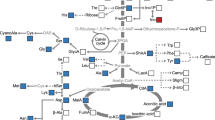
Abstract
Background
Phosphoenolpyruvate carboxylase (PEPC) plays an important role in the primary metabolism of higher plants. Several studies have revealed the critical importance of PEPC in the interaction of carbon and nitrogen metabolism. However, the function mechanism of PEPC in nitrogen metabolism is unclear and needs further investigation.
Results
This study indicates that transgenic rice expressing the sugarcane C4-PEPC gene displayed shorter primary roots and fewer crown roots at the seedling stage. However, total nitrogen content was significantly higher in transgenic rice than in wild type (WT) plants. Proteomic analysis revealed that there were more differentially expressed proteins (DEPs) responding to nitrogen changes in transgenic rice. In particular, the most enriched pathway “glutathione (GSH) metabolism”, which mainly contains GSH S-transferase (GST), was identified in transgenic rice. The expression of endogenous PEPC, GST and several genes involved in the TCA cycle, glycolysis and nitrogen assimilation changed in transgenic rice. Correspondingly, the activity of enzymes including GST, citrate synthase, 6-phosphofructokinase, pyruvate kinase and ferredoxin-dependent glutamate synthase significantly changed. In addition, the levels of organic acids in the TCA cycle and carbohydrates including sucrose, starch and soluble sugar altered in transgenic rice under different nitrogen source concentrations. GSH that the substrate of GST and its components including glutamic acid, cysteine and glycine accumulated in transgenic rice. Moreover, the levels of phytohormones including indoleacetic acid (IAA), zeatin (ZT) and isopentenyladenosine (2ip) were lower in the roots of transgenic rice under total nutrients. Taken together, the phenotype, physiological and biochemical characteristics of transgenic rice expressing C4-PEPC were different from WT under different nitrogen levels.
Conclusions
Our results revealed the possibility that PEPC affects nitrogen metabolism through regulating GST, which provide a new direction and concepts for the further study of the PEPC functional mechanism in nitrogen metabolism.
Similar content being viewed by others
Background
PEPC widely exists in vascular plants, cyanobacteria, green algae, non-photosynthetic bacteria and archaea, but not in fungi and animals [1, 2]. PEPC is recognized for its special role in the carbon metabolism of plants. In C4 and crassulaceae plants, PEPC plays a critical role in the initial fixation of atmospheric CO2 in C4 photosynthesis and crassulacean acid metabolism photosynthesis. In C3 plants and most non-photosynthetic tissues, PEPC primarily catalyzes irreversible β-carboxylation of phosphoenolpyruvate (PEP) in the presence of HCO3− and Me2+ to form oxaloacetate (OAA) and inorganic phosphate (Pi) [1]. OAA is the carbon skeleton for the synthesis of aspartic acid (Asp) and asparagine (Asn), and an important material in the tricarboxylic acid (TCA) cycle, which generates intermediates involved in other physiological functions and provides carbon skeletons for biosynthesis and nitrogen assimilation [2].
PEPC is an abundant plant protein that is used for nitrogen storage [3]. Moreover, PEPC has an important role in carbon and nitrogen interactions [4]. Knockdown of Osppc4 encoding a plant-type PEPC targeted to chloroplasts in rice resulted in a decrease of OAA, citric acid and isocitric acid. Meanwhile, glutamate and aspartate contents decreased, and the nitrogen content of the whole plant is reduced in Osppc4 knockdown lines, which indicates that the down-regulation of Osppc4 suppresses NH4+ assimilation and amino acid synthesis [5]. A double mutant of PPC1 and PPC2 encoding PEPC in Arabidopsis showed a growth-arrest phenotype and decreased PEPC activity, and further reduced the synthesis of malate and citrate and severely suppressed ammonium assimilation [6]. Transgenic rice overexpressing maize PEPC had increased carbon levels, higher grain yield per plant and were more tolerant to low nitrogen stress with up-regulation of photorespiratory genes [7]. Overexpression of Zea mays PEPC in Arabidopsis thaliana resulted in increasing protein and free amino acid content in transgenic plants [8]. Seed-specific expression of a bacterial PEPC in Vicia narbonensis resulted in higher seed-dry weight, elevating crude protein and free amino acids content [9]. These studies suggested that a change in PEPC expression in plants altered physiology and biochemistry with changes of biomass, organic acid content, protein and amino acid levels. In addition, Dof1 transcription factor regulated the expression of genes, such as PEPC, to modulate carbon and nitrogen metabolites. The expression of maize Dof1 in Arabidopsis thaliana and rice significantly enhanced PEPC gene expression, increased PEPC activity and amino acid content and improved growth under low nitrogen conditions [10, 11]. The research indirectly proves the function of PEPC in carbon and nitrogen metabolism. Moreover, overexpression of GS1;1 and GS1;2 encoding glutamine synthetase, a key enzyme involved in ammonium assimilation, induced a decrease of PEPC genes expression [12], and glutamine synthetase 2 co-suppressed plants showed a significant decrease of PEPC1 and PEPC2 gene expression [13], which suggested that PEPC was associated with nitrogen metabolism. PEPC is involved in nitrogen metabolism, but it may not directly participate in nitrogen assimilation. The effects of PEPC in the modulation of carbon and nitrogen interactions need to be further clarified.
