abundances of species comprising underwater communities are also decreasing, e.g., S. denticulatum and I. lacustris, with a concurrent increase in Myriophyllum alterniflorum and E. canadensis cover.
Similar content being viewed by others
Background
Luronium natans (L.) Raf. (floating water-plantain) is a European endemic species distributed in North-western and Central Europe [1] in the sub-Atlantic and Atlantic climate zones [2,3,4,5]. In most European countries, Luronium is considered a rare species and in danger of extinction, and in some of them, it is already extinct [6, 7]. It is protected under the Berne Convention, as well as the so-called Habitats Directive within the Natura 2000 Program. Natura 2000 is a network of core breeding and resting sites for rare and threatened species, together with certain rare natural habitat types, which are protected. The Natura 2000 network includes all EU countries, both on land and at sea (European Commission; https://environment.ec.europa.eu/index_en). The Council Directive 92/43/EEC [8], on the conservation of natural habitats, and wild fauna and flora, determined the legal framework for creating the European ecological network Natura 2000.
Floating water-plantain Luronium natans (L.) Raf. (synonyms: Alisma natans L., Elisma natans (L.) Buchenau) is an angiosperm (Angiospermae) and a monocotyledonous species (Monocotyledones) in the Alismataceae plant family. It is a perennial and evergreen plant characterized by clonal growth. Each individual is comprised of unevenly aged ramets connected with stolons. Moreover, it is a rhizophyte and isoetid.
It mainly propagates vegetatively [9], but such vegetative propagation is lower compared to populations of Lobelia and Littorella [9, 10]. This can result from Luronium stolons breaking apart easily in disturbed habitats, and uprooted ramets which fail to root again in the substrate [11]. Offspring ramets fail to obtain assimilates when disconnected from maternal ramets [12]. As with other clonal aquatic plants, the effectiveness of generative propagation is very low [13], and is especially exacerbated in disturbed habitats [14].
L. natans is distributed in aquatic and semi-aquatic environments, where it develops into two morphologically distinct forms: underwater and semi-aquatic [15]. The underwater form comprises of a bottom-dwelling plant with a rosette of linear leaves growing on a shortened shoot with a filamentous flowering stem. The leaves in the rosette are ensiform and pointed; however, unlike other isoetids, they are flexible. The root system is of the beam type, while stolons grow from the rosette base. The semi-aquatic form includes shallow-growing individuals, as a nymphaeid plant with long-petioled oval leaves floating on the water surface [16]. This form can be found in several types of aquatic environments, from dystrophic to eutrophic lakes, as well as in anthropogenically transformed reservoirs. To date, the differences in the community structures composed of both forms, as well as their environmental conditions, have not yet been studied.
Literature has demonstrated that the floating water-plantain L. natans grows in soft water, but which is highly very variable in terms of pH, ranging from strongly acidic [17,18,19], slightly acidic, neutral [20,21,22], and even alkaline [17, 23]. These are most often oligotrophic waters [21, 24,25,26,27,28,29,30], and less often mesotrophic [2, 31, 32] or eutrophic [30]. It can be found on mineral substrates which are mainly sandy [2, 17, 18, 30], or a mix of sand and gravel [2, 17], but can be also be found on substrates rich in calcium [27, 33] and organic material [30, 34], such as silt [17, 35] or peat [36, 37]. These substrates are usually moderately fertile [38] and moderately rich in organic matter [2, 18]. Moreover, these are often variable in pH, similar to water, ranging from acidic and neutral, to alkaline [27].
Literature has shown that floating water-plantain is distributed throughout numerous communities of aquatic plants, as well as aquatic and wetland plants [22, 32, 39,40,41,42]. It is firstly a component of the Littorelletea uniflorae Br.-Bl. et R.Tx. 1943 class communities [21, 42, 43]. However, it is not yet clear with which isoetids it coexists most frequently, and whether it occurs in the same or different habitats from other isoetids.
In most countries, L. natans is an increasingly rare and endangered species. England is an exception, since this species, although disappearing, has not yet been classified as endangered [6, 44]. The extinction of L. natans populations is significant, but more thorough underwater research, as well as the discovery of new habitats where they occur (e.g., dykes, ditches, equalization ponds, water reservoirs formed due to mineral extraction and peat mines) mean that the total number of known populations have not yet decreased drastically [6]. Previous studies have shown that L. natans is a species with a high phenotypic plasticity, specifically carbon acquisition plasticity, and can adapt to different habitat conditions [33, 45].
The following hypotheses are presented here:
-
1.
The community structure of Luronium differs according to water gradient.
-
2.
The environmental conditions of Luronium differ according to the water gradient.
-
3.
Luronium natans has a broader ecological spectrum than other isoetids found in softwater lobelia lakes (habitat 3110); Oligotrophic waters containing very few minerals of sandy plains (Littorelletalia uniflorae).
-
4.
A slight increase in anthropogenic pressure leads to an increase in Luronium abundance. In turn, the strong anthropogenic pressure on Luronium habitats affects environmental conditions and community structure. Additionally, Luronium is found in increasingly shallower habitats, in decreasing abundance, and in communities with decreasing species numbers.
The aim of this study was to compare the community structures and environmental conditions of shallow and deep-water habitats of L. natans subjected to anthropogenic pressures of varying intensities. Moreover, the study aimed to identify the main differences in Luronium habitats as compared to habitats of other isoetids that are found in the lakes of north-western Poland.
Results
Structure of Luronium natans community
A total of 38 plant species were found in the L. natans community, including a large group of helophytes (11 species). On average, there were 3.3 ± 1.7 species per 0.1 m2 (1–10, Me = 4). Only 9 species had a frequency exceeding 5%, while 6 species had a frequency exceeding 10%. Isoëtes lacustris had the highest frequency in the community (36.4%), with Lobelia dortmanna being similar (36.1%), and Sphagnum denticulatum also having a relatively high frequency (25.9%). The other species were much less frequent (Table 1; Fig. 1).
Isoëtes lacustris as observed in the community also had the highest abundance (cover of 35.7 ± 29.3%; 1–100%; Me = 25%), with L. natans having only slightly less cover (35.3 ± 30.6%; 1–100%; Me = 25%). A high abundance was also found for L. dortmanna (25.8 ± 20.8%; 1–100%; Me = 20%), while significantly lower abundances were found for S. denticulatum, Myriophyllum alterniflorum, Warnstorfia exannulata, Elodea canadensis and L. uniflora (Table 1). The highest Luronium abundance was observed in patches composed with I. lacustris and S. denticulatum, whereas the lowest abundance occurred in patches with L. uniflora and Juncus bulbosus (Fig. 2).
