Abstract
Background
Lentil is an essential cool-season food legume that offers several benefits in human nutrition and cropping systems. Drought stress is the major environmental constraint affecting lentil plants’ growth and productivity by altering various morphological, physiological, and biochemical traits. Our previous research provided physiological and biochemical evidence showing the role of silicon (Si) in alleviating drought stress in lentil plants, while the molecular mechanisms are still unidentified. Understanding the molecular mechanisms of Si-mediated drought stress tolerance can provide fundamental information to enhance our knowledge of essential gene functions and pathways modulated by Si during drought stress in plants. Thus, the present study compared the transcriptomic characteristics of two lentil genotypes (drought tolerant-ILL6002; drought sensitive-ILL7537) under drought stress and investigated the gene expression in response to Si supplementation using high-throughput RNA sequencing.
Results
This study identified 7164 and 5576 differentially expressed genes (DEGs) from drought-stressed lentil genotypes (ILL 6002 and ILL 7537, respectively), with Si treatment. RNA sequencing results showed that Si supplementation could alter the expression of genes related to photosynthesis, osmoprotection, antioxidant systems and signal transduction in both genotypes under drought stress. Furthermore, these DEGs from both genotypes were found to be associated with the metabolism of carbohydrates, lipids and proteins. The identified DEGs were also linked to cell wall biosynthesis and vasculature development. Results suggested that Si modulated the dynamics of biosynthesis of alkaloids and flavonoids and their metabolism in drought-stressed lentil genotypes. Drought-recovery-related DEGs identified from both genotypes validated the role of Si as a drought stress alleviator. This study identified different possible defense-related responses mediated by Si in response to drought stress in lentil plants including cellular redox homeostasis by reactive oxygen species (ROS), cell wall reinforcement by the deposition of cellulose, lignin, xyloglucan, chitin and xylan, secondary metabolites production, osmotic adjustment and stomatal closure.
Conclusion
Overall, the results suggested that a coordinated interplay between various metabolic pathways is required for Si to induce drought tolerance. This study identified potential genes and different defence mechanisms involved in Si-induced drought stress tolerance in lentil plants. Si supplementation altered various metabolic functions like photosynthesis, antioxidant defence system, osmotic balance, hormonal biosynthesis, signalling, amino acid biosynthesis and metabolism of carbohydrates and lipids under drought stress. These novel findings validated the role of Si in drought stress mitigation and have also provided an opportunity to enhance our understanding at the genomic level of Si’s role in alleviating drought stress in plants.
Similar content being viewed by others
Background
Lentil is an important cool season food legume crop and is sensitive to drought stress, especially during anthesis with devastating effects on production and yield [1,2,3,4]. Responses of lentil towards drought stress have been studied, based on various agronomical, morphological, physiological, biochemical, and molecular approaches [2, 5,6,7,8,9,10,11]. However, an appropriate and sustainable management strategy to maintain crop performance under drought is still lacking. Therefore, identifying a suitable stress alleviator to promote sustainable drought management is essential for lentil adaptation in adverse environments.
Silicon enhances plant growth, yield and tolerance to various environmental stresses and thus gained attention as an essential element in agriculture [12,13,14]. Silicon alleviates drought stress in both monocot and dicot plants by modulating physiological and biochemical mechanisms [12, 15,16,17,18,19], but the underlying molecular mechanisms of its effect remain poorly understood. In plants, Si modulates complex metabolic activities including, photosynthesis, osmotic adjustments, antioxidant metabolism, phytohormonal interactions and metabolism related to protein, carbohydrates, lipids, and secondary metabolites under abiotic stress environments [20,21,22,23,24]. The ameliorative effects of Si on abiotic stresses have been investigated at the molecular levels in plants [25,26,27,28,29,30,31,32]. Silicon nanoparticles (SiNp) modulated the expression of salt stress genes in salinity-stressed tomato seedlings by enhancing the expression of abscisic acid responsive element-binding protein (AREB), abscisic acid and environmental stress-inducible protein (TAS14), 9-cis-epoxycarotenoid dioxygenase (NCED30 and cysteine-rich receptor-like protein kinase genes (CRK1) and decreasing the expression of respiratory burst oxidase (RBOH1), cytosolic ascorbate peroxidase (APX2), mitogen-activated protein kinase (MAPK2), ethylene response factor (ERF5) dwarf and delayed flowering genes (DDF2) [33]. These up- or downregulated genes are involved in regulating the responses of abscisic acid (ABA), and the activation of the antioxidant defense system in plants under stress. Silicon-mediated regulation of the expression of genes Csa3G199590 (transcription factor MYB44-like), Csa6G091830 (AP2 domain transcription factor RAP2), Csa1G033310 (Chlorophyll a-b binding protein P4, chloroplastic-like), Csa6G104650 (auxin-induced protein 5NG4-like gene) and Csa2G000790 (oxidative stress) have contributed to enhanced salt tolerance in cucumber plants. These genes regulate photosynthesis, oxidative stress and auxin signaling pathway in cucumber plants under stress [34]. Silicon-mediated alleviation of Cd toxicity is reported in rice and wheat plants with alterations in the expression patterns of Cd transporter genes (OsNramp5, OsHMA2, TaNramp5, TaTM20 and TaHMA3) responsible for Cd uptake and translocation [35,36,37]. However, from a molecular perspective, limited studies have been published to explore the mechanisms of Si-mediated drought stress tolerance in plants. In rice, Si application mitigated drought stress through enhanced expression of the transcription factors, dehydration-responsive element-binding protein DREB2A, and NAC5 [no apical meristem (NAM), Arabidopsis thaliana activating factor (ATAF), and cup-shaped cotyledon (CUC)], which control various defence pathways involved in drought stress tolerance [38]. Silicon application increased the relative expression levels of genes encoding antioxidant enzymes in the ascorbate glutathione cycle (TaSOD, TaCAT, TaAPX, TaGR, TaDHAR, TaMDHAR, and TaGS) and restored the gene expressions of TaPAL, TaCHS, TaF3H, TaDFR, and TaANS, which encode enzymes involved in the flavonoid biosynthesis pathway, in drought-stressed wheat plants [39]. Most recently, Boora et al. [40] reported that the application of biosynthesised SiNPs could mitigate drought stress in wheat plants through the upregulation of stress-related genes such as DREB2 (dehydration response), TaMYB33 (osmotic equilibrium recovery and ROS detoxification), WRKY 19 (hormone signaling, secondary metabolite biosynthesis), and SnRK (ROS scavenging and ABA-dependent signal transduction).
Silicon supplementation is also known to modulate the signalling mechanisms of one or more phytohormones such as abscisic acid (ABA), gibberellic acid (GA), jasmonic acid (JA), brassinosteroid (BR), ethylene (ET), salicylic acid (SA), cytokinin (CK) and auxin (AUX) by overexpressing the genes that control their production under abiotic stress in plants [41,42,43,44,45]. Silicon significantly boosted the expression of AUX biosynthesis genes, OsYUCCA1 and OsTAA1 in rice plants under arsenate stress [46], and regulated the expression of genes in CK signalling pathway (Csa4G647490, Csa1G589070, Csa7G392940 and Csa3G150100) in salt-stressed cucumber plants [34] and downregulated the expression levels of ABA biosynthetic genes (SlNCEDI) and SA signalling genes (SlR1b1, SlPR-P2, SlICS, and SlPAL) in thermotolerant tomato plants [47]. However, studies detailing the molecular mechanisms underpinning Si’s function in controlling hormonal signalling are limited, especially under drought stress [48,49,50,51].
Silicon protects photosynthetic machinery and enhances chlorophyll fluorescence/gas exchange parameters such as maximum photochemical efficiency of PSII (Fv/Fm), basal quantum yield (Fv/Fo), photochemical quenching (qP), non-photochemical quenching (NPQ), actual photochemical efficiency of PSII (ΦPII), net photosynthesis (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (Tr) and photosynthetic enzymes such as RuBP carboxylase and PEP carboxylase [18, 52,53,54,55]. Silicon upregulated genes encoding PS I and PSII core proteins (PsbH, PsbB, PsbP, PsbQ, PsbW, Psb28 and PsbD) in heat-stressed wheat plants and drought-stressed tomato plants and maintained photosynthetic electron transport rate (ETR) and photochemical efficiency [56, 57].
Most of the studies mentioned above are conducted in high Si accumulator plants and to the best of our knowledge, no studies are published on Si-mediated drought stress tolerance responses, at the molecular level based on high throughput RNA seq analysis, in a legume plant (a low Si accumulator). Even though the physiological and biochemical mechanisms of Si-mediated drought stress tolerance in lentil plants have been studied recently [7, 8, 19, 54] concerning the molecular response still needs to be investigated. Thus, this study comprehensively explored Si-mediated stress tolerance responses in drought-tolerant and sensitive lentil genotypes using high-throughput RNA-seq analysis and provided novel insight into gene regulation of various pathways involved in Si-mediated drought stress tolerance.
Results
Total RNA integrity and cDNA library preparation
All the RNA samples passed the quality check for library construction and sequencing, with RNA integrity number (RIN) values ranging between 8.3 and 9.2 (Table 1). The concentration of RNA samples (ng/μL) ranged from 273–896 and A260/A280 ratio was greater than 2.1 for all the samples (Fig. 1; Table 1).
Mapping and identification of differentially expressed genes
RNA samples from the leaves of the two lentil genotypes, grown under different treatments, were used for sequencing by the Illumina Hiseq Analyzer to reveal the role of Si in various molecular regulatory mechanisms to alleviate the adverse effect of drought stress in selected lentil genotypes. Approximately 22 million reads were generated from each sample with an average data yield of 4.52 Gbp (Table 2). Nearly 85% of the pseudo-aligned reads were mapped to the lentil draft reference genome (Table 2).
A core set of DEGs from the two lentil genotypes, under various treatments, were examined and analysed to identify the key genes involved in Si-induced drought stress tolerance. The distribution of samples (after normalization and the clustering of three replicates from each sampling group) are shown in the normalization plot and multidimensional scaling (MDS) plot (Fig. 2a and b). The biological replicates clustered together for each treatment, suggesting high reliability and accuracy of the RNA sequencing data.