In this study, the sugarcane C4-PEPC gene was introduced into the indica cultivar Hang2. To further investigate phenotype and physiological-biochemical changes, transgenic plants and WT (indica rice cultivar, Hang2) plants were cultured in nutrient solution with different concentrations of nitrogen source. Then, proteomic analysis was performed to identify DEPs and the relevant metabolic pathways. Additionally, gene expression, enzyme activities, metabolites and hormones were measured and comparatively analyzed. Finally, we identified DEPs with significant changes in transgenic rice expressing C4-PEPC. We also summarized and obtained the differences in the physiology and biochemistry between transgenic rice expressing C4-PEPC and WT plants.
Results
Plant phenotype under different nitrogen source concentrations
Previously, we introduced the sugarcane PEPC gene containing the promoter and coding region into indica cultivar Hang2 using Agrobacterium-mediated transformation and generated tPEPC transgenic lines. The C4-PEPC was successfully expressed at the transcriptional and protein level in tPEPC lines. Correspondingly, all tPEPC lines showed higher PEPC enzyme activities compared with WT [14]. Moreover, the total nitrogen content of tPEPC lines increased at both the seedling and tillering stages (Supplementary Figure S1). This suggested that the expression of C4-PEPC influenced both carbon metabolism and nitrogen metabolism in transgenic rice. Then we investigated the phenotype and biochemical characteristics of tPEPC and WT plants under different nitrogen source concentrations. The tPEPC and WT plants were cultured in total nutrient solution containing normal levels of nitrogen and labeled as tPEPC-T and WT-T. To investigate the difference of sensitivity to nitrogen, tPEPC and WT plants were cultured in low nitrogen solution containing trace nitrogen and labeled as tPEPC-L and WT-L. To better investigate the growth difference between tPEPC and WT plants, they were also cultured in nitrogen deficiency solution and labeled as tPEPC-D and WT-D. The plant height and shoot length measurements of tPEPC plants were significantly higher than that of WT plants in different conditions. The primary root length of tPEPC plants was shorter than that of WT under total nutrients, while there were no differences between tPEPC and WT plants under low nitrogen and nitrogen deficiency (Fig. 1a-d). Notably, the crown root number of tPEPC plants was lower than that of WT plants under total nutrients (Fig. 1e). The chlorophyll a (Chl a) content of tPEPC-L and tPEPC-D was greater than that of WT-L and WT-D, respectively (Fig. 1f). In addition, tPEPC plants showed higher root dehydrogenase activity (Fig. 1 g). Correspondingly, the Chl a content and root dehydrogenase activity increased in 6-day-old seedlings of tPEPC lines (Supplementary Figure S2). Compared with that of WT, the total carbon content of tPEPC plants increased by about 14 % under total nutrients. By contrast, they decreased by approximately 22 and 20 % under low nitrogen and nitrogen deficiency, respectively (Fig. 1 h). The total nitrogen content of tPEPC plants increased significantly under different nitrogen source concentrations (Fig. 1i). These results suggested that the expression of C4-PEPC influenced growth and physiology in Oryza sativa.