The vertical structure of the Luronium community was poorly developed. Specifically, it was a monolayered community dominated by isoetids and Sphagnum. However, elodeids were also found in it, mainly M. alterniflorum and E. canadensis. These two species comprised the vertical structure, and their percentage frequency exceeded about 12%. Similar results were observed for average cover, but on occasion they were found in community patches with up to 90% cover (Table 1). In contrast, the proportion of rush plants (Eleocharis palustris, Juncus effusus, J. bulbosus and Lysimachia thyrsiflora) was small, and their frequencies and covers had small percentage values. The vertical structure was also occasionally formed by nymphaeids (Table 1). The spatial variability of the community was small and mainly related to depth gradient changes, and only at community edges did Luronium have a small cover (< 5%), in contrast to the other isoetids or S. denticulatum. This variability was noticeable primarily in the depth gradient (cf. section on the “Shallow- and deep-water populations of L. natans”).
Environmental conditions
Luronium natans habitats occurred at a depth of 1.0 ± 0.6 m (Me = 0.5) in water with a very different pH (4.52 – 8.76; Me = 5.88), and characterized by a low conductivity (38.3 ± 20.9 µS cm−1; 19.0 – 106.1) and calcium concentration (3.9 ± 2.4 mg dm−3; 1.6 – 11,7). Moreover, the water was clear and well oxygenated, but significantly colored by humic acids (Table 2). The sediment within the community was characterized by acidic reactions and, similar to the water, also had a low conductivity and calcium concentration. It was also rich in mineral content and poorly hydrated.
Luronium communities primarily occurred in waters having the highest (slightly alkaline) pH, together with M. alterniflorum and E. canadensis, whereas acidic waters were characterized by S. denticulatum, W. exannulata, I. lacustris, and Eleocharis palustris (see Additional file 1: Fig. S1). Luronium, as well as W. exannulata, S. denticulatum, J. bulbosus and E. palustris, also occurred in habitats with the highest water redox potential, while in low redox habitats were characterized by M. alterniflorum, E. canadensis, I. lacustris, and L. dortmanna. Myriophyllum alterniflorum and E. canadensis co-occurred with Luronium in waters rich in calcium, whereas other species were found in waters with low Ca2+ levels; these mainly included S. denticulatum and W. exannulata. Sphagnum denticulatum and W. exannulata also occurred concurrently with Luronium in significantly colored habitats, whereas I. lacustris, M. alterniflorum and E. canadensis were mainly found in less colored waters.
Most species coexisted with Luronium in very shallow waters (at a depth of ca. 0.5 m), while W. exannulata and I. lacustris mainly occurred in deeper zones (ca. 1.5–3.5 m; see Additional file 1: Fig. S1).
Myriophyllum alterniflorum and E. canadensis, together with Luronium, grew mainly in waters with the highest oxygen levels, while other species were found in less oxygenated waters. Warnstorfia exannulata and S. denticulatum were specifically found in the least oxygenated waters (see Additional file 1: Fig. S2). Eleocharis palustris was found together with Luronium in the warmest and most illuminated waters, whereas colder waters were mainly characterized by J. bulbosus, and deep-water species such as W. exannulata, occurred in poorly lit waters. Isoёtes lacustris occurred mainly in waters with the highest transparency, together with Luronium, and waters with the lowest transparency were characterized by E. palustris and J. bulbosus.
Littorella uniflora, E. canadensis, and M. alterniflorum, together with Luronium, grew in sediments with the highest pH (see Additional file 1: Fig. S3), whereas sediments with the lowest pH (strongly acidic) were characterized mainly by W. exannulata and S. denticulatum, as well as E. palustris. Sediments with the highest electrolytic conductivity, organic matter density, and hydration levels were characterized by Luronium assemblages with W. exannulata. Sediment features failed to significantly differentiate the habitats of other L. natans assemblages.
In conclusion: Luronium habitats were very similar in terms of environmental conditions compared to S. denticulatum and I. lacustris, as well as W. exannulata (group A; Fig. 3). Luronium assemblages that had these species in common were mainly found in medium depth phytolitoral zones, and in acidic waters that were poor in calcium, light, and oxygen levels, but that were significantly colored. Lobelia dortmanna and L. uniflora (group C) were distributed with Luronium mainly in shallow waters with a higher pH than group A, as well as being well lit and oxygenated, while being poorly colored. Eleocharis palustris and J. bulbosus (group B) were also found in shallow water habitats that were rich in nitrogen and humic acids, and where water transparency was very low. significantly with the depth gradient. canadensis (group D; Fig. 3) occurred together with Luronium mainly in waters with a high electrolytic conductivity, and that were rich in calcium and phosphorus, well oxygenated, very poorly colored and with high pH levels.
Shallow- and deep-water populations of L. natans
Luronium natans was found in lakes at a depth of up to 3.5 m, and its abundance changed significantly with the depth gradient (p < 0.0001; Fig. 4). The largest cover occurred at a depth of 1.5 m (44.8 ± 35.3%), and was larger as compared to the shallow zone 0.5 m (p < 0.0001; RIR Tukey test for unequal numbers) and deep zone at 2.5 m (p = 0.037; Fig. 4, Table 3). Luronium was only occasionally found in the deepest zone (3.5 m). Vegetation structure and environmental conditions changed evidently in relation to the depth gradient for Luronium habitats. The shallow-water population was found up to a depth of 1 m (average depth of 0.5 m), which was different compared to the deep-water population that was characterized by a range of 1–4 m (average depth of 2.5 m) and the occurrence of 24 plant species. Only 14 species were common to both populations (Table 4).
In shallow-water habitats (0–1 m), L. natans usually produced numerous floating leaves (Fig. 5), whereas underwater rosettes were poorly developed. Luronium occurred with 30.4 ± 26.4% (1–100%) cover, and the community had 34 additional species. However, most of these were not very frequent (Table 4). Only 9 species had a frequency above 5%, while 7 had a frequency above 10% (L. dortmanna 54.0%, S. denticulatum 26.7%, I. lacustris 23.6%, M. alterniflorum 16.9%, E. canadensis 15.5%, J. bulbosus 11.7%, L. uniflora 11.2%). The largest Luronium cover occurred with L. dortmanna (27.2 ± 21.7%) and I. lacustris (21.4 ± 18.9%) in shallow-water habitats, while S. denticulatum (19.9 ± 18.5%) and W. exannulata (19.1 ± 28.0%) also had significant abundances. The Luronium shallow-water habitats were characterized by a lightly acidic water pH (4.52–8.76; Me = 6.01), a low conductivity and calcium concentration, as well as significant PAR intensity and a high oxygen level. The sediments in this zone were mineral, and were poorly hydrated and strongly reduced (Table 5).