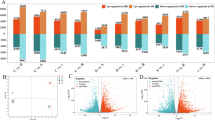
High-throughput RNA-sequencing analysis was performed in the following five combinations from the two genotypes: (i) control vs. drought (C vs. D), (ii) control vs. Si alone (C vs. Si), (iii) drought vs. drought stress supplemented with Si (D vs. DSi) (iv) Si alone vs. drought stress supplemented with Si (Si vs. DSi), and (v) control vs. drought stress supplemented with Si (C vs. DSi). The specific and common DEGs were identified for all the possible combinations, from drought tolerant and sensitive genotypes, as shown in the Venn diagram (Fig. 3a and b). Among the DEGs, 14 unique genes were identified as common to all the comparisons and all the possible combinations for the drought-tolerant genotype (ILL 6002), whereas 169 DEGs were found from the drought-sensitive (ILL 7537) genotype.
Venn diagram showing genes differently expressed for each comparison and all the possible combinations for (a) the drought tolerant genotype (ILL 6002) and (b) the drought sensitive genotype (ILL 7537). Abbreviations used include (i) control vs. drought (C vs. D), (ii) control vs. silicon alone (C vs. Si), (iii) drought vs drought stress supplemented with Si (D vs. DSi) (iv) silicon alone vs. drought stress supplemented with Si (Si vs. DSi), (v) control vs. drought stress supplemented with Si (C vs. DSi)
When the two genotypes were compared for different treatments such as (i) G1 drought vs. G2 drought, (ii) G1 control vs. G2 control (iii) G1DSi vs. G2 DSi and (iv) G1 Si alone vs. G2 Si alone, a total of 1223 genes were differentially expressed (Fig. 4a). Interestingly, when two genotypes were compared for treatments involving Si (G1 Si alone vs. G2 Si alone and G1DSi vs. G2 DSi, 4884 genes were expressed differently (Fig. 4b).
a Venn diagram showing DEGs across four comparisons of various treatments in ILL 6002 (G1) and ILL 7537 (G2) under drought stress supplemented with Si and Si alone treatments. Abbreviations used include (i) G1D vs. G2D (G1 drought vs. G2 Drought), (ii) G1C vs. G2C (G1 control vs. G2 control) (iii) G1DSi vs. G2 DSi (G1 drought silicon vs. G2 drought stress supplemented with Si) (iv) G1Si vs. G2Si (G1 silicon alone vs. G2 silicon alone). b shows co-expressed and specifically expressed genes in two genotypes. Arrows point to the DEGs exclusively found in each comparison
During drought stress, the drought-sensitive genotype, ILL 7537, showed a slightly higher number of DEGs (6683) than the drought-tolerant genotype, ILL 6002 (6246), when compared with their respective controls (Table 3). Interestingly, different types of genes were expressed in response to drought stress by the two genotypes and more importantly, the drought-sensitive one showed higher DEGs (4781) in the C vs. Si treatments, compared with the 127 DEGs in the drought-tolerant genotype, ILL 6002. However, a total of 7164 genes were differentially expressed in the tolerant genotype, which comprised 3695 upregulated and 3469 downregulated genes, compared with a total of 5576 DEGs in the sensitive genotypes, including 3077 upregulated and 2499 downregulated genes, under D vs. DSi. Overall, Si supplementation under drought stress expressed the specific sets of genes in both the sensitive and tolerant genotypes (Table 3). The volcano plot showed the overall distribution of data points. The significance of the measured differences in expression levels and the selection of upregulated and downregulated DEGs from the two genotypes, under drought stress and Si vs. drought, and control vs. Si alone treatments, is illustrated in Fig. 5a—d. The most exciting finding from this comparison was the higher number of DEGs being detected from ILL 7537 (drought-sensitive) under control vs. Si alone treatments, which suggests that Si interacted with multiple and specific defence pathways/mechanisms in the drought-sensitive genotype to mitigate drought stress. Furthermore, these results indicate that Si supplementation can enhance a drought-sensitive plant’s ability to perform better, even under non-stress conditions.
a-d The volcano plot of DEGs for drought stress and Si Vs drought treatments of and for control vs. Silicon alone treatments of (c) ILL 6002 and (d) ILL 7537. The abscissa indicates the level of expression as log2 of the folds change (log 2F) and the ordinates are -log10 (p-value). Each symbol represents a gene. The red circle represents the down regulated DEGs, blue squares represent the up regulated DEGs, and green triangle represent non-DEGs. DEG- differentially expressed gene
The current study identified the candidate genes behind Si-mediated drought stress tolerance from both the genotypes under drought vs. drought stress with Si treatments, ascertaining the significance (p ≤ 05) of the DEGs related to drought tolerance mechanisms. The heatmap shows these selected DEGs' expression patterns (Figs. 6 and 7). Interestingly, the major group of genes among these highly expressed DEGs were related to photosynthetic processes, osmoprotective function, antioxidant metabolisms, hormonal regulation and signalling, cell wall and vasculature biogenesis, carbohydrate and lipid metabolism, protein and amino acid metabolism, secondary metabolite production, flowering, drought recovery and water homeostasis.
Heatmap representing the expression of differentially expressed candidate genes in response to Si treatment during drought stress in ILL 6002 in DSi vs. D treatment (p ≤ 0.05). The colour key represents the normalised log transformed counts. Red indicates low expression, black indicates intermediate expression and green indicates high expression. Each column represents an experimental condition, and each row represents a gene (p ≤ 0.05, ≥ 1.5—folds change). The significantly (P ≤ 0.05) enriched biological process GO terms are shown on the right side of each cluster. Abbreviations used in this Figure are DSi—Drought stress supplemented with Si and D—Drought stress
Heatmap representing the expression of differentially expressed candidate genes in response to Si application during drought stress in ILL 7537 in DSi vs. D treatment (p ≤ 0.05). The colour key represents the normalised log transformed counts. Red indicates low expression, black indicates intermediate expression and green indicates high expression. Each column represents an experimental condition, and each row represents a gene (p ≤ 0.05, ≥ 1.5-folds change). The significantly (P ≤ 0.05) enriched biological process GO terms are shown on the right side of each cluster. Abbreviations used in this Figure are DSi—Drought stress supplemented with Si and D—Drought stress
Photosynthetic process, osmoprotective function and antioxidant metabolism related genes expression in response to Si-mediated drought stress tolerance
The DEGs related to photosynthetic processes, such as chlorophyll biosynthesis, qP, relocation of chloroplast, guard cell synthesis, carbon fixation, and plastoquinone biosynthesis, were upregulated. In contrast, the genes controlling movements and openings of stomata were downregulated with Si supplementation under drought stress in both genotypes (Table 4). Upregulated DEGs related to chlorophyll biosynthetic processes were found in both genotypes. DEGs responsible for osmotic stress response were upregulated with the downregulation of proline biosynthesis and metabolism genes in both genotypes under drought stress with Si supplementation. More DEGs were positively regulated in response to oxidative stress and antioxidant metabolism in drought-sensitive genotype, ILL 7537, compared to the tolerant one (ILL 6002). Moreover, genes related to defence responses and detoxification of cellular oxidants were upregulated and differentially expressed in the drought-sensitive genotype with Si supplementation during drought stress, compared with drought stress without Si supplementation.
Role of important phytohormones in S-induced drought tolerance in lentil
In the present study, different expression levels of genes related to hormone and hormone signalling pathways were observed in both tolerant and sensitive genotypes (Table 5). Upregulated DEGs were identified for AUX, karrikin (KAR) and polyamines (PA), while downregulated DEGs were found for ET and JA metabolism in both the genotypes, in response to Si supplementation under drought stress. Furthermore, the drought-sensitive genotype showed upregulated DEGs for BR and downregulated DEGs for CK which were not identified from the drought-tolerant genotypes. Upregulated DEGs for negative regulation of the GA-mediated signalling pathway were identified in the drought-tolerant genotype. Interestingly, while the drought-tolerant genotype showed the downregulation of the expression of genes involved in SA biosynthesis, drought sensitive-genotype showed the reverse. Additionally, the gene related to AUX biosynthetic processes was found to be common for both genotypes.
Differentially regulated genes involved in cell wall development, synthesis of cell wall materials and vasculature biogenesis
In this study, Si supplementation of drought stressed lentil genotypes resulted in the upregulation of DEGs related to primary and secondary cell wall biogenesis and organization (Table 6). Furthermore, Si upregulated the genes responsible for synthesising and metabolising cell wall material such as cellulose, lignin, xyloglucan, chitin and xylan. Genes regulating phenylpropanoid pathway, a source of lignin formation in plant cells and lignin metabolic processes, were also upregulated, suggesting Si’s role in physical defence mechanisms in lentils under drought stress. Upregulated DEGs were identified for the histogenesis, development and patterning of the vascular tissues (xylem and phloem) and phloem transport in both genotypes, in response to Si under drought stress.
Differentially regulated genes encoding carbohydrate and lipid metabolism
The current study showed an upregulation in carbohydrate metabolism with higher upregulated DEGs in both genotypes under drought stress in response to Si (Table 7). Among the upregulated DEGs, the most important ones were related to the metabolism of starch and trehalose (synthesized especially during freezing and drought stress in plants) [58]. Additionally, downregulated DEGs were found for sucrose metabolism. Downregulated DEGs involved in the metabolism of phospholipids, galactolipids, fatty acids and fatty acid beta-oxidation were identified in both the genotypes under drought stress in response to Si. In addition, downregulated DEGs were also identified for the activity of major enzymes involved in lipid metabolism, such as omega-3 fatty acid desaturase, acyl-CoA dehydrogenase and acetyl-CoA C-acyltransferase, which further supports the downregulation of lipid metabolism.
Differentially expressed genes involved in protein and amino acid metabolism
Protein and amino acid metabolism-related DEGs were differentially expressed in the studied genotypes under Si-mediated drought stress tolerance, with more upregulated DEGs for protein phosphorylation, protein kinase activity and positive regulation of amino acids (Table 8). Among DEGs for amino acids, DEGs related to phenylalanine and tryptophan were upregulated and those for leucine were downregulated, in both genotypes. Intriguingly, DEGs involved in arginine, asparagine, glutamate and threonine were downregulated in the drought-sensitive genotype under drought stress.