Phenotype of transgenic rice expressing C4-PEPC (tPEPC). a-d Plants of tPEPC and WT under total nutrients, low nitrogen and nitrogen deficiency. e Crown root number. f Chlorophyll (Chl) and carotenoid (Car x) content. g Root dehydrogenase activity. h Total carbon content. i Total nitrogen content. Bar = 1 cm. (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001)
Proteomic analysis
To clarify the molecular mechanisms of C4-PEPC regulating plant growth in Oryza sativa, we conducted protein profile analysis by the tandem mass tag (TMT) method. Proteins with fold changes (FC) greater than 1.3 and less than 1/1.3 were identified as DEPs. Overall, 685, 775 and 75 DEPs were identified in tPEPC-L/tPEPC-T, tPEPC-D/tPEPC-T, and tPEPC-D/tPEPC-L, respectively, which were more than the 538, 446 and 32 DEPs identified in WT-L/WT-T, WT-D/WT-T, and WT-D/WT-L. This implied that more genes were involved in the response to nitrogen changes in tPEPC plants. Overall, 47 DEPs (27 up-regulated and 20 down-regulated), 80 DEPs (27 up-regulated and 53 down-regulated) and 44 DEPs (25 up-regulated and 19 down-regulated) were identified in tPEPC-T/WT-T, tPEPC-L/WT-L and tPEPC-D/WT-D, respectively (Fig. 2a). Then, we focused on the DEPs identified in tPEPC-T/WT-T, tPEPC-L/WT-L and tPEPC-D/WT-D. DEPs were annotated and classified into the three major Gene Ontology (GO) categories: biological process, cellular component and molecular function. The primary functional GO terms were cellular process and metabolic process in the biological process category; cell, membrane and organelle in the cellular component category; and binding and catalytic activity in the molecular function category (Supplementary Figure S3). The DEPs were localized predominantly in chloroplasts and cytoplasm (Supplementary Figure S4), which implied a change of metabolic activity in tPEPC plants. GO enrichment analysis was performed to identify significant functional classifications. Then, comparative cluster analysis was carried out to indicate a comparison between the groups (Fig. 2b-d, Supplementary Figure S5). In molecular function ontology, the prominent classifications were transferase activity and metal/iron-sulfur cluster in tPEPC-T/WT-T, including enzyme inhibitor/regulator activity, molecular function regulator, hydrolase activity, sulfur compound binding, nutrient reservoir activity and manganese ion binding in tPEPC-L/WT-L and peptidase activity, cation transmembrane transporter activity, cysteine-type peptidase activity and chlorophyll-binding in tPEPC-D/WT-D. In cellular component ontology, the prominent classifications were apoplast in tPEPC-T/WT-T, mitochondrial protein complex and extracellular region in tPEPC-L/WT-L and classed of thylakoid and photosystem in tPEPC-D/WT-D. In biological process ontology, the prominent classifications were cellular response to chemicals/stress in tPEPC-T/WT-T, defense response and metal ion transport in tPEPC-L/WT-L and purine-containing compound/ribose phosphate/glycosyl compound biosynthetic process and photosynthesis in tPEPC-D/WT-D. The results suggested that the nitrogen responsiveness of tPEPC plants significantly differed from WT plants and there was a significant difference between the tPEPC-T/WT-T, tPEPC-L/WT-L and tPEPC-D/WT-D groups.
Subsequently, the enriched pathways were identified in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and assessed by cluster analysis (Fig. 2e; Table 1, Supplementary Figure S6). In tPEPC-T/WT-T, an up-regulated and a down-regulated pathway “GSH metabolism” was identified and included two GSTs. In this study, tPEPC plants with down-regulated GST had shorter primary roots and fewer crown roots, which was consistent with a loss-of-function rice mutant with a T-DNA insertion in GST showed reduced primary root elongation and lateral root formation [15]. In tPEPC-L/WT-L, the up-regulated pathway category “photosynthesis - antenna proteins” included “light-harvesting II chlorophyll a/b binding protein 1 (Lhcb1)” and “light-harvesting complex I chlorophyll a/b binding protein 2 (Lhca2)”. The down-regulated pathway “plant hormone signal transduction/MAPK signaling pathway–plant/plant-pathogen interaction” included “pathogenesis-related proteins (PRs)”. In tPEPC-D/WT-D, the enriched pathway “carbon metabolism” included up-regulated proteins “isocitrate lyase” and “malic enzyme” (ME), and down-regulated protein “pyruvate phosphate dikinase” (PPDK). The down-regulated pathway “photosynthesis” included “photosystem I reaction center subunit XI, chloroplastic”. The enriched pathway “metabolic pathways/photosynthesis-antenna proteins” included up-regulated “alpha-amylase”, and down-regulated “chlorophyll a-b binding protein, chloroplastic” (Lhcb1) and “lipoxygenase” (LOX). These results indicated that the DEPs in tPEPC-L/WT-L and tPEPC-D/WT-D were mainly related to photosynthesis and carbon metabolism.