In deep-water habitats (1–4 m), Luronium specimens were characterized by very well-developed underwater rosettes and a low number of floating leaves, which had very long and delicate petioles (Fig. 6). Such habitats occurred at a depth of 1.7 ± 0.4 m and were characterized by a much higher L. natans cover of 42.1 ± 34.4% (p < 0.001) compared to shallow-water zones. There were much fewer accompanying species (20; Table 4), with 9 species featuring a frequency of over 5% and 6 above 10% per 0.1 m2. Isoëtes lacustris had nearly double the cover of other species (44.3 ± 31.1%; p < 0.001), with E. canadensis also having a high cover of 21.9 ± 13.0 (p = 0.005), together with Batrachospermum (11.1 ± 7.2%; p < 0.027), while L. dortmanna (16.5 ± 9.3%; p = 0.011) had a low cover. Deep-water habitats were poorer in species compared to shallow-water zones. Specifically, these were marked by a lower pH (4.75–7.35; Me = 5.62; p < 0.001), conductivity, lower calcium, phosphorus and nitrogen (p < 0.001) concentrations, and low oxygen levels and PAR intensities (p < 0.001), whereas water color (p = 0.035) and its redox potential (p < 0.001) were higher, as well as sediment hydration, organic matter content, and sediment redox potential (p < 0.001; Table 5).
Considering the community structures and the features of the aquatic environments, the shallow-water habitat of Luronium occurred in a different part of the PCA graph compared to deep-water zones (Fig. 7). Although sample diversity was larger along the first axis (due to environmental conditions), the separation of shallow and deep-water habitats was mainly determined by features correlated with the second axis. These primarily included positively correlated water nitrogen (0.91) and phosphorus (0.35) concentrations, and negatively correlated abundances of I. lacustris (-0.31). Habitats diversities along the first axis were determined by environmental conditions. The strongest positive correlations with this axis were calcium concentration (0.92), pH (0.88), conductivity (0.85), phosphorus concentration (0.73), while water color (-0.56) was negatively correlated.
Luronium natans versus other isoetids
The average depth of Luronium was 1.0 ± 0.6 m (Me = 0.5 m) and was greater compared to shallow-water species, such as L. dortmanna and L. uniflora, and much smaller than the deep-water I. lacustris (see Additional file 1: Fig. S4). Moreover, in terms of PAR radiation intensity, Luronium revealed indirect features compared to other isoetids, as it occurred in waters with a higher PAR than I. lacustris, and a lower than L. dortmanna and L. uniflora. However, compared to other isoetids, Luronium preferred waters with a higher temperature (23.8 ± 2.7 °C) and that were less oxygenated (96.6 ± 20.0%). The values of these traits were associated with higher concentrations of dissolved organic matter, as indicated by the strongest water color (32 ± 25 mg Pt dm−3) and were thus characterized by the poorest water transparency (3.3 ± 1.1 m). Moreover, L. natans occurred in waters with the lowest pH, conductivity, and calcium concentration values, while in terms of redox potential, it occurred in habitats similar to I. lacustris, with a slightly higher potential than L. dortmanna, and a lower potential than L. uniflora (see Additional file 1: Fig. S5). Luronium natans also occurred in habitats that were characterized by a very low concentration of nitrogen and phosphorus (see Additional file 1: Fig. S6). In Luronium habitats, mean carbon dioxide concentration was 3.39 ± 2.73 mg CO2 dm−3 (0.0–9.42; Me = 2.30), and does not differ from the values found in the habitats of the other isoetids (Additional file 1: Fig. S8). Mean bicarbonate concentration in Luronium habitats was 13.96 ± 10.81 mg HCO3− dm−3 (6.86–91.08; Me = 11.44), and were the same as in I. lacustris and L. dortmanna habitats. However, the bicarbonate concentration found in Littorella habitats stood out, as it was higher than the habitats of all isoetids with p < 0.0001. Isoetid habitats differed slightly in terms of sediment features. Luronium was found in sediments similar to those preferred by L. dortmanna and L. uniflora, but that were poorer in organic matter and distinguished by a lower redox potential (see Additional file 1: Figs. S7, S8).
The PCA analysis showed that the L. natans community did not differ from other isoetids (Fig. 8) when accounting for the occurrence of other species and environmental conditions. It should therefore be assumed that they occurred in one habitat and formed one community. The diversity of isoetid habitats along the first axis was primarily determined by water color (-0.65), PAR intensity (0.56), and water oxygenation (0.51). The diversity of habitats along the second PCA axis was determined by positively correlated environmental characteristics, such as water color (0.73), PAR intensity (0.63), and water oxygenation (-0.25).
Anthropogenic pressure on Luronium habitats
The increase in the intensity of anthropogenic pressure significantly limited the depth range of the aquatic plants analyzed, but also increased the number of existing species (Table 6). However, no significant correlation was found between pressure intensity and Luronium cover, as well as general underwater plant cover. Importantly, pressure impacts that were directed at the lake and its catchment were very similar.
Anthropogenic pressure directed at Luronium habitats resulted in a clear transformation of its environmental conditions. Specifically, waters subject to anthropogenic pressure had a higher conductivity, and a lower color and PAR light intensity (Fig. 9).
The impact of the intensity of anthropogenic pressure on L. natans habitats. Explanations: L; blue color—the impact of the intensity of anthropogenic pressure on the lake; C; green color—the impact of the intensity of anthropogenic pressure on the catchment area;—conductivity (respectively L and C: y = 44.37–21.94x + 7.53x2; y = -18.27 + 47.71x-6.66x2);—water color (respectively L and C: y = 40.34–5.95x + 0.18x2; y = 73.58–44.24x + 8.74x2);—PAR intensity (respectively L and C: y = 4.45 + 25.08x-4.92x2; y = -6.73 + 38.19x-8.15x.2)
A slight increase in anthropogenic pressure intensity led to an increase in the abundance of L. natans (Fig. 10); however, a larger pressure resulted in a clear reduction in cover. It should be noted that the impacts on L. natans abundance were very similar regarding pressure directed at the lake and its catchment; however, this was slightly larger for catchment transformations.
The increase in anthropogenic impact on L. natans habitats resulted in the community area shifting towards an increasingly shallow littoral (Fig. 11), while the number of species in the community increased simultaneously, and consequently also the competition from other macrophytes.
The impact of the anthropogenic pressure on the occurrence depth of plants and the number of species. Explanations: L; blue color—the impact of the lake on the occurrence depth of plants (y = 2.20–0.62x + 0.09x2) and the number of species (y = 4.03–1.89x + 0.47x2); C; green color—the impact on the catchment area on the occurrence depth of plants (y = 1.87–0.39x + 0.05x2) and the number of species (y = 2.04 + 0.32x-0.01x.2)
The impact of anthropogenic pressure on L. natans referred to both the transformation of environmental conditions and community structure (Fig. 12). There is no clear difference in the effects of these transformations regardless of whether the human impact was directed at the lake or its catchment. The intensity of anthropogenic pressure primarily caused an increase in water conductivity and a decrease in water transparency. It is worth emphasizing that, as a result of the pressure, Luronium occurred in waters with increasingly less color (despite the decrease in water transparency); this is related to community formation at more shallow depth (cf. Fig. 11), where the water is better oxygenated. The increase in anthropogenic pressure intensity influenced the decrease in the abundance of L. natans in the community, but primarily also the decrease in the abundance of other species such as S. denticulatum and I. lacustris, with a concurrent increase in M. alterniflorum and E. canadensis cover, as well as L. dortmanna to a lesser extent.