Differentially regulated genes involved in the production of secondary metabolites in drought sensitive genotype (ILL 7537)
It was somewhat surprising that no DEGs related to the production of secondary metabolites were identified in the drought-tolerant genotype (ILL 6002), in response to Si under drought stress (Table 9). However, upregulated DEGs were found for the biosynthesis and metabolising secondary metabolites, such as alkaloids and flavonoids, in the drought-sensitive genotype (ILL7537). Upregulated DEGs were also identified for isopentenyl diphosphate, the precursor of isoprenoid, involved in the biosynthesis of terpenes and terpenoids. Silicon triggers vital secondary metabolite biosynthetic pathways in plants, especially under stress conditions [59]. The results suggest that Si may also act as a regulatory molecule under drought stress to protect the plant by effectively synthesizing secondary metabolites in drought-sensitive genotypes. It also indicates the existence of genetic differences among different plant genotypes and species for the regulatory mechanism related to secondary metabolite synthesis and their role in stress tolerance.
Gene expression related to drought recovery and defence response
The most important finding from this study, which confirmed the role of Si as a ‘drought stress alleviator’ in lentils, was the upregulation of genes related to drought tolerance recovery in both the genotypes under Si-mediated drought tolerance (Table 10). The relatively low expression of these DEGs in the control and the drought stress treatments (Table 10) and their high expression in drought stress in response to Si strongly confirm the positive role of Si in drought stress tolerance of lentil genotypes. Additional upregulated genes related to water homeostasis and defence responses, found in drought-sensitive genotype, further underlines the essentiality and potential of Si supplementation to drought-sensitive genotypes under drought stress.
Gene ontology (GO) annotation of differentially expressed genes
The functional annotation and gene ontology enrichment (GO) analysis was performed to categorize the top 100 upregulated and downregulated DEGs from both the genotypes during drought stress and non-stress conditions with Si supplementation. Gene ontology terms were classified into three principal categories: biological processes, cellular components, and molecular functions. GO enrichment analysis of both upregulated and downregulated DEGs in tissue samples led to recognising many GO terms in both tolerant and sensitive genotypes (Fig. 8). In the biological process category, the most represented and enriched categories were ‘signalling’, ‘regulation of RNA synthetic process’ and ‘cell communication’ in the drought-tolerant genotype, along with ‘protein phosphorylation’, ‘cell wall biogenesis/organization’, and ‘regulation of transcription’ (Fig. 9). However, genes associated with ‘defence response’, ‘signal transduction’, ‘secondary metabolite production’ and ‘oxidation–reduction process’ were the most enriched categories in the drought-sensitive genotype. For cellular components, the genes associated with 'intracellular', ‘plasma membrane', ‘intrinsic component of membrane’ and 'intracellular membrane-bounded organelle' were the most enriched categories in both genotypes. Furthermore, many other GO terms were also significantly enriched in the cellular component category (Fig. 10). Under the broad ‘molecular functions’ category, DEGs were significantly annotated for ‘DNA binding transcription activity’ and ‘protein kinase/kinase activity’, followed by ‘sequence-specific DNA binding’ and ‘carbohydrate binding’ in both genotypes (Fig. 10).
Discussion
Response of photosynthetic process, osmoprotective function and antioxidant metabolism related genes
As photosynthesis involves various organelles (stomata, chloroplast, photosynthetic pigments), systems and pathways (photosystems, the electron transport system, and CO2 reduction), any damage, at any level, caused by drought stress may reduce the overall photosynthetic efficiency of plants. The current study showed that Si increases photosynthetic efficiency under drought stress in lentil plants. The increase is attributed to the upregulation of genes related to stomatal and chloroplast movements, chlorophyll biosynthesis, photochemical quenching and gas exchange parameters (Table 4). These findings also validated our controlled and field-based research on Si-mediated drought stress tolerance in lentil plants, enhancing photosynthetic efficiency [54]. Our studies corroborate the findings of various researchers regarding Si’s positive role in mitigating drought stress by improving chlorophyll content and water use efficiency, while also protecting photosynthetic machinery from ROS. Additionally, it interacts with other physiological processes such as the absorption of macro and micronutrients and phytohormones, which also influence photosynthetic activity [55, 60,61,62,63,64,65,66]. Silicon demonstrated the capability to maintain irregular swelling and disintegrated thylakoid and chloroplast membranes [25, 52], altered stomatal aperture that influences the water uptake and water use efficiency [64, 67] and regulated the expression of photosynthetic genes (PsbY, PsaH, PetC, PetH, Os09g26810, PetF, PsbP, PsbQ, PsbW and Psb28) in plants during stress conditions, thus contributing to the efficient photosynthetic process. The present study’s findings reaffirm the role of Si in enhancing photosynthetic efficiency, as reported in numerous previous studies mentioned here.
In plants, ROS such as superoxide anion (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2) and singlet oxygen (1O2) play a vital role in the activation of stress-response networks, especially during stress conditions [68,69,70,71]. In this study, Si supplementation downregulated DEGs related to ROS (H2O2 and O2.−), revealing the crucial role of Si in ROS detoxification under stress (Table 4). Interestingly, Si application downregulated genes related to proline biosynthesis and metabolism in this study. In higher plants, proline is synthesized from glutamate mainly by the action of two enzymes: Δ1-pyrroline-5-carboxylic acid synthesis (P5CS) and pyrroline-5-carboxylic acid reductase (P5CR). Silicon-mediated downregulation of genes involved in proline synthesis and metabolism could be attributed to the accumulated proline-mediated feedback inhibition of P5CS, a rate-limiting enzyme for proline synthesis, consequently leading to downregulation of P5CR [72,73,74,75,76]. In the current study, the concentration of proline within the cells may have exceeded a certain threshold due to drought stress. With Si supplementation, the previously accumulated proline might have become bound to the active site of P5CS, consequently inhibiting its activity, leading to proline-mediated feedback inhibition in the cells. This result supports prior reports on Si-induced decrease in proline accumulation as a sign of plant stress injury alleviation [77, 78]. As the proline biosynthesis pathway is a conserved pathway, further studies could help identify the signal transduction pathways associated with proline biosynthesis, metabolism and the coordination of gene expressions, and other transcription factors under drought stress in response to Si supplementation.
Furthermore, upregulated genes related to the ‘glutathione metabolic process’ were identified in drought-stressed lentil plants in response to Si supplementation (Table 5). Glutathione is a non-enzymatic antioxidant playing a pivotal role in the ROS-scavenging strategies in plants [79]. The observed higher expression of genes related to photosynthetic process, osmoprotective mechanisms and antioxidant systems appears to be a common response among the two genotypes expressing Si-induced drought tolerance. The present findings strongly align with previous results of drought-stressed lentils, where Si application mitigated drought stress by regulating the accumulation of the metabolites mentioned above [7, 19].
Phytohormones and Si-induced drought tolerance in lentil
Phytohormones (AUX, PA, ET, JA, ABA, GA, BR, and CK) are the key regulators in sensing and signalling numerous environmental stresses [80,81,82,83]. They induce drought stress tolerance in plants via synergistic and antagonistic interactions [84, 85]. Analysis of DEGs related to phytohormones, in the drought-stressed lentil genotypes in responses to Si, revealed that AUX, Karrikin (KAR), PA, ET and JA were the most important hormones involved in Si-mediated drought tolerance responses (Table 5). Silicon might have triggered the upregulation of DEGs related to AUX metabolism to develop a prolific root system in the plants, which is vital for drought tolerance. Previous reports also suggested that Si can accelerate the growth and development of the root system of drought-stressed plants [86]. Helaly’s [87] showed that Si supplementation increased AUX concentration in mango plants under drought stress. Silicon also upregulated the expression of an AUX-induced protein 5NG4-like gene (Csa6G104650), involved in the AUX signalling pathway, in salinity-stressed cucumber plants.
One novel finding of this study is the discovery of upregulated DEGs related to KAR response, under Si-mediated drought tolerance, in both genotypes. This is the first report of Si interaction with KARs under drought stress in plants. Karrikins are a class of butenolide compounds found in smoke promoting seed germination, seedling establishment and ecological diversity of plants [88, 89]. Karrikins have significant roles in mediating abiotic stress tolerance in plants due to their structural similarity with strigolactones [90, 91]. KAR enhanced drought tolerance in creeping bentgrass (Agrostis stolonifera) in association with antioxidative protection and regulation of stress-responsive gene expression [92]. Ma et al. [39] reported the upregulated expression of a secretory protein (33 kDa) related to KAR in rice plants under long-term Si-mediated Cd stress tolerance. A possible explanation for the observed upregulation of KAR in this study may be based on the previous experiments, where Si improved seed germination under drought stress in lentil [19]. It can be assumed that under drought stress, Si might have triggered the production of KARs, which interacted with other phytohormones and antioxidant compounds to induce a tolerance response in lentil plants. Li et al. (2017)’s [89] findings support these assumptions of KARs’ interaction with AUX and ABA, leading to the closure of stomata, activation of the antioxidant machinery and maintenance of oxidative homeostasis, in Arabidopsis under drought stress.
Polyamines are effective anti-senescence agents and ROS scavengers in plants [93]. Ethylene is a senescing hormone, whose inhibition can retard leaf senescence in plants [94]. Polyamines and ethylene share a biosynthetic association in terms of competitive demand for a limited pool of the common precursor, S-adenosyl methioninamine (SAM) [95]. Even though these two hormones share a common precursor, they act in antagonistic ways to senescence. The balance between these two opposite functions is crucial for one or the other adaptive strategy in plants and triggers their tolerance to various environmental stresses. Polyamines induce drought stress tolerance in plants by regulating genes encoding ABA biosynthesis enzymes [96].
The current study’s findings further confirm this balance and association between PA and ET through differentially regulated genes related to their biosynthesis in lentil genotypes under Si-mediated drought stress tolerance. These findings demonstrated that under drought stress Si upregulated genes associated with PA biosynthesis, which contributed to the efficient scavenging of ROS. Furthermore, under Si supplementation, downregulation of genes involved in ET production might have contributed to delayed senescence, with the help of upregulated genes for chlorophyll biosynthesis. This study strongly supports Biju et al.’s [19, 54] findings that Si supplementation effectively mitigated drought stress in lentil plants by regulating ROS and maintaining chlorophyll pigments under drought stress. Silicon application upregulated the expression of crucial ET biosynthesis genes (1-aminocyclopropane-1-carboxylic acid oxidase and 1-aminocyclopropane-1-carboxylic acid synthase) and reduced the expression of PA biosynthetic genes (Spermidine Synthase 1, Spermine Synthase, and Polyamine Oxidase 1), which resulted in an enhanced stress tolerance in tobacco plants [21, 97] However, this contradicts Manivannan and Ahn’s [27] and Yin et al. [26] reports on Sorghum plants where Si-mediated alleviation of salt stress was correlated with a decrease in ET biosynthesis. The interactions between ET and PAs imply that Si can influence ET production by regulating PA synthesis, thus maintaining the balance of PAs and ET biosynthesis.