Gene expression analysis
Proteomic analysis indicated significant differences between tPEPC and WT. To complement the changes at the transcriptional level, the gene expression of several DEPs was analyzed using quantitative real-time PCR (qRT-PCR). GST (968), GST1 and GST4 were remarkably down-regulated in tPEPC-T plants compared with WT-T, which was consistent with the regulation of expression at the protein level. However, the expressions of GST (968), GST1 and GST4 were up-regulated in tPEPC-L and tPEPC-D, which were also higher than those in WT-L and WT-D, respectively. In contrast with WT plants, the ferredoxin-nitrite reductase (NiR) and FD-GOGAT genes involved in nitrogen assimilation were up-regulated in tPEPC plants under different nitrogen source concentrations (Fig. 3a). The expression of Lhcb1 and Lox genes were higher in tPEPC-T plants than in WT-T, but they were down-regulated in tPEPC-L and tPEPC-D, which were lower than that in WT-L and WT-D, respectively. The ME gene was up-regulated in tPEPC plants, especially in tPEPC-T (Fig. 3b). The results indicated that the expression patterns of genes involved in GSH metabolism, nitrogen assimilation and carbon metabolism in tPEPC plants were different from that in WT plants.
Analysis of gene expression by qRT-PCR. a Expression of GST, NiR and FD-GOGAT. GST (968): BGIOSGA010968. b Expression of Lhcb, LOX and ME. c Expression of C4-PEPC and endogenous PEPC genes including Osppc1, Osppc2a, Osppc2b, Osppc3 and Osppc4. d Expression of genes involved in the TCA cycle (α-KGDH, ICDHc and CS) and glycolysis (HK, PFK and PK)
To investigate whether the introduction of C4-PEPC affected the expression of endogenous PEPC genes, we examined them using qRT-PCR. First, we analyzed the expression of C4-PEPC and found that it was gradually down-regulated in tPEPC-L and tPEPC-D. There was no obvious difference in the expression of Osppc1 and Osppc2a between tPEPC and WT plants. Osppc2b and Osppc3 were up-regulated under low nutrient and nitrogen deficiency, especially in tPEPC-L and tPEPC-D. Osppc4 was up-regulated in tPEPC plants and it was significantly up-regulated in tPEPC-D (Fig. 3c). These results suggested that the expression of some endogenous PEPC genes indeed changed in tPEPC plants. In C3 plants, PEPC was mainly involved in the anaplerotic reaction of the TCA cycle, which is also in connection with glycolysis. Therefore, we analyzed the expression of rate-limiting enzyme genes in the TCA cycle and glycolysis. The expression of α-ketoglutarate dehydrogenase (α-KGDH), isocitrate dehydrogenase (ICDHc), hexokinase (HK) and PFK genes was higher in tPEPC-T compared with WT-T, but was no different between tPEPC-L and WT-L or tPEPC-D and WT-D. On the contrary, the expression of CS and PK genes were down-regulated in tPEPC-T compared with in WT-T (Fig. 3d). The results indicated that these TCA cycle and glycolysis genes had different expression patterns in tPEPC relative to WT under different nitrogen source concentrations.