Discussion
Luronium natans is a very rare European endemic, although it is found in many different communities, both aquatic and aquatic-terrestrial plants [46]. Literature shows that it is the basic unit of Littorelletea uniflorae Br.-Bl. et R.Tx. 1943 class communities [21, 42, 43]. The occurrence of this species was confirmed in patches of Isoëtetum echinosporae Koch 1926 em. Dierss. 1975 [39], Isoëto-Lobelietum Koch 1926 em. Tx. 1937 [32, 35, 39], Eleocharitetum multicaulis (Allorge, 1922) R. Tx. 1937 [17, 39], Hyperico-Potamogetonetum oblongi (Allorge, 1921) Br.-Bl. Et Tx. 1952 [39], Samolo-Littorelletum Westhoff 1943 [39], Pilularietum globuliferae Tx. 1955 ex Müller und Görs 1960 [39], Eleocharitetum acicularis (Bauman, 1911) Koch 1926 [17, 21, 39], Eleocharito-Littorelletum uniflorae Chouard 1924 [32], and Myriophyllo-Nupharetum Koch 1926 [32]. Luronium was also found in Potametea R. Tx. et Prsg. class communities, Isoëto-Nanojuncetea Br.-Bl. Et R. Tx. 1943 class communities [21], and Phragmitetea R.Tx. et Prsg. 1942 class communities [47]. It was occasionally found in Utricularietea intermedio-minoris Den Hartog et Segal 1964 em. Pietsch 1965 [40, 48, 49] community. Some authors report that L. natans forms its own community, namely Luronietum natantis Szańkowski ex Šumberová, Čtvrtlíková et Bauer in Chytrý 2011 ass. nova [50], in which it is a characteristic and dominant species. According to Šumberová [50], the Luronium community forms within the shallow littoral zone at a depth of between 0.1 to 2.5 m. In Poland, it also occurs at similar depths (1–2 m) [9], although as indicated by our research in Pomeranian lakes, it is occasionally found up to a depth of 3.5 m (cf. Fig. 3, Table 4). In Czech Republic reservoirs it forms a community mainly with Callitriche hamulata, Juncus bulbosus and Potamogeton natans [50]. In Poland, due to harsh winters, Luronium mainly forms part of aquatic plant communities, most often in the Littorelletea uniflorae Br.-Bl. et R.Tx. 1943 class, and occurs with other isoetids, predominantly I. lacustris and L. dortmanna (Table 1). It often forms a community with S. denticulatum, and slightly less frequently with M. alterniflorum and E. canadensis. In addition, a common element of this community, as in the Czech Republic, is J. bulbosus (11.2%, Table 1), and less frequently P. natans (0.2%), but never C. hamulata.
The main factor limiting the development of Luronium, and the reason for the decrease in the number of its sites, relates to its intolerance of competition [27, 33], even when resulting from the natural succession of communities. This is also why Luronium retreats from anthropogenic habitats, such as ditches and canals subjected to rapid overgrowing [51]. This intolerance may result for several reasons, including physical overgrowth, and competition for light and nutrients. All of these factors likely play a significant role, since they often occur concurrently. This is particularly important in the present, since the pressure on the lakes is very high and the anthropogenic transformations of Luronium habitats are significant, for example, as a result of their eutrophication. One of the effects of advanced eutrophication includes mass blooms of plankton algae and the development of filamentous algae, which leads to a decrease in light intensity, as well as transformations in the structures of biocenoses developed by isoetids [52,53,54,55]. However, it is worth emphasizing that Luronium, as a plant capable of producing surface leaves, is quite resistant to the effects of eutrophication (Fig. 13) and occurs in highly transformed lakes. Floating leaves enable improved access to light, the intensity of which may be low in stained eutrophic water. Floating surface leaves also allow access to atmospheric carbon dioxide, which may be in short supply in eutrophic waters since it is not available at a pH higher than 8.3. Luronium prefers free CO2 for photosynthesis, but is able to use also bicarbonate [45]. Specimen sizes decrease with increases in trophies, while population densities decrease, and their ranges shift closer towards the shore [9]; however, population reproductive potential is not significantly disturbed. Literature shows that, under conditions of trophic growth, slow-growing isoetids are displaced by faster-growing elodeids [56, 57]. However, Luronium growth rates are quite significant for isoetids, so it can be assumed that it is not as easily eliminated from communities as other isoetids. On the other hand, Luronium rosettes are less durable compared to other isoetids and decompose much faster, especially in highly disturbed conditions, which primarily applies to underwater leaves.
It is also worth noting decreased water levels, resulting from climate change, can contribute to a significant reduction in the typical, and usually very abundant, deep-water populations. Poor rooting in organic sediments causes a mass release and floating up of entire patches of isoetids (Fig. 14), and not only for Luronium natans. This may soon contribute to a significant reduction in the abundance of deep-water isoetid populations, including, but not limited to, Luronium. Uprooted individuals of deep-water Luronium natans populations can remain in water for extended time periods due to stolons. Completely uprooted plants can then remain on the water surface among rush plants, or with floating leaves, where they bloom and fruit or are drifted by wave action to the shore (Fig. 14), where they root again and establish terrestrial populations of floating water-plantain.