Jasmonic acid is a positive regulator of drought stress in plants [98]. Allene oxide synthase (AOS) and allene oxide cyclase (AOC) are enzymes in the octadecanoid pathway that lead jasmonic acid and biosynthesis.The current study identified downregulated genes related to the activities of AOS and AOC and metabolism of JA-mediated signalling pathways. These findings demonstrate that Si might have triggered the crosstalk interactions of JA with other phytohormones leading to increased drought stress tolerance. The results support Hamayun et al.’s [99] findings in drought-stressed soybean and Kim et al.’s [49] in rice under heavy metal stress, where JA synthesis was negatively affected in response to Si treatment. Furthermore, Si-induced negative regulation of JA can also be explained partially through Dhakarey et al.’s [100] studies, which suggested that JA might be a negative regulator of drought stress tolerance in rice.
Abscisic acid is a plant stress hormone crucial for plant growth and development. ABA significantly integrates various stress signalling pathways and controls downstream stress responses [101]. This study identified upregulated DEGs for the biosynthesis of ABA and the activity of abscisic acid 8'-hydroxylase, an enzyme responsible for the first step in the oxidative degradation of ABA [102]. This demonstrates the role of Si in maintaining ABA homeostasis in lentils under drought stress. Likewise, downregulated DEGs were found for the negative regulation of the ABA-activated signalling pathway, indicating the regulatory role of Si in modulating ABA responses under drought stress. These results support previous experimental results where Si improved seed germination in lentil under polyethylene glycol (PEG) induced drought stress [19] since the seed germination processes are regulated by ABA and GA homeostasis [103]. Kim et al. [48] also found that Si enhanced the expression of ABA-biosynthetic genes, Zeaxanthin epoxidase (ZEP) and 9-cis-epoxycarotenoid oxygenase 1 and 4 (NCED1, NCED4) in salt-stressed rice, by showing an antagonistic relationship between ABA and Si.
Gibberellins are essential phytohormones known for their role in plant internode elongation [104]. Silicon is also known for altering endogenous GA levels in plants under stress and non-stress conditions [41, 48, 49, 105]. From the current study, it seems possible that Si might have affected lentil shoot growth and shoot proliferation under drought stress by upregulating the expression of genes related to GA synthesis and signalling pathways. These results agree with Hamayun et al.’s [99] and Lee et al.’s [106] findings, where they reported a higher accumulation of GA levels upon Si addition in soybean under drought and salt stress, respectively. Furthermore, upregulation of a protein related to GA (gibberellin 20 oxidase), involved in the gibberellin signalling pathway was found in salt-stressed tomato plants in response to Si supply [107].
Maintaining a suitable concentration SA concentration in plants can alleviate various stresses by regulating different biochemical pathways [108]. Downregulated DEGs were found for SA metabolic processes in the drought-tolerant genotype, while upregulated DEGs related to the SA biosynthetic process were found in the sensitive genotype. Differential regulation of the phenolic phytohormone, SA, in response to Si in both the genotypes might be related to the concentration of SA accumulated in cells and different drought tolerance levels of the genotypes. There are reports on decreased endogenous SA content in soybean plants, grown under drought stress [99] and downregulated DEGs encoding SA-binding proteins under biotic stress in tomato plants [109]. response to Si supplementation. However, no studies have compared SA accumulation and expression levels in drought-tolerant and sensitive plants in response to Si application. Moreover, additional upregulated DEGs for BRs and downregulated DEGs for CK synthesis were found only in the drought-sensitive genotype (ILL 7537), further suggesting a detailed investigation of the role of Si in regulating the phytohormonal interactions in plants under drought stress via comparison in drought-tolerant and sensitive genotypes is required.
Silicon triggers cell wall development, and vasculature biogenesis
Silicon supplementation of drought-stressed lentil genotypes modulated the expression of genes related to cell wall development and vasculature biogenesis (Table 6). This finding provides strong evidence for the possible interaction or the binding of polymerized Si with cell wall components as investigated in previous studies [110,111,112,113]. These findings also support Si-dependent strengthening and reinforcement of the cell wall, as a protective adaptation strategy in plants, especially in the dicots, which are low Si accumulators compared with the monocots. Furthermore, these results strongly re-establish the role of Si in improving the mechanical properties and regeneration of cell walls in plants [113,114,115]. Lignin is the most abundant structural polymer found in the plant cell walls, after cellulose. The presence of Si is reported in plant epidermal cell walls associated with lignin-carbohydrate complexes [110]. These results are consistent with Si-induced enhancement of lignin deposits in roots of salinity-stressed rice plants and the formation of silica bodies in tall fescue (Festuca arundinacea) and bentgrass (Agrostis stolonifera) plants [116, 117]. Silicon also improved the lodging resistance of rape (Brassica napus) stems by improving lignin accumulation and the mechanical tissue structure [28]. Furthermore, Si is also known to enhance lignin accumulation in plants under biotic stresses [118, 119].
Vascular tissues (xylem and phloem) provide mechanical strength and facilitate the transport of water, nutrients, hormones and other signalling molecules throughout the plant. Even though the formation of the vascular system is a well-organized developmental process in plants, it can also be flexible in response to environmental changes [120]. Plant hormones, peptide signalling, and transcriptional regulators are known to regulate the development and patterning of the xylem and phloem in plants [119]. Based on the current findings, it can be inferred that Si plays a crucial role in maintaining the structural components and regulating the source to sink transport in lentil via maintaining phytohormonal homeostasis under drought stress. The role of Si in the development and differentiation of vascular tissues certainly needs to be addressed.
Carbohydrate and lipid metabolism in response to Si supplementation
Silicon supplementation significantly regulated the genes related to carbohydrate and lipid metabolism in lentil plants under drought stress (Table 7). These results suggest that added Si might have positively regulated the loading and unloading of sucrose via phloem by maintaining homeostasis between starch and sucrose levels as a protective adaptation strategy under drought stress in plants. These results differ from Yin et al.’s [26] findings in drought-stressed sorghum; however, they are consistent with the findings of Zhu et al. [34], who noticed an increase in starch and a decrease in sucrose contents in cucumber under salinity stress with Si treatment. Silicon-induced upregulation of trehalose metabolism could also be a part of the plant’s adaptive strategy to combat drought stress. These results support the findings of Manivannan et al. [121], who reported Si mitigated salinity stress in capsicum by regulating the expression of proteins and carbohydrate metabolism.
The downregulated DEGs for oxidation of unsaturated fatty acids indicate reduced lipid peroxidation, preventing cell membrane damage under drought stress [122]. Previous results demonstrated that Si mitigated drought stress by reducing lipid peroxidation in lentil plants under similar experimental conditions [7]. Furthermore, Si might have also maintained the optimal membrane fluidity to prevent structural and functional deterioration of cell membrane by downregulating the lipid concentrations to a minimum level under drought stress. Thus, it can be inferred that Si might have some regulatory effects on lipid composition and the degree of fatty acid unsaturation in plants under stress. Several plant studies have also reported Si-induced drought tolerance via reduced lipid peroxidation [16, 61, 77, 78].
Silicon modulated the expression of genes related to the metabolism of amino acids, secondary metabolites and drought recovery
Silicon triggered the expression of genes related to protein phosphorylation and protein kinase activity in lentil plants under drought stress (Table 8). Protein kinases act to phosphorylate and dephosphorylate their targets (proteins/amino acids), thereby maintaining drought-signalling homeostasis in plants [123, 124]. Ubiquitin plays a key role in plant hormone synthesis, hormonal signalling cascades and other defence mechanisms [125]. Downregulation of the ubiquitin protein ligase and ubiquitin protein transferase activity by Si, in drought-stressed lentil plants, might have contributed to the fine adjustment of hormonal signalling pathways and other defence mechanisms at the molecular level. The ability of Si to modulate the expression of proteins involved in the ubiquitin-mediated nucleosome pathway was identified in salt-stressed capsicum [121]. Although this study cannot rule out the differential regulation of protein and amino acid metabolism in response to Si under drought stress for both genotypes, it is suggested that Si might have maintained amino acid homeostasis by regulating the activity of protein kinases to enhance drought stress tolerance. These results corroborate the findings of Pereira et al. [126], where a positive corrripathielation was found between increased amino acid contents and osmotic adjustment in response to Si in drought-stressed capsicum. Silicon triggers vital secondary metabolite biosynthetic pathways in plants, especially under stress conditions [127]. Results from the present study suggest that Si may also act as a regulatory molecule under drought stress to protect the plant by effectively synthesizing secondary metabolites in drought-sensitive genotypes (Table 9). This result supports our previous findings, where Si supplementation increased the accumulation of flavonoids in drought-stressed lentil plants [8]. It also indicates the existence of genetic differences among different plant genotypes and species for the regulatory mechanism related to secondary metabolite synthesis and their role in stress tolerance. Furthermore, the role of Si as a ‘drought stress alleviator’ in lentils is confirmed by the findings of differentially regulated genes related to drought tolerance recovery in both the genotypes under Si-mediated drought tolerance (Table 10). Upregulation of genes involved in water homeostasis validated all other findings in this study.