Enzyme activity assay
Given the changes in the proteome and gene expression, we conjectured a change in enzyme activity. Therefore, we measured the enzyme activity in tPEPC and WT plants. GST activity in tPEPC-T and tPEPC-L was lower than that in WT-T and WT-L, respectively. Meanwhile, GOGAT activity in tPEPC plants was higher than in WT plants under different nitrogen source concentrations (Fig. 4a). Compared with under total nutrients, PEPC activity and pyruvate dehydrogenase (PDH) activity significantly decreased under low nutrient and nitrogen deficiency in both tPEPC and WT plants. PEPC activity was higher in tPEPC plants than in WT plants, while PDH activity showed inverse activity. ME activity was higher in tPEPC-T and tPEPC-L than in WT-T and WT-L, respectively (Fig. 4b). This indicated that the activity of these enzymes correlated in response to TCA cycle changes in tPEPC plants. CS activity decreased under low nutrient and nitrogen deficiency and was significantly lower in tPEPC plants than in WT plants. The activity of α-KGDH was higher and ICDHc activity was lower in tPEPC-T than in WT-T, but there was no difference in activity between tPEPC-L and WT-L, tPEPC-D and WT-D (Fig. 4c). In addition, PFK activity was higher in tPEPC plants compared to in WT plants under different nitrogen source concentrations. On the contrary, HK was lower in tPEPC plants, especially in tPEPC-L and tPEPC-D plants. PK activity in tPEPC-T plants was lower than that in WT-T plants, whereas it was higher in tPEPC-L and tPEPC-D plants (Fig. 4d). Together, these results suggested that the activity of rate-limiting enzymes in the TCA cycle and glycolysis changed to some extent in tPEPC plants and there were significant changes in CS, PFK and PK activity.
Analysis of organic acid content
Subsequently, we considered whether the content of metabolic intermediate products in the TCA cycle and glycolysis changed correspondingly. We further measured organic acid content in tPEPC and WT plants. Compared with that in WT plants, OAA content was lower in tPEPC-T, while it was significantly higher in tPEPC-D. With the decrease of nitrogen concentration, the OAA contents decreased in WT plants, while they increased in tPEPC plants. The fumaric acid (FA) content in tPEPC-T and tPEPC-D was higher than that in WT-T and WT-D plants. The levels of α-ketoglutaric acid (α-KG) and malic acid (MA) were lower in tPEPC-L and tPEPC-D than in WT-L and WT-D. The citric acid (CA) and succinic acid (SA) content in tPEPC-T were significantly higher than in WT-T, but they were lower in tPEPC-D than in WT-D (Fig. 5a and b). These results indicated that the levels of these organic acids in the TCA cycle changed. Moreover, the changing trends in organic acids in tPEPC plants differed from those in WT plants under different nitrogen source concentrations. In addition, the levels of fructose-1,6 diphosphate (FDP) and pyruvic acid (PA) in tPEPC-T were higher than in WT-T, and lactic acid (LA) levels were higher in tPEPC-L plants (Fig. 5c). There were no differences between the other two comparative groups, which suggested little change in the content of these organic acids in glycolysis in tPEPC plants.
Changes in GSH, amino acid and carbohydrate content
In this study, GST was evidently down-regulated in tPEPC-T according to proteomic analysis. GST plays an important role in cell detoxification and catalyzes the combination of reduced GSH and toxic substances, GSH is a tripeptide synthesized from Glu, Cys and Gly. Subsequently, the content of GSH and the three amino acids were determined in tPEPC and WT plants. Compared with total nutrients, GSH content increased under low nutrient and nitrogen deficiency both in tPEPC and WT plants. Moreover, GSH levels in tPEPC-T and tPEPC-L were higher than those in WT-T and WT-L, respectively (Fig. 6a). This probably implied that GSH accumulated in tPEPC-T and tPEPC-L due to lower GST activity. Glu, Gly and Cys levels in tPEPC-T were significantly higher than in WT-T, while they were lower in tPEPC-D relative to WT-D (Fig. 6b). Additionally, OAA, an important raw material for the TCA cycle, is also the carbon skeleton of Asp and Asn. Asp levels decreased both in WT and tPEPC plants with decreasing nutrient supply. Meanwhile, Asp levels in tPEPC-T were higher than in WT-T but lower in tPEPC than in WT under low nitrogen and nitrogen deficiency (Fig. 6b). This suggested that different levels of Asp occur in WT and tPEPC plants under different nitrogen source concentrations.