Uprooted individuals of Luronium natans. Top photos—for an extended time period uprooted individuals of deep-water populations remain in water due to stolons; Bottom photos—completely uprooted individuals of Luronium remain on the water surface among rush plants or with floating leaves where they bloom and fruit
Literature indicates that floating water-plantain seems to have a very wide range of tolerance to the physical and chemical properties of aquatic environment [6, 58, 59]. However, this information is often contradictory and differs for local populations. In the Czech Republic L. natans occupies habitats with clear and colorless water of pH 6–7, containing small amounts of dissolved substances, especially nitrogen and phosphorus, and up to 30 mg Ca dm−3 of calcium [50]. Similar habitats occur in Germany [20]. In Poland, L. natans grows in waters with very similar characteristics [9], but mainly in oligotrophic water, with a slightly lower pH of 4.8–6.3, and a very low calcium content (1–2 mg Ca dm−3). In the Atlantic part of the area, where Luronium has an ecological optimum, it has often been observed in eutrophic waters with very high concentrations of calcium [27] and in waters with an exceptionally wide pH range (3.6–8.0) [6]. This study confirms that Luronium is characterized by a large ecological range, but this only applies to a few features of the aquatic environment. For some features, the range of variability for Luronium habitats is slightly wider than other isoetids, but it should be assumed that it still occurs in the same habitats as other isoetids (cf. Fig. 8), thereby usually forming a common community. Previous studies [22] have indicated that significant differences exist between L. natans, and I. lacustris and L. dortmanna communities, specifically in their waters, but also in their substrates. Despite its adaptation to adverse conditions (surface leaves), Luronium is not found in alkaline waters that are fertile and rich in calcium, as in the case of L. uniflora (cf. Additional file 1: Figs. S4-S8). Bazydło [9] reports that high levels of pH (pH > 8.0), carbon (> 6.0 mg C dm−3), and phosphorus (> 30.0 µg TP dm−3) impede the growth of Luronium populations. These specific environmental conditions, and a wide range of variability in Luronium habitats, are primarily demonstrated by its occurrence in very poor habitats of dystrophic lakes. Here, floating leaves enable it to compete even with abundant bryophytes of the Sphagnum genus. It also allows Luronium to survive in waters that are intensely colored by humic substances (cf. Table 2, Fig. 3). Luronium’s range of tolerance to water coloration is the largest among isoetids (see Additional file 1: Fig. S6), but it also tolerates lower levels of pH, conductivity, calcium, and trophy (cf. Additional file 1: Figs. S5-S8), which other indicator species of lobelia lakes do not tolerate [60]. This is confirmed by Szańkowski and Kłosowski [22], and indicates a clear preference of Luronium for growing in oligotrophic, extremely soft, and calcium-poor waters. Therefore, Luronium natans is a species that significantly expands the diversity of habitat 3110 in the Natura 2000 [61], to include mainly dystrophic basins where the remaining isoetids can no longer be found.
Conclusion
The community structure and environmental conditions of Luronium differ distinctly between shallow- and deep-water zones. Luronium natans has a broader ecological spectrum than other isoetids due to being abundant in extremely oligotrophic, acidic, and soft waters. Low-intensity anthropogenic pressure focused on a lake or its catchment area leads to an increase in L. natans abundance. In turn, a strong anthropogenic pressure on L. natans habitats negatively affects both environmental conditions in the lake and the structure of underwater communities, from which L. natans regresses or moves towards the shallowest phytolittoral zones.
Our work confirms that Luronium occurs mainly with other isoetids, primarily with I. lacustris and L. dortmanna. It often forms a community only with S. denticulatum, and that is why Luronium is a species that significantly expands the diversity of habitat 3110 in the Natura 2000 network, to include mainly dystrophic lakes where the remaining isoetids can no longer be found. Therefore, it is worth considering Luronium as an indicator species of lobelia lakes because it is the only isoetid in habitats with such advanced natural succession. Dystrophic lakes without Lobelia, Isoëtes, and Littorella are now treated as former lobelia lakes/degraded lobelia lakes, although they still contain a very rare isoetid, namely Luronium, which is especially valuable for the preservation of biodiversity in Europe.
Methods
Samples collection and environmental analysis
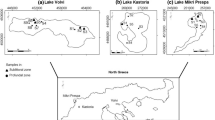
The material for the study was collected each July from 2010 to 2020, from 21 lakes of the Pomeranian Lakeland, where Luronium natans is distributed (Fig. 15; Table 7). All studied lakes are low-trophy reservoirs; 18 of them are lobelia lakes with indicator species, and three are dystrophic lakes with L. natans and without the presence of indicator species, namely Lobelia, Isoëtes and Littorella (Nos. 1, 2, and 16). Of the lobelia lakes, 11 are nutrient-balanced (typical) and characterized by low trophy, a pH close to neutral, and the usual presence of all indicator species. Two of the surveyed reservoirs were eutrophic lobelia lakes (lakes 8 and 20), distinguished by higher trophy than nutrient-balanced lobelia lakes, a higher pH (alkaline) and a higher water color, and the occurrence of mainly L. uniflora and M. alterniflorum. The development of these lakes, and the succession of vegetation, are moving towards a eutrophic state. Five of the studied lakes were dystrophic lobelia lakes (5, 6, 7, 9 and 10), characterized by a very acidic pH, brown water color and very low trophy. The indicator species of these lakes are primarily representatives of the genus Isoëtes, and a large proportion of Sphagnum and Luronium exists in their underwater communities. The development of this group of lobelia lakes, and the succession of vegetation, leads to the formation of dystrophic lakes and, consequently, peatbogs.
Location of the study sites (1–21; for explanations, see Table 7)
The samples were collected using the diving method, in transects perpendicular to the lakeside (as per the methodology of Chmara et al. [62]). Diving was performed within the phytolittoral zone of each lake, at depths ranging from the shallowest zone (0.5 m) to the lower limit of plant occurrence. In total, the research was conducted in 241 depth zones within 43 transects, each being approximately 250 m wide (Table 7). In each zone (a bottom strip at 1.0 m intervals), a non-invasive diving method was applied to describe an average of about 50 random plant samples. Phytosociological relevés were taken, each with an area of 0.1 m2. Total plant cover was determined for each sample. A total of 8 732 plant samples were described from the lakes, with Luronium occurring in 723 samples. Only 34 samples (4.7%) had Luronium cover < 5%, and only 23 had a cover of 1%, which were samples at the edges of community ranges. In such samples, Luronium usually occurred with a low cover, but often without the contribution of other species.
Environmental condition assessments were performed based on the samples of near-sediment water and sediments from depth zones. Field measurements were made in the depth profile from a pontoon anchored in the deepest part of the lake. From each depth zone where the plant samples were described, three samples of near-sediment water (approx. 5 cm above the bottom, where plants grow) were collected, with a volume of 0.5 dm3, and three samples of sediment of similar volume were taken in the area where plants are rooted. Water and sediment samples were taken at the beginning of each littoral zone (depth zone), as well as the middle and at the end. Samples were taken by the diver at the beginning of the underwater work, and were allowed to determine the maximum extent of submerged plants, as well as dive to plants in each zone. Plant samples were generally taken starting from the deepest zone, then from successively more shallow zones, and finally from the shallowest, to avoid disturbing the environment by the diver. In total, 543 water and sediment samples, respectively, were collected in the analyzed lakes, with 87 water samples and 87 sediment samples from 29 depth zones for the analyses of physical and chemical characteristics of Luronium habitats. Water samples were analyzed as per the methods proposed by Eaton et al. [63]. Water pH, conductivity, concentration of calcium, CO2, HCO3−, nitrogen, phosphorus and humic acids, and color were determined, whereas in the depth profile, water oxygenation, temperature, and intensity of photosynthetically active radiation (PAR) were measured in the field. In sediments, pH, redox potential, and electrolytic conductivity were measured, and the content of organic and mineral matter, as well as sediment hydration, were determined. Sampling depths were determined using the Eagle TriFinder depth finders, and water transparency by a Secchi disk without a glass pane on water surface. Water and sediment pH were measured using a pH-meter 320/SET1 with a SENTIX 97 T electrode. Water oxygenation and temperature were measured by a WTW OXI 197 oxygen meter with an EOT 196 electrode. PAR light intensity were measured by a Licor LI–250 Light Meter, and given as a percentage of the light reaching the water surface. Calcium concentration was determined with complexometric methods. Humic acid concentration was determined spectrophotometrically using a UV–VIS Aquamate spectrophotometer at 330 nm. The concentration of dissolved forms of inorganic carbon (CO2 and HCO3−) was assessed in the collected water samples by titration, using phenolphthalein and methyl orange, respectively. Water color was determined with a comparative method using the Platinum-Cobalt Reference Standards. Finally, total nitrogen and phosphorus were determined using Merck Spectroquant Cuvette Tests.