Differential expression of genes related to biological processes, cellular components and molecular functions as revealed by gene ontology (GO) annotation
The findings shown in Figs. 8, 9 and 10 indicate Si’s crucial role in regulating all the biological processes in lentil genotypes, to alleviate the adverse effects of drought stress. Results of the cellular component category demonstrate that Si might act as a signal molecule in regulating cell metabolism and maintaining the structural integrity of cells and membranes under drought stress, as He et al. [128] (2015) suggested in rice plants. The findings from this study are well supported by previous work done in this area, such as polymerized Si accumulation in the epidermal cell walls of rice [129], and high root endodermal silicification in sorghum [130], under Si-mediated drought stress tolerance. Moreover, many reviews are also available on the protective role of Si on cell walls and membranes in plants under environmental stresses [20, 21]. The molecular category results further confirm the involvement of Si in phosphorylation, active transport of ions and its binding with other cellular molecules to lessen cell or membrane damage during drought stress (Fig. 10). The GO terms related such as ‘chloroplast’, “thylakoid’ and ‘plastid envelop’ demonstrate the active engagement of Si in photosynthetic processes. A recent study in rice also showed that Si improved the photosynthetic performance by maintaining thylakoid membrane protein components such as PSI core binding LHCI (Light harvesting complex I), PSI core, F1-ATPase binding Cytb6/f complex, PSII core, trimeric LHCII and monomeric LHCII, under drought stress [52]. These results corroborate the observations of Kang et al. [131] in the succulent xerophytic plant, Zygophyllum xanthoxylum, where they found this C3 plant accumulated high amounts of Si and utilized it as an osmoregulator to improve photosynthetic activity and antioxidant enzyme activities under drought stress.
Methods
Plant materials and drought stress treatments
Two lentil genotypes, ILL 6002 (drought tolerant) and ILL 7537 (drought sensitive) were selected as experimental materials and the seeds were procured from The Australian Grains Genebank (AGG), Horsham, Victoria. These genotypes were identified as drought tolerant and drought sensitive from a previous drought stress tolerance screening experiment [6]. This experiment was conducted in a growth room (temperature: 23 ± 2 °C; relative humidity: 45–50%; photoperiod: 12 h; light intensity: 300–325 μmol m2 s−1 from metal halide illumination lamps (MH 400 W/640 E40 CLU 1SL/6, Netherlands) of the University of Melbourne, Parkville. Lentil seeds were sown, after surface-sterilisation (30% [v/v] hydrogen peroxide solution), in 950 mL plastic pots filled with 700 g lentil potting mix (70% garden soil and 30% composted pine bark with 1.6 kg dolomite lime per 60 L potting mix, pH 7.00). The source of silica is sodium metasilicate (Na2SiO3) and 2 mM of Na2SiO3 solution (500 ml kg−1 potting mix) was added to the pots before sowing seeds. Silicon is soluble in the soil only at pH < 9 and concentration at or below 2 mM [132,133,134,135]. Our previous experiments also showed that 2 mM Si improved the drought stress tolerance in lentil plants [6,7,8, 19]. Therefore, this experiment was designed with 2 mM of Si solution. The molarity of Na2SiO3 was calculated based on the potting mix volume and solution’s final pH was made to 7.5 using 0.1 N hydrochloric acid. Plants were fertilised with Nitrosol (Amsgrow) during the vegetative stage to maintain normal plant growth. The experiment was carried out as a completely randomized design in three replicates with two lentil genotypes and four treatments. Lentil plants were given severe drought stress (20% field capacity) in this study as our previous research showed that maximum plant damage occurs at severe drought stress (Biju et al. 2018; 2021a). The treatments were as follows: (i) control (C -well watered, 100% FC), (ii) severe drought stress (D-20% FC), (iii) severe drought stress with supplemented Si (DSi), and (iv) Si alone (Si). The control treatments were supplied with sodium sulphate (Na2SO4; 2 mM) to balance the sodium levels in treatments (ii) and (iv) supplied with Si. Drought stress was imposed at the reproductive growth stage (R1 stage-anthesis) and continued for 28 days at respective field capacities. Furthermore, to maintain uniformity of growing conditions and elimination of light and air flow stress biases, pots were reorganised weekly in the growth room. Leaf samples for RNA analysis were harvested at the end of the drought stress treatment period when the plants reached the R3 stage (pod development stage). The growth stages of the plants were assessed using the descriptors for stages of development in lentil plants [136].
RNA isolation, cDNA library preparation and Illumina sequencing
Total RNA from leaf samples of various treatments were extracted using RNeasy plant mini kit along with DNase treatment, according to the manufacturer’s instruction (Qiagen, USA). The quantification of RNA was determined using a NanoDrop ND8000 (Thermo Scientific, USA). The integrity and quality control check for RNA was done on the tape station and the Agilent bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). The samples were normalised to an input RNA weight of 1 µg for processing. The samples underwent high-throughput sequencing on the Illumina HiSeq platform at the Australian Genome Research Facility (Melbourne, Australia). RNA seq libraries were constructed using the illumina TruSeq Stranded mRNA kit, following the manufacturer’s instructions (Illumina, USA). The RNA seq experiment (including library preparation) was completed with three biological replicates (only 2 replicates for S24, which failed the library preparation). Image analysis was performed in real-time by the HiSeq Control Software (HCS) v2.2.68 and real-time analysis (RTA) v1.18.66.3, running on the instrument’s computer. The RTA performs real-time base calling on the HiSeq instrument computer. Then the Illumina bcl2fastq 2.20.0.422 pipeline was used to generate the sequence data.
Mapping of RNA-Seq reads and differential gene expression analysis
Lens culinaris genome v1.2 and annotation v1.2b were used for the analysis [137]. Transcripts were extracted using gffread v0.9.10 (https://github.com/gpertea/gffread). The reads were pseudo aligned and transcript abundance was estimated using Kallisto v0.44.0 [138]. The estimated counts were loaded, and differential gene expression analysis was performed using DEApp (https://gallery.shinyapps.io/DEApp/) [139]. The Raw Count Data file containing summarized count results of all samples in the experiment, and the Meta-data Table file containing summarized experimental design information for each sample were used as input data. Low expression genetic features were removed after alignment if the count per million (CPM) value was ≤ 1 in less than two samples. After filtering out the low expression genomic features, the samples’ normalization and multidimensional scaling (MDS) plots were also obtained to illustrate the samples’ distribution and relationship. DE analysis was performed on the raw count data from all the treatments using edgeR, using cut‐off values of log Folds Change (log FC)—1.5 and a false discovery rate (FDR) adjusted to a P value < 0.05. A dispersion plot, overall DE analysis results, and statistically significant DE results were generated, together with a volcano plot related to the specified parameters and cut-off values. Venn diagrams were created with differentially expressed genes (DEGs) for each comparison and all the possible combinations from the drought-tolerant ILL 6002 and the drought-sensitive ILL 7537, using the website (http://bioinformatics.psb.ugent.be/webtools/Venn/). The Heatmapper program (http://www.heatmapper.ca/) was used to draw the heatmap of the significant DEGs, in response to various treatments. All the raw data is deposited in Github repository https://github.com/SajithaBiju/Data-LentilSilicon.
Functional annotation and Gene Ontology (GO) enrichment
The DEGs were annotated for gene ontology (GO) terms and categorized into Molecular Function (MF), Cellular Component (CC), and Biological Process (BP) categories. GO enrichment was performed using top GO v2.34.0 [140] using the ‘weight’ method to adjust for multiple comparisons. Lens culinaris genes were annotated with GO terms by transferring A. thaliana GO annotation (ATH_GO_GOSLIM.txt; https://www.arabidopsis.org/) to the best L. culinaris match as established by BLASTP v2.6.0 [141] comparison (-max_target_seqs 1 -num_threads 16 -evalue 1e-5 -outfmt '6 qseqid sseqid pident length mismatch gapopen qstart qend sstart send evalue bitscore qcovs qlen'). The GO enrichment (p value ≤ 0.05) was investigated by subjecting all DEGs to the GO database (http://www.geneontology. org/) to further classify genes, or their products, into terms of molecular function, biological process and cellular component which helps understand the genes’ biological functions (https://github.com/SajithaBiju/Data-LentilSilicon).
Conclusion
The current study determined the role of Si in mitigating drought stress in two lentil genotypes employing RNA sequencing to identify biological cellular and molecular pathways in continuation of the physiological and biochemical experiments undertaken in lentils under Si-mediated drought tolerance. The results have provided considerable evidence to demonstrate that lentil adaptation to drought stress is a diverse approach involving the modulation of the expression of several genes that regulate various metabolic functions like photosynthesis, antioxidant defence system, osmotic balance, hormonal regulation and crosstalk, signalling, amino acid biosynthesis, carbohydrate and lipid metabolism and other defence related pathways that assist lentil in drought tolerance recovery (Fig. 11). This study has provided novel insights into plants’ responses to drought and new leads for functional studies of genes involved in Si-induced drought tolerance. Furthermore, the findings confirmed that additional protective strategies are induced in sensitive genotypes compared with the tolerant genotype under Si-mediated drought tolerance, as evidenced by identifying upregulated DEGs related to secondary metabolite synthesis. The differential upregulation noticed in the hormonal cascade prioritises the need for a detailed exploration to unveil the role of Si in maintaining hormonal homeostasis under drought stress. Furthermore, this data suggests that the studies focussing on Si-induced drought tolerance in open field conditions should also focus on the interaction of Si with the synthesis and development of cell walls and vascular tissues. These findings imply that further bioinformatics and metabolomics should be conducted to extricate the complex control networks involved in Si-mediated drought tolerance in plants. With a better understanding of the role and mechanism of action of Si in stress responses, it would be possible to develop new breeding or Si-mediated stress management strategies to enhance plant survival in adverse environmental conditions.
Availability of data and materials
All data generated or analysed during this study are included in this published article and raw data are provided in Github repository https://github.com/SajithaBiju/Data-LentilSilicon.
Abbreviations
- ABA:
-
Abscisic acid
- ATAF:
-
Arbidopsis thaliana Activating factor
- AUX:
-
Auxin
- BR:
-
Brassinosteroid
- Ci:
-
Intercellular CO2 concentration
- CPM:
-
Count per million
- CUC:
-
Cup-shaped cotyledon
- CK:
-
Cytokinin
- DEGs:
-
Differentially expressed genes
- DREB2A :
-
Dehydration responsive element-binding protein
- ET:
-
Ethylene
- ETR:
-
Electron transport rate
- Fv/Fm:
-
Maximum photochemical efficiency of PSII
- GA:
-
Gibberellic acid
- GO:
-
Gene ontology
- gs:
-
Stomatal conductance
- JA:
-
Jasmonic acid
- KAR:
-
Karrikin
- MDS:
-
Multidimensional scaling
- NAM:
-
No apical meristem
- NPQ:
-
Non-photochemical quenching
- PA:
-
Polyamine
- Pn:
-
Net photosynthetic rate
- qP:
-
Photochemical quenching
- RIN:
-
RNA integrity number
- RNA Seq:
-
RNA Sequencing analysis
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- Si:
-
Silicon
- TF:
-
Transcription factors
- Tr:
-
Transpiration rate
References
Kumar J, Basu PS, Srivastava E, Chaturvedi SK, Nadarajan N, Kumar S. Phenotyping of traits imparting drought tolerance in lentil. Crop Pasture Sci. 2012. https://doi.org/10.1071/CP12168.