Lake pressure was determined on a scale of 0–3, where 0 represents no pressure (e.g., protection in lake reserves), 1 represents a low pressure (e.g., amateur fishing), 2 represents a medium intensity (e.g., intensive fishing, poorly developed recreation), and 3 represents intense pressure (fishing management, recreation, inflow of drainage waters from drained bog habitats, etc.). The pressure on the lake catchment was also determined on a scale of 0–3; where 0 represents no pressure (the catchment is natural, forest), 1 represents a low pressure (small transformations in the catchment, small deforestations), 2 represents medium transformations in the catchment (significant deforestation, mainly grassland, small areas of arable land), and 3 represents large catchment transformations (arable land dominant, urban, rural and summer buildings).
Statistical methods
The data collected on the environmental conditions and vegetation of individual lakes was catalogued in Microsoft Excel. Each entry recorded the number of species and the related plant cover, and all samples from a given depth zone were assigned to appropriately characterized environmental conditions, i.e., the average of the three water and sediment samples collected in each depth zone.
A total of 38 macrophyte species were found in the lakes analyzed. Average cover and frequency were determined for each species, as well as basic cover statistics (Table 1). All samples with L. natans (723) were used for further analysis of the community structure and its environmental conditions.
Community structure was described to verify the first hypothesis, and nine species with the highest frequency (> 5%, Fig. 1) were distinguished and further analyzed. Differences in L. natans cover in patches with these species were also determined (Fig. 2) using a nonparametric Kruskal–Wallis test. To indicate how Luronium cover changes with depth gradient, a Kruskal–Wallis test (Fig. 4) and HSD Tukey's post hoc test for unequal abundances (Table 3) were performed, while statistically significant differences between the community structure of shallow- and deep-water populations of Luronium were performed using the Mann–Whitney U test (Tables 4 and 5).
The environmental conditions of L. natans in the studied lakes were described to verify the second hypothesis (Table 2). Differences in habitat patches formed by Luronium with the highest frequency species (9 species with F > 5%, Additional file 1: Figs. S1-S3) were also determined using the non-parametric Kruskal–Wallis test. In addition, the rank of environmental factors in the formation of these Luronium patches (Fig. 3) was determined using Canonical-correlation analysis (CCA) in the CANOCO 5.1 software [64]. Statistically significant differences between environmental conditions of the shallow- and deep-water Luronium populations were determined using the Mann–Whitney U test (Table 5) using Statistica 13.1.
The two hypotheses were also verified by distributing shallow- and deep-water patches of Luronium, taking into account both the coverages of all species and the environmental features studied, using Principal Component Analysis (PCA) in CANOCO 5.1 (Fig. 7).
To verify the third hypothesis, we used the results from our underwater plant database AquaPlant, containing 8,662 samples of I. lacustris, 4,022 samples of L. dortmanna, and 2,214 samples of L. uniflora. Differences in environmental conditions of Luronium and other isoetids were also determined (see Additional file 1: Figs. S4-S8). The habitat distribution of L. natans and other isoetids was determined using PCA analysis based on species occurrence and environmental variables (Fig. 8). Calculations were performed in PAST v. 4.05 based on the correlation matrix.
To determine the relationship between the structure of the Luronium community and environmental conditions, and the intensity of anthropogenic pressure (the fourth hypothesis), the following were determined: (1) Spearman's rank correlation—correlation pressure in the lake and catchment on species depth, number of species, and coverage of Luronium, and total plant number (Table 6); (2) the effect of anthropogenic pressure intensity on L. natans habitat and coverage using the non-parametric Kruskal–Wallis test (Figs. 9, 10 and 11); and (3) the effect of anthropogenic pressure on environmental conditions and plant abundance based on Redundancy analysis (RDA; Fig. 12) in CANOCO 5.1 [64].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hultén E, Fries M. Atlas of north european vascular plants. Königstein: Koeltz Scientific Books; 1986. p. 1172.
Arts GHP, den Hartog C. Phytogeographical aspects of the West European softwater macrophyte flora. Acta Botanica Neerlandica. 1990;39:369–78.
Haury J, Thiebaut G, Muller S. Les associations rhéophiles des rivières acides du Masssif Armoracain, de Lozère et des Vosges du Nord, dans un contexte Ouest-Européen. Colloques Phytosociologiques. 1994;23:145–68.
Greulich S. Competition, perturbations et productivite potentielle dans la definition de l’habitat d’especes rares: Etudes experimentale du macrophyte aquatique Luronium natans (L.) Rafin. Lyon 1, France: Université Claude Bernard. 1999.
Szmeja J. Luronium natans L. (Raf.) in: Zarzycki, K., Kaźmierczakowa, R. (Eds.) Polska Czerwona Księga roślin [Polish Red Book of Plants], Kraków: PAN. 2001. p. 395–396. (in Polish).
Lansdown RV, Wade PM. Ecology of the Floating Water-plantain, Luronium natans. Conserving Natura 2000 Rivers Ecology Series No. 9. Peterborough: English Nature. 2003.
Szmeja J. Luronium natans (L.) Raf. in: Sudnik-Wójcikowska, B., Werblan-Jakubiec, H. (Eds.). Poradnik ochrony siedlisk i gatunków Natura 2000 – podręcznik metodyczny [Natura 2000 Habitat and Species Conservation Guide - methodological manual]. T. 9, Warszawa: Ministerstwo Środowiska. 2004. p. 155–159. (in Polish).
European Commission. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora, Official Journal of European Communities L 206, 1992; p. 7–50
Bazydło E. Effect of environmental conditions on the populations of Luronium natans (L.) Raf. Polish J Ecol. 2004;52(2):181–189.
Szmeja J. Struktura, organizacja przestrzenna i demografia populacji isoetydów [Structure, Spatial Organization and Demography of Isoetid Populations]. Ecological Studies of Submerged Aquatic Plants. Gdańsk: Uniwersytet Gdański. Rozprawy i Monografie, 1992;175:1–137. (in Polish).
Barrat-Segretain MH, Henry CP, Bornette G. Regeneration and colonization of aquatic plant fragments in relation to the disturbance frequency of their habitats. Arch Hydrobiol. 1999;145:111–27.
Price EAC, Marshall Ch. Clonal plants and environmental heterogeneity. Plant Ecol. 1999;141:3–7.
Erikson O. Clonal life histories and the evolution of seed recruitment. In: de Kroon H, van Groenendael J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. p. 211–26.
Combroux I, Bornette G, Willby NJ, Amoros C. Regenerative strategies of aquatic plants in disturbed habitats: the role of propagule bank. Arch Hydrobiol. 2001;152:215–35.
Casper SJ, Krausch HD. Süβwasserflora von Mitteleuropa. Pteridophyda und Anthophyta, Bd 23. Stuttgart: Gustav Fischer Verlag; 1980.
Rich TCG, Jermy AC. Plant Crib 1998. London: Botanical Society of British Isles; 1998.
Schoof-Van Pelt MM. A study of the vegetation of some amphiphytic communities of Western Europe. Stichting Studentenpers Nijmegen. 1973.
Smits AJM, Laan P, Their RH, van der Velde G. Root aerenchyma, oxygen leakage patterns and alcoholic fermentation ability of the roots of some nymphaeid and isoetid macrophytes in relation to the sediment type of their habitat. Aquat Bot. 1990;38:3–17.
Smits AJM, Kleufers RMJC, Kok CJ, Van Der Velde G. Alcohol dehydrogenase isoenzymes in the roots of some nymphaeid and isoetid macrophytes. Adaptations to hypoxic sediment conditions. Aquat. Bot. 1990;38:19–27.
Hanspach D, Krausch HD. Zur Verbreitung und Ökologie von L. natans (L.) Raf. in der DDR. Limnologica. 1987;18:167–175.
Szmeja J, Clément B. Comparaison de la structure et du déterminisme des Littorelletea uniflorae en Poméranie (Pologne) et en Bretagne (France). Phytocoenologia. 1990;19:123–48.
Szańkowski M, Kłosowski S. Habitat conditions of the phytocoenoses dominated by Luronium natans (L.) Raf. in Poland. Hydrobiologia. 2001;455:213–222.
Broyer J, Curtet L, Maillier S, Bove JJ. Incidences de la gestion des étangs piscicoles de la Dombes sur la flore aquatique remarquable. Ecologie. 1997;28:323–36.
Witting R, Pott R. Die Verbreitung von Littorelletea-Arten in der Westfalischen Bucht. Decheniana. 1982;135:14–21.
Van Katwijk MM, Roelofs JGM. Vegetaties van waterplanten in relatie tot het milieu. Laboratorium voor Aquatische Oecologie. Nijmegen: Katholieke Universiteit Nijmegen; 1988.
Fritz O. Flytsvalting, L. natans, funnen i Halland (1988) (A find of L. natans in the province of Halland, SW Sweden). Svensk botanisk tidskrift. 1989;83(2):135–136.
Willby NJ, Eaton JW. The distribution, ecology and conservation of Luronium natans (L.) Rafin. in Britain. J Aquat Plant Manag. 1993;31:70–76.
Lockton A, Whild S. Rare plants of Shropshire. Shrewsbury: Shrewsbury Museums Service. 1995.
Van den Munckhof PJJ. Glauconietrijke afzettingen in the Peel region. Een ijzersterke basis voor behoud en ontwikkeling van voedselarme, natte milieus. (Glauconite rich deposits in the Peel region. An iron basis for conservation and development of nutrient poor, wet environments). Natuurhistorisch Maandblad 89, maart, 2000. p. 43–52.
Bazydło E, Szmeja J. The effect of pH, dissolved organic carbon and total phosphorus concentration on selected life history traits of Luronium natans (L.) Raf. Pol J Ecol. 2004;52(2):191–200.
Meriaux JL, Wattez JR. Groupements vegetaux aquatiques et subaquatiques de la valée de la Somme. Colloques Phytosociologiques. 1981;10:369–413.
Cook CDK. Aquatic plants endemic to Europe and the Mediterranean. Botanische Jahrbücher für Systematik. 1983;103:539–82.
Greulich S, Bornette G, Amoros C, Roelofs JGM. Investigation on the fundamental niche of a rare species: an experiment on establishment of Luronium natans. Aquat Bot. 2000;66:209–24.
Szańkowski M. Ekologiczny status roślinności jezior lobeliowych w Polsce, PhD Dissertation. Warszawa: Warsaw University, (in Polish). 1998.
Dąmbska I. Roślinność litoralu jezior lobeliowych Pojezierza Kartuskiego [Vegetation of the littoral of the lobelia lakes of the Kartuskie Lake District]. Poznańskie Towarzystwo Przyjaciół Nauk. Prace Komisji Biologicznej. 1965;30:1–53. (in Polish).
Van Ooststroom SJ, Reichgelt TJ. Alismataceae Flora Neerlandica. 1964;1:1–15.
Weeda EJ, Westra R, Westra C, Westra T. Nederlandse oecologische flora, deel 4 IVN (Dutch ecological flora). Amsterdam: VARA en Vewin; 1991.
Schaminée JHJ, Weeda EJ, Westhoff V. Plantengemeenschappen van wateren, moerassen en natte heiden; de vegetatie van Nederland, deel 2 (Plant communities of waters, marshes and wet heath; the vegetation of the Netherlands, part 2). Uppsala-Leiden: Opulus Press; 1995.
Tüxen R. Prodromus der europäischen pflanzengesellschaften. Vaduz. 1975.
Pietsch W. Beitrag zur Soziologie und Őkologie der europaischen Littorelletea- und Utricularietea-Gesellschaften. Feddes Repertorium. 1977;88:141–245.
Roelofs JGM. Impact of acidification and eutrophication on macrophyte communities in soft waters in the Netherlands. I Field observations Aquatic Botany. 1983;17:139–55.
Schaminée JHJ, Westhoff V, Arts GHP. Die Strandlingsgesellschaften (Littorelletea Br -BL et Tx 43) der Niederlande, in europäischen Rahmen gefabt. Phytocoenologia. 1992;20:529–58.
Kłosowski S. Ekologia zbiorowisk głównych roślin wodnych z klasy Littorelletea uniflorae Br.-Bl. et R. Tx. 1943 w Polsce [Ecology of communities of major aquatic plants of the class Littorelletea uniflorae Br.-Bl. et R. Tx. 1943 in Poland]. in: Kraska, M. (Ed.) Jeziora Lobeliowe. Charakterystyka, funkcjonowanie i ochrona [Characteristics, functioning and conservation]. Cz. I. Idee ekologiczne, T. 6, Seria Szkice. 1994;4:93–104. (in Polish).
Stewart A, Pearman DA, Preston CD. Scarce plants in Britain. Peterborough: Joint Nature Conservation Committee; 1994.
Hyldgaard B, Brix H. Plasticity in carbon acquisition of the heterophyllous Luronium natans: An endangered freshwater species in Europe. Aquat Bot. 2011;94:127–33.