Mishra BK, Srivastava JP, Lal JP. Drought resistance in lentil (Lens culinaris Medik.) in relation to morphological, physiological parameters and phenological developments. Int. J. Curr. Microbiol. Appl. Sci. 2018. https://doi.org/10.20546/ijcmas.2018.701.277.
Sehgal A, Sita K, Bhandari K, Kumar S, Kumar J, Vara Prasad PV, Siddique KH, Nayyar H. Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant Cell Environ. 2019. https://doi.org/10.1111/pce.13328.
Kumari VV, Roy A, Vijayan R, Banerjee P, Verma VC, Nalia A, Pramanik M, Mukherjee B, Ghosh A, Reja MH, Chandran MA. Drought and heat stress in cool-season food legumes in sub-tropical regions: consequences, adaptation, and mitigation strategies. Plants. 2021. https://doi.org/10.3390/plants10061038.
Singh D, Singh CK, Taunk J, Tomar RS, Chaturvedi AK, Gaikwad K, Pal M. Transcriptome analysis of lentil (Lens culinaris Medikus) in response to seedling drought stress. BMC Genom. 2017. https://doi.org/10.1186/s12864-017-3596-7.
Biju S, Fuentes S, Gupta D. The use of infrared thermal imaging as a non-destructive screening tool for identifying drought-tolerant lentil genotypes. Plant Physiol Biochem. 2018. https://doi.org/10.1016/j.plaphy.2018.03.005.
Biju S, Fuentes S, Gupta D. Silicon modulates nitro-oxidative homeostasis along with the antioxidant metabolism to promote drought stress tolerance in lentil plants. Physiol Plant. 2021. https://doi.org/10.1111/ppl.13437.
Biju S, Fuentes S, Gonzalez Viejo C, Torrico DD, Inayat S, Gupta D. Silicon supplementation improves the nutritional and sensory characteristics of lentil seeds obtained from drought-stressed plants. J Sci Food Agric. 2021. https://doi.org/10.1002/jsfa.10759.
Gupta D, Dadu RH, Sambasivam P, Bar I, Singh M, beera N, Biju S. Toward climate-resilient lentils: challenges and opportunities. In: Kole, C. editors. Genomic designing of climate-smart pulse crops. Springer: Cham. 2019. https://doi.org/10.1007/978-3-319-96932-9_4.
Foti C, Kalampokis IF, Aliferis KA, Pavli OI. Metabolic Responses of two contrasting lentil genotypes to PEG-induced drought stress. Agron. 2021. https://doi.org/10.3390/agronomy11061190.
Zeroual A, Baidani A, Idrissi O. Drought Stress in Lentil (Lens culinaris, Medik) and Approaches for Its Management. Horticulturae. 2022. https://doi.org/10.3390/horticulturae9010001.
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Zia-ur-Rehman M, Qayyum MF, Abbas F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res. 2015. https://doi.org/10.1016/j.ecoenv.2015.05.011.
Zargar SM, Mahajan R, Bhat JA, Nazir M, Deshmukh R. Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech. 2019; doi:https://doi.org/10.1007/s13205-019-1613-z.
Naidu S, Pandey J, Mishra LC, Chakraborty A, Roy A, Singh IK, Singh A. Silicon nanoparticles: Synthesis, uptake and their role in mitigation of biotic stress. Ecotoxicol Environ Saf. 2023. https://doi.org/10.1016/j.ecoenv.2023.114783.
Gong H, Zhu X, Chen K, Wang S, Zhang C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005. https://doi.org/10.1016/j.plantsci.2005.02.023.
Crusciol CA, Pulz AL, Lemos LB, Soratto RP, Lima GP. Effects of silicon and drought stress on tuber yield and leaf biochemical characteristics in potato. Crop Sci. 2009. https://doi.org/10.2135/cropsci2008.04.0233.
Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol. 2010. https://doi.org/10.1016/j.jplph.2010.04.011.
Ashfaq W, Fuentes S, Brodie G, Gupta D. The role of silicon in regulating physiological and biochemical mechanisms of contrasting bread wheat cultivars under terminal drought and heat stress environments. Front Plant Sci. 2022. https://doi.org/10.3389/fpls.2022.955490.
Biju S, Fuentes S, Gupta D. Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol Biochem. 2017. https://doi.org/10.1016/j.plaphy.2017.09.001.
Ma JF. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr. 2004. https://doi.org/10.1080/00380768.2004.10408447.
Liang Y, Zhang W, Chen Q, Liu Y, Ding R. Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ Exp Bot. 2006. https://doi.org/10.1016/j.envexpbot.2005.05.012.
Cooke J, Leishman MR. Consistent alleviation of abiotic stress with silicon addition: a meta-analysis. Funct Ecol. 2016. https://doi.org/10.1111/1365-2435.12713.
Ahmed SR, Anwar Z, Shahbaz U, Skalicky M, Ijaz A, Tariq MS, Zulfiqar U, Brestic M, Alabdallah NM, Alsubeie MS, Mujtaba H. Potential role of silicon in plants against biotic and abiotic stresses. SILICON. 2023. https://doi.org/10.1007/s12633-022-02254-w.
Prisa D. Increasing plant resistance with silicon applications. World J Adv Res Rev. 2023. https://doi.org/10.30574/wjarr.2023.17.3.0413.
Song A, Li P, Fan F, Li Z, Liang Y. The effect of silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0113782.
Yin L, Wang S, Liu P, Wang W, Cao D, Deng X, Zhang S. Silicon-mediated changes in polyamine and 1-aminocyclopropane-1-carboxylic acid are involved in silicon-induced drought resistance in Sorghum bicolor L. Plant Physiol Biochem. 2014. https://doi.org/10.1016/j.plaphy.2014.04.014.
Manivannan A, Ahn YK. Silicon regulates potential genes involved in major physiological processes in plants to combat stress. Front Plant Sci. 2017. https://doi.org/10.3389/fpls.2017.01346.
Hu AY, Che J, Shao JF, Yokosho K, Zhao XQ, Shen RF, Ma JF. Silicon accumulated in the shoots results in down-regulation of phosphorus transporter gene expression and decrease of phosphorus uptake in rice. Plant Soil. 2018. https://doi.org/10.1007/s11104-017-3512-6.
Zhu Y, Guo J, Feng R, Jia J, Han W, Gong H. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil. 2016. https://doi.org/10.1007/s11104-016-2877-2.
Wang Y, Zhang K, Lu L, Xiao X, Chen B. Novel insights into effects of silicon-rich biochar (Sichar) amendment on cadmium uptake, translocation and accumulation in rice plants. Environ Pollut. 2020. https://doi.org/10.1016/j.envpol.2020.114772.
Zhang Y, Chen H, Liang Y, Lu T, Liu Z, Jin X, Hou L, Xu J, Zhao H, Shi Y, Ahammed GJ. Comparative transcriptomic and metabolomic analyses reveal the protective effects of silicon against low phosphorus stress in tomato plants. Plant Physiol Biochem. 2021. https://doi.org/10.1016/j.plaphy.2021.05.043.
Kapoor B, Kumar P, Gill NS, Sharma R, Thakur N, Irfan M. Molecular mechanisms underpinning the silicon-selenium (Si-Se) interactome and cross-talk in stress-induced plant responses. Plant Soil. 2023. https://doi.org/10.1007/s11104-022-05482-6.
Almutairi ZM. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics. 2016. https://doi.org/10.3316/informit.888088806058398.
Zhu Y, Yin J, Liang Y, Liu J, Jia J, Huo H, Wu Z, Yang R, Gong H. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol Environ Saf. 2019. https://doi.org/10.1016/j.ecoenv.2019.02.075.
Wu J, Mock HP, Giehl RF, Pitann B, Mühling KH. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J Hazard Mater. 2019. https://doi.org/10.1016/j.jhazmat.2018.10.052.
Zhou J, Zhang C, Du B, Cui H, Fan X, Zhou D, Zhou J. Soil and foliar applications of silicon and selenium effects on cadmium accumulation and plant growth by modulation of antioxidant system and Cd translocation: Comparison of soft vs. durum wheat varieties. J Hazard Mater. 2021. https://doi.org/10.1016/j.jhazmat.2020.123546.
Sun C, Liang X, Gong X, Chen H, Liu X, Zhang S, Li F, Zhao J, Yi J. Comparative transcriptomics provide new insights into the mechanisms by which foliar silicon alleviates the effects of cadmium exposure in rice. J Environ Sci. 2022. https://doi.org/10.1016/j.jes.2021.07.030.
Khattab HI, Emam MA, Emam MM, Helal NM, Mohamed MR. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol Plant. 2014. https://doi.org/10.1007/s10535-014-0391-z.
Ma D, Sun D, Wang C, Qin H, Ding H, Li Y, Guo T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J Plant Growth Regul. 2016. https://doi.org/10.1007/s00344-015-9500-2.
Boora R, Sheoran P, Rani N, Kumari S, Thakur R, Grewal S. Biosynthesized silica nanoparticles (Si NPs) helps in mitigating drought stress in wheat through physiological changes and upregulation of stress genes. SILICON. 2023. https://doi.org/10.1007/s12633-023-02439-x.
Kim YH, Khan AL, Lee IJ. Silicon: a duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit Rev Biotechnol. 2016. https://doi.org/10.3109/07388551.2015.1084265.
Moradtalab N, Weinmann M, Walker F, Höglinger B, Ludewig U, Neumann G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front Plant Sci. 2018. https://doi.org/10.3389/fpls.2018.00420.
Merhij IE, Al-Timmen WM, Jasim AH. the effect of silicon, tillage and the interaction between them on some antioxidants and phytohormones during drought stress of maize (Zea mays L.) plants. Plant Arch. 2019.
Hosseini SA, Naseri Rad S, Ali N, Yvin JC. The ameliorative effect of silicon on maize plants grown in Mg-deficient conditions. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20040969.