Goldsmith B, Shilland E, Shilland J, Turner S., Floating Water Plantain Luronium natans (L) Raf.: Current distribution and status in Llyn Padarn and Llyn Cwellyn, Wales. ECRC Research Report Number 161, London: Ensis Ltd. Environmental Change Research Centre, University College London, Pearson Building, Gower St., WC1E 6BT. 2014
Rejewski M. Roślinność jezior rejonu Laski w Borach Tucholskich [Vegetation of the lakes of the Laski area in Bory Tucholskie]. Toruń: UMK, Rozprawy. 1981. (in Polish).
Gos K, Bociąg K, Banaś K. Roślinność podwodna w kwaśnych jeziorach Pomorza [Underwater vegetation in the acidic lakes of Pomerania]. In: Banaszak J, Tobolski K, editors. Park Narodowy Bory Tucholskie. Bydgoszcz: Wydawnictwo Uczelniane WSP w Bydgoszczy; 1998. p. 261–78 (in Polish).
Matuszkiewicz W. Przewodnik do oznaczania zbiorowisk roślinnych Polski [Guide to the identification of plant communities of Poland]. in: Faliński, J.B. (red.) Vademecum geobotanicum 3, Warszawa: PWN. 2001. (in Polish).
Šumberová K, Čtvrtlíková M, Bauer P. Luronietum natantis. In: Chytry M. editor. Vegetace České republiky 3. Vodni a mokřadni vegetace (Vegetation of the Czech Republic 3. Aquatic and wetland vegetation). Praha: Academia. 2011. p. 296–99.
Nielsen UN, Riis T, Brix H. The effect of weed cutting on Luronium natans. Aquat Conserv Mar Freshwat Ecosyst. 2006;16:409–17.
Arts GHP, Roelofs JGM, De Lyon MJH. Differential tolerances among soft-water macrophyte species to acidification. Can J Bot. 1990;68:2127–34.
Arts GHP, van der Velde G, Roelofs JGM, van Swaay CAM. Successional changes in the soft-water macrophyte vegetation of (sub) Atlantic, sandy, lowland regions during this century. Freshw Biol. 1990;24:287–94.
Rørslett B. Principal determinants of aquatic macrophyte richness in Northern European lakes. Aquat Bot. 1991;39:173–93.
Vestergaard O, Sand-Jensen K. Alkalinity and trophic state regulate aquatic plant distribution in Danish lakes. Aquat Bot. 2000;67:85–107.
Chambers PA. Light and nutrients in the control of aquatic plant community structure II. In situ observations. J Ecol. 1987;75:621–8.
Szmeja J. Effect of disturbances and interspecific competition in isoetid populations. Aquat Bot. 1994;48:225–38.
Szmeja J, Bazydło E. The effect of water conditions on the phenology and age structure of Luronium natans (L.) Raf. population. Acta Societatis Botanicorum Poloniae. 2005;74:253–262.
Lockton AJ. Species account: Luronium natans. Botanical Society of the British Isles, www.bsbi.org.uk. Accessed Nov 2022.
Szmeja J, Banaś K, Bociąg K. Ecological conditions and tolerance limits of isoetids along the southern Baltic coast. Ekologia Polska. 1997;45:343–59.
Willby NJ, Eaton J, Clarke S. Monitoring the Floating Water-plantain. Conserving Natura 2000 Rivers Monitoring Series No. 11, Peterborough: English Nature. 2003.
Chmara R, Banaś K, Szmeja J. Changes in the structural and functional diversity of macrophyte communities along an acidity gradient in softwater lakes. Flora. 2015;216:57–64.
Eaton AD, Clesceri LS, Fanson M, Rice EW, Greenberg AE. Standard methods for the examination of water and wastewater Tom 21. Washington: American Public Health Association, American Water Works Association, Water Environment Federation; 2005.
Ter Braak CJF, Smilauer P. Canoco reference manual and user’s guide: software for ordination (version 5.10). Ithaca, NY, USA: Microcomputer Power. 2018.
Acknowledgements
Research was done with permissions No. RDOŚGd-WZG.6400.115.2018.AO.3 and RDOŚ-GdWZG.6400.115.2018.APO.1.
Funding
Field studies was supported by the National Fund for Environmental Protection and Water Management [Grant No. WFOŚ/D/210/118/2018 and WFOŚ/D/805/5556/2022].
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception: BK, RR, SJ; design of the work: BK, RR; field work: BK, RR, CR; data analysis: BK, RR, CR; interpretation of data: BK, RR; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Water conditions in the Luronium natans community with species with a frequency > 5%. Explanations: 1- I. lacustris, 2- L. dortmanna, 3- S. denticulatum, 4- M. alterniflorum, 5-J. bulbosus, 6- E. canadensis, 7- W. exannulata, 8-L. uniflora. 9- E. palustris. Figure S2. Oxygenation and water temperature, PAR light intensity and water transparency in the Luronium community with species with a frequency > 5%. Explanations see Fig. S1. Figure S3. Environmental conditions in the sediment in Luronium community with species with a frequency > 5%. Explanations see Fig. S1. Figure S4. Differences in the depth of occurrence, PAR intensity, water oxygenation and temperature between habitats of L. natans (Ln) and other isoetids (Il - I. lacustris, Ld - L. dortmanna and Lu - L. uniflora). Statistically significant differences were determined with p<0.001 - ***, p<0.01- **, p<0.05 - *. Figure S5. Difference in pH, redox potential, calcium concentration and water conductivity between habitats of L. natans (Ln) and other isoetids (Il - I. lacustris, Ld - L. dortmanna and Lu - L. uniflora). Statistically significant differences were determined with p<0.001 - ***, p<0.01- **, p<0.05 - *. Figure S6. Differences in water transparency and color, nitrogen and phosphorus concentration between habitats of L. natans (Ln) and other isoetids (Il - I. lacustris, Ld - L. dortmanna and Lu - L. uniflora). Statistically significant differences were determined with p<0.001- ***, p<0.01 - **, p<0.05 - *. Figure S7. Differences in pH, redox potential, calcium concentration and sediment conductivity between habitats of L. natans (Ln) and other isoetids (Il - I. lacustris, Ld - L. dortmanna and Lu - L. uniflora). Statistically significant differences were determined with p<0.001 - ***, p<0.01- **, p<0.05 - *. Figure S8. Differences in nitrogen and phosphorus concentration, organic matter and sediment hydration between habitats of L. natans (Ln) and other isoetids (Il -I. lacustris, Ld - L. dortmanna and Lu - L. uniflora). Statistically significant differences were determined with p<0.001 - ***, p<0.01- **, p<0.05 - *.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Banaś, K., Ronowski, R., Chmara, R. et al. Community structure, environmental conditions and anthropogenic pressure on the habitat of the European endemic aquatic plant Luronium natans (L.) Raf.. BMC Plant Biol 23, 596 (2023). https://doi.org/10.1186/s12870-023-04518-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04518-y