Khan I, Awan SA, Rizwan M, Brestic M, Xie W. Silicon: an essential element for plant nutrition and phytohormones signaling mechanism under stressful conditions. Plant Growth Regul. 2022. https://doi.org/10.1007/s10725-022-00872-3.
Tripathi DK, Rai P, Guerriero G, Sharma S, Corpas FJ, Singh VP. Silicon induces adventitious root formation in rice under arsenate stress with involvement of nitric oxide and indole-3-acetic acid. Jour Exp Bot. 2021. https://doi.org/10.1093/jxb/eraa488.
Khan A, Khan AL, Imran M, Asaf S, Kim YH, Bilal S, Numan M, Al-Harrasi A, Al-Rawahi A, Lee IJ. Silicon-induced thermotolerance in Solanum lycopersicum L. via activation of antioxidant system, heat shock proteins, and endogenous phytohormones. BMC Plant Biol. 2020. https://doi.org/10.1186/s12870-020-02456-7.
Kim YH, Khan AL, Waqas M, Shim JK, Kim DH, Lee KY, Lee IJ. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J Plant Growth Regul. 2014. https://doi.org/10.1007/s00344-013-9356-2.
Kim YH, Khan AL, Kim DH, Lee SY, Kim KM, Waqas M, Jung HY, Shin JH, Kim JG, Lee IJ. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014. https://doi.org/10.1186/1471-2229-14-13.
Souri Z, Khanna K, Karimi N, Ahmad P. Silicon and plants: current knowledge and future prospects. Plant Growth Regul. 2021. https://doi.org/10.1007/s00344-020-10172-7.
Thakur A, Singh A, Tandon A, Sharma V. Insights into the molecular mechanisms of uptake, phytohormone interactions and stress alleviation by silicon: a beneficial but non-essential nutrient for plants. Plant Growth Regul. 2023. https://doi.org/10.1007/s10725-023-01002-3.
Wang Y, Zhang B, Jiang D, Chen G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ Exp Bot. 2019. https://doi.org/10.1016/j.envexpbot.2018.11.022.
Rastogi A, Yadav S, Hussain S, Kataria S, Hajihashemi S, Kumari P, Yang X, Brestic M. Does silicon really matter for the photosynthetic machinery in plant? Plant Physio Biochem. 2021. https://doi.org/10.1016/j.plaphy.2021.11.004.
Biju S, Fuentes S, Gupta D. Regulatory role of silicon on photosynthesis, gas-exchange and yield related traits of drought-stressed lentil plants. SILICON. 2023. https://doi.org/10.1007/s12633-023-02492-6.
Haghighi TM, Saharkhiz MJ, Ramezanian A, Zarei M. The use of silicon and mycorrhizal fungi to mitigate changes in licorice leaf micromorphology, chlorophyll fluorescence, and rutin content under water-deficit conditions. Plant Physiol Biochem. 2023. https://doi.org/10.1016/j.plaphy.2023.107662.
silicon on photosynthesis of tomato seedlings under water stress. J Int Agric. 2018; doi: https://doi.org/10.1016/S2095-3119(18)62038-6.
Hassan H, Alatawi A, Abdulmajeed A, Emam M, Khattab H. Roles of Si and SiNPs in improving thermotolerance of wheat photosynthetic machinery via upregulation of PsbH, PsbB and PsbD genes encoding PSII core proteins. Horticulturae. 2021. https://doi.org/10.3390/horticulturae7020016.
Iordachescu M, Imai R. Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol. 2008. https://doi.org/10.1111/j.1744-7909.2008.00736.x.
Tripathi DK, Singh S, Singh S, Chauhan DK, Dubey NK, Prasad R. Silicon as a beneficial element to combat the adverse effect of drought in agricultural crops: capabilities and future possibilities. Water stress and crop plants: a sustainable approach. Ahmad, P.editors. 2016a. https://doi.org/10.1002/9781119054450.ch39.
Chen W, Yao X, Cai K, Chen J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res. 2011. https://doi.org/10.1007/s12011-010-8742-x.
Habibi G, Hajiboland R. Alleviation of drought stress by silicon supplementation in pistachio (Pistacia vera L.) plants. Folia Hortic. 2013; doi:https://doi.org/10.2478/fhort-2013-0003.
Cao BL, Ma Q, Zhao Q, Wang L, Xu K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci Hortic. 2015. https://doi.org/10.1016/j.scienta.2015.07.037.
Maghsoudi K, Emam Y, Ashraf M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk J Bot. 2015. https://doi.org/10.3906/bot-1407-11.
Verma KK, Song XP, Verma CL, Chen ZL, Rajput VD, Wu KC, Liao F, Chen GL, Li YR. Functional relationship between photosynthetic leaf gas exchange in response to silicon application and water stress mitigation in sugarcane. Biol Res. 2021. https://doi.org/10.1186/s40659-021-00338-2.
Xu J, Guo L, Liu L. Exogenous silicon alleviates drought stress in maize by improving growth, photosynthetic and antioxidant metabolism. Environ Exp Bot. 2022. https://doi.org/10.1016/j.envexpbot.2022.104974.
Raza MA, Zulfiqar B, Iqbal R, Muzamil MN, Aslam MU, Muhammad F, Amin J, Aslam HM, Ibrahim MA, Uzair M, Habib-ur-Rahman M. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci Rep. 2023. https://doi.org/10.1038/s41598-023-29784-6.
Yonny K, Sukendah S, Edi P, Djoko P. Stomatal behaviour of soybean under drought stress with silicon application. Ann Agri Bio Res. 2020.
Mahawar L, Popek R, Shekhawat GS, Alyemeni MN, Ahmad P. Exogenous hemin improves Cd2+ tolerance and remediation potential in Vigna radiata by intensifying the HO-1 mediated antioxidant defence system. Sci Rep. 2021. https://doi.org/10.1038/s41598-021-82391-1.
Mahawar L, Shekhawat GS. EsHO 1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO 1 in seedlings of Eruca sativa: underutilized oil yielding crop of arid region. Physiol Mol Biol Plants. 2019. https://doi.org/10.1007/s12298-019-00663-7.
Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot. 2014;65:15.
Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol. 2022. https://doi.org/10.1038/s41580-022-00499-2.
Hong Z, Lakkineni K, Zhang Z, Verma DP. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant physiol. 2000. https://doi.org/10.1104/pp.122.4.1129.
Choudhary NL, Sairam RK, Tyagi A. Expression of Δ1-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.). Indian J Biochem. Biophys. 2005.
Sharma S, Verslues PE. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant, Cell Environ. 2010. https://doi.org/10.1111/j.1365-3040.2010.02188.x
Reddy PS, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, Varshney RK, Kishor PK. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol Biochem. 2015. https://doi.org/10.1016/j.plaphy.2015.05.014.
Ghosh D, Sen S, Mohapatra S. Modulation of proline metabolic gene expression in Arabidopsis thaliana under water-stressed conditions by a drought-mitigating Pseudomonas putida strain. Ann Microbiol. 2017. https://doi.org/10.1007/s13213-017-1294-y.
Gunes A, Pilbeam DJ, Inal A, Bagci EG, Coban S. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J Plant Interact. 2007. https://doi.org/10.1080/17429140701529399.
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul. 2010. https://doi.org/10.1007/s00344-009-9120-9.
Ogawa KI. Glutathione-associated regulation of plant growth and stress responses. Antioxid Redox Signal. 2005. https://doi.org/10.1089/ars.2005.7.973.
Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009. https://doi.org/10.1007/s11103-008-9435-0.
Santner A, Calderon-Villalobos LI, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol. 2009. https://doi.org/10.1038/nchembio.165.
Verma V, Ravindran P, Kumar PP. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016. https://doi.org/10.1186/s12870-016-0771-y.
Wani SH, Kumar V, Shriram V, Sah SK. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016. https://doi.org/10.1016/j.cj.2016.01.010.
Iqbal S, Wang X, Mubeen I, Kamran M, Kanwal I, Díaz GA, Abbas A, Parveen A, Atiq MN, Alshaya H, Zin El-Abedin TK. Phytohormones trigger drought tolerance in crop plants: outlook and future perspectives. Front Plant Sci. 2022. https://doi.org/10.3389/fpls.2021.799318.
Wahab A, Abdi G, Saleem MH, Ali B, Ullah S, Shah W, Mumtaz S, Yasin G, Muresan CC, Marc RA. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants. 2022. https://doi.org/10.3390/plants11131620.
Eneji AE, Inanaga S, Muranaka SL, Li J, Hattori T, An P, Tsuji W. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J Plant Nutr. 2008. https://doi.org/10.1080/01904160801894913.
Helaly MN, El-Hoseiny H, El-Sheery NI, Rastogi A, Kalaji HM. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol Biochem. 2017. https://doi.org/10.1016/j.plaphy.2017.05.021.
Ghebrehiwot HM, Kulkarni MG, Kirkman KP, Van Staden J. Smoke‐water and a smoke‐isolated butenolide improve germination and seedling vigour of Eragrostis Tef (Zucc.) Trotter under high temperature and low osmotic potential. J Agron Crop Sci. 2008. https://doi.org/10.1111/j.1439-037X.2008.00321.x.
Li W, Nguyen KH, Chu HD, Ha CV, Watanabe Y, Osakabe Y, Leyva-González MA, Sato M, Toyooka K, Voges L, Tanaka M. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017. https://doi.org/10.1371/journal.pgen.1007076.
Smith SM, Li J. Signalling and responses to strigolactones and karrikins. Curr Opin Plant Biol. 2014. https://doi.org/10.1016/j.pbi.2014.06.003.
Li W, Tran LS. Are karrikins involved in plant abiotic stress responses? Trends Plant Sci. 2015. https://doi.org/10.1016/j.tplants.2015.07.006.
Tan ZZ, Wang YT, Zhang XX, Jiang HY, Li Y, Zhuang LL, Yu JJ, Yang ZM. Karrikin1 enhances drought tolerance in creeping bentgrass in association with antioxidative protection and regulation of stress-responsive gene expression. Agron. 2023. https://doi.org/10.3390/agronomy13030675.
Evans PT, Malmberg RL. Do polyamines have roles in plant development? Ann Rev Plant Biol. 1989. https://doi.org/10.1146/annurev.pp.40.060189.001315.
John I, Drake R, Farrell A, Cooper W, Lee P, Horton P, Grierson D. Delayed leaf senescence in ethylene-deficient ACC-oxidase antisense tomato plants: molecular and physiological analysis. The Plant J. 1995. https://doi.org/10.1046/j.1365-313X.1995.7030483.x.
Pandey S, Ranade SA, Nagar PK, Kumar N. Role of polyamines and ethylene as modulators of plant senescence. J Biosci. 2000. https://doi.org/10.1007/BF02703938.
Napieraj N, Janicka M, Reda M. Interactions of polyamines and phytohormones in plant response to abiotic stress. Plants. 2023. https://doi.org/10.3390/plants12051159.
Flora C, Khandekar S, Boldt J, Leisner S. Silicon alleviates long-term copper toxicity and influences gene expression in Nicotiana tabacum. J Plant Nutr. 2019. https://doi.org/10.1080/01904167.2019.1589508.
Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013. https://doi.org/10.1093/aob/mct067.
Hamayun M, Sohn EY, Khan SA, Shinwari ZK, Khan AL, Lee IJ. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pak J Bot. 2010.
Dhakarey R, Raorane ML, Treumann A, Peethambaran PK, Schendel RR, Sahi VP, Hause B, Bunzel M, Henry A, Kohli A, Riemann M. Physiological and proteomic analysis of the rice mutant cpm2 suggests a negative regulatory role of jasmonic acid in drought tolerance. Front Plant Sci. 2017. https://doi.org/10.3389/fpls.2017.01903.
Swamy PM, Smith BN. Role of abscisic acid in plant stress tolerance. Current Sci. 1999;76:9.
Krochko JE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ. (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol. 1998;118:3.
Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006. https://doi.org/10.1111/j.1469-8137.2006.01787.x.
Potts WC, Reid JB, Murfet IC. Internode length in Pisum. Gibberellins and the slender phenotype. Physio Plant. 1985. https://doi.org/10.1111/j.1399-3054.1985.tb02310.x.
Sahebi M, Hanafi MM, Azizi P. Application of silicon in plant tissue culture. In Vitro Cell Dev Biol Plant. 2016. https://doi.org/10.1007/s11627-016-9757-6.
Lee SK, Sohn EY, Hamayun M, Yoon JY, Lee IJ. Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor Syst. 2010. https://doi.org/10.1007/s10457-010-9299-6.
Muneer S, Jeong BR. Proteomic analysis of salt-stress responsive proteins in roots of tomato (Lycopersicon esculentum L.) plants towards silicon efficiency. Plant Growth Regul. 2015. https://doi.org/10.1007/s10725-015-0045-G.
Hara M, Furukawa J, Sato A, Mizoguchi T, Miura K. Abiotic stress and role of salicylic acid in plants. In: Ahmad P, Prasad M, editors. Abiotic stress responses in plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-0634-1_13.
Jiang N, Fan X, Lin W, Wang G, Cai K. Transcriptome analysis reveals new insights into the bacterial wilt resistance mechanism mediated by silicon in tomato. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20030761.
Inanaga S, Okasaka A. Calcium and silicon binding compounds in cell walls of rice shoots. Soil Sci Plant Nutr. 1995. https://doi.org/10.1080/00380768.1995.10419563.
Currie HA, Perry CC. Silica in plants: biological, biochemical and chemical studies. Ann Bot. 2007. https://doi.org/10.1093/aob/mcm247.
Kido N, Yokoyama R, Yamamoto T, Furukawa J, Iwai H, Satoh S, Nishitani K. The matrix polysaccharide (1; 3, 1; 4)-β-D-glucan is involved in silicon-dependent strengthening of rice cell wall. Plant Cell Physiol. 2015. https://doi.org/10.1093/pcp/pcu162.
He C, Wang L, Liu J, Liu X, Li X, Ma J, Lin Y, Xu F. Evidence for ‘silicon’within the cell walls of suspension-cultured rice cells. New Phytol. 2013. https://doi.org/10.1111/nph.12401.
Zhang J, Zou W, Li Y, Feng Y, Zhang H, Wu Z, Tu Y, Wang Y, Cai X, Peng L. Silica distinctively affects cell wall features and lignocellulosic saccharification with large enhancement on biomass production in rice. Plant Sci. 2015. https://doi.org/10.1016/j.plantsci.2015.07.014.
Guerriero G, Hausman JF, Legay S. Silicon and the plant extracellular matrix. Front Plant Sci. 2016. https://doi.org/10.3389/fpls.2016.00463.
Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK. Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J Exp Bot. 2011. https://doi.org/10.1093/jxb/erq392.
Qian S, Poffenbarger H, Unrine JM. Effects of silicon supplementation on growth and silicon accumulation in tall fescue (Festuca arundinacea) and bentgrass (Agrostis stolonifera). J Plant Nutr. 2023. https://doi.org/10.1080/01904167.2023.2206435.
Fortunato AA, da Silva WL, Rodrigues FÁ. Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathol. 2014. https://doi.org/10.1094/PHYTO-07-13-0203-R.
Fukuda H, Ohashi-Ito K. Vascular tissue development in plants. Curr Top Dev Biol. 2019. https://doi.org/10.1016/bs.ctdb.2018.10.005.
Yang JH, Wang H. Molecular mechanisms for vascular development and secondary cell wall formation. Front Plant Sci. 2016. https://doi.org/10.3389/fpls.2016.00356.
Manivannan A, Soundararajan P, Muneer S, Ko CH, Jeong BR. Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang.’ BioMed Res Inter. 2016. https://doi.org/10.1155/2016/3076357.
Mishra A, Choudhuri MA. Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant. 1999. https://doi.org/10.1023/A:1002469303670.
Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001. https://doi.org/10.1016/S1360-1385(00)01838-0.
Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005. https://doi.org/10.1016/j.tplants.2005.05.009.
Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Ann Bot. 2007. https://doi.org/10.1093/aob/mcl255.
Pereira TS, da Silva LAK, Tan DK, da Costa DV, Uchoa EB, Nascimento FR, dos Santos PE, Avila FW, Marques DJ, Silva GEM. Positive interference of silicon on water relations, nitrogen metabolism, and osmotic adjustment in two pepper (‘capsicum annuum cultivars’) under water deficit. Aust J Crop Sci. 2013;7:8.
Tripathi DK, Singh VP, Ahmad P, Chauhan DK, Prasad SM. Silicon in plants: advances and prospects. Tripathi DK, Singh, VP, Ahmad P, Chauhan DK, Prasad SM, editors. CRC Press; Florida. 2016b. https://doi.org/10.1201/9781315369310.
He C, Ma J, Wang L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015. https://doi.org/10.1111/nph.13282.
Agarie S, Hanaoka N, Ueno O, Miyazaki A, Kubota F, Agata W, Kaufman PB. Effects of silicon on tolerance to water deficit and heat stress in rice plants (Oryza sativa L.), monitored by electrolyte leakage. Plant Prod Sci. 1998. https://doi.org/10.1626/pps.1.96.
Lux A, Luxová M, Hattori T, Inanaga S, Sugimoto Y. Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol Plant. 2002. https://doi.org/10.1034/j.1399-3054.2002.1150110.x.
Kang J, Zhao W, Zhu X. Silicon improves photosynthesis and strengthens enzyme activities in the C3 succulent xerophyte Zygophyllum xanthoxylum under drought stress. J Plant Physiol. 2016. https://doi.org/10.1016/j.jplph.2016.05.009.
Sommer M, Kaczorek D, Kuzyakov Y, Breuer J. Silicon pools and fluxes in soils and landscapes—a review. J Plant Nutr Soil Sci. 2006. https://doi.org/10.1002/jpln.200521981.
Haynes RJ. The nature of biogenic Si and its potential role in Si supply in agricultural soils. Agric Ecosyst Environ. 2017. https://doi.org/10.1016/j.agee.2017.04.021.
Mandlik R, Thakral V, Raturi G, Shinde S, Nikolić M, Tripathi DK, Sonah H, Deshmukh R. Significance of silicon uptake, transport, and deposition in plants. J Exp Bot. 2020. https://doi.org/10.1093/jxb/eraa301.
Ma JF, Miyake Y, Takahashi E. Silicon as a beneficial element for crop plants. Studies in plant science. In: Datnoft IE, Snyder GH, Korndorfer GH, editors. Silicon in agriculture. 2001. https://doi.org/10.1016/S0928-3420(01)80006-9.
Erskine W, Muehlbauer FJ, Short RW. Stages of development in lentil. Exp Agric. 1990. https://doi.org/10.1017/S0014479700018457.
Ficklin SP, Sanderson LA, Cheng CH, Staton ME, Lee T, Cho IH, Jung S, Bett KE, Main D. Tripal: a construction toolkit for online genome databases. Database. 2011. https://doi.org/10.1093/database/bar044.
Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechol. 2016. https://doi.org/10.1038/nbt.3519.
Li Y, Andrade J. DEApp: an interactive web interface for differential expression analysis of next generation sequence data. Source Code Biol Med. 2017. https://doi.org/10.1186/s13029-017-0063-4.
Alexa A, Rahnenführer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinform. 2006. https://doi.org/10.1093/bioinformatics/btl140.
Camacho DM, Collins KM, Powers RK, Costello JC, Collins JJ. Next-generation machine learning for biological networks. Cell. 2018. https://doi.org/10.1016/j.cell.2018.05.015.
Acknowledgements
The authors are grateful to the University of Melbourne for the Australian Government Research Training Program Scholarship and the Grains Research and Development Corporation (GRDC), Australia for the Grain Industry Research Scholarship (GRS-11011) given to Sajitha Biju.
Funding
The research was supported by the University of Melbourne (Australian Government Research Training Program Scholarship) and the Grains Research and Development Corporation (GRDC), Australia (GRDC Research Scholarship (GRS-11011).
Author information
Authors and Affiliations
Contributions
Sajitha Biju: Study conception, design and execution of experiments, sample preparation, methodology, data collection, data analysis, prepared the first draft, reviewed, and edited the draft, approved the final version. Sigfredo Fuentes: Study conception and experiment design, reviewed the draft and approved the final version. Dorin Gupta: Study conception and experiment design, reviewed the draft and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Biju, S., Fuentes, S. & Gupta, D. Novel insights into the mechanism(s) of silicon-induced drought stress tolerance in lentil plants revealed by RNA sequencing analysis. BMC Plant Biol 23, 498 (2023). https://doi.org/10.1186/s12870-023-04492-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04492-5