As PEPC has an anaplerotic role in the TCA cycle in most non-photosynthetic tissues and C3 plants, it was probably the reason that the carbohydrate content changed in tPEPC plants. We determined the levels of sucrose, starch, soluble sugar in tPEPC and WT plants under different nitrogen source concentrations. The sucrose contents in tPEPC-T and tPEPC-L were about 2.5 times of that in WT-T and WT-L, respectively. Starch and soluble sugar content in tPEPC-T and tPEPC-L were significantly higher than in WT-T and WT-L. By contrast, sucrose, starch and soluble sugar content were no different between tPEPC-D and WT-D. Moreover, sucrose and soluble sugar content increased obviously in WT-D compared with WT-T, while they had a relatively higher level in tPEPC plants and there were no differences between tPEPC-D and tPEPC-T (Fig. 6c). The results indicated that these carbohydrates changed differently between tPEPC and WT with decreasing nutrient supply.
Changes in IAA, ZT and 2iP content
The growth and development of plants were closely related to plant endogenous hormone content. In view of the phenotypic difference between tPEPC and WT plants, we measured the levels of IAA, ZT and 2iP separately in plant aerial parts and roots. The levels of IAA were lower in both aerial parts and roots of tPEPC-T compared with WT-T. While, the levels of IAA were higher in the aerial part of tPEPC-L and tPEPC-D compared with WT-L and WT-D, respectively. The level of IAA was lower in the roots of tPEPC-L compared with WT-L, but there was no change in the IAA level in roots between tPEPC-D and WT-D (Fig. 7a). The ZT content increased in the aerial parts of tPEPC and WT under low nutrient and nitrogen deficiency compared with that under total nutrient. The levels of ZT were lower in the aerial parts of tPEPC-T and tPEPC-L compared with WT-T and WT-L, respectively, but higher in the aerial parts of tPEPC-D than in WT-D. In roots, the levels of ZT were lower in tPEPC-T and tPEPC-D than in WT-T and WT-D, and higher in tPEPC-L than in WT-L (Fig. 7b). Both in aerial parts of tPEPC and WT, the levels of 2ip under low nutrient and nitrogen deficiency were about half of those under total nutrients, and lower in the aerial parts of tPEPC-L than in WT-L. In roots, the level of 2ip in tPEPC-T was only about 35 % of that in WT-T, in tPEPC-L it was about 47 % of that in WT-L, but higher in tPEPC-D than in WT-D (Fig. 7c). It seemed that the levels of these hormones were closely related to nitrogen levels in the culture solution. It was most likely that the different hormone levels induced phenotypic differences between tPEPC and WT plants.
Discussion
PEPC, a key enzyme located in a branch of carbohydrate metabolism in plants, replenishes PEP for the TCA cycle to support anabolism. In this study, transgenic rice expressing C4-PEPC displayed great changes in phenotype, gene expression, proteome, metabolites and hormone content.
Proteomic analysis indicated that GST significantly decreased in tPEPC-T plants. Correspondingly, GST activity was lower in tPEPC-T plants. GST plays an important role in cellular detoxification by conjugating tripeptide GSH (γ-glutamyl-cysteinyl-glycine) with toxic xenobiotic and oxidation products [16,17,18,19,20,21,22]. However, several studies have shown that transgenic rice expressing C4-PEPC were more tolerant to photooxidation and photoinhibition and had enhanced oxidative tolerance and drought tolerance via Ca2+, NO and saccharide responses [23,24,25,26,27,28,29,30]. It is most likely that other defense pathways differed from GST pathways in C4-PEPC-expressing transgenic rice, which is also probably affected by environmental conditions such as nitrogen supply. Notably, GST also participates in modulating plant growth and development [31]. GST interacting with far-red insensitive 219 was involved in the regulation of cell elongation and plant development [32]. Mutation of OsGST4 remarkably inhibited primary root elongation, lateral root formation and shoot growth [15]. In our study, the level of GST was significantly decreased at the protein and transcriptional levels in C4-PEPC transgenic rice under normal conditions. Moreover, GSH, the substrate of GST and its components, Glu, Cys and Gly, accumulated in C4-PEPC transgenic rice, and the C4-PEPC transgenic plants had shorter roots and fewer crown roots, but plant height was taller and shoot length was longer. This suggests that the introduction of C4-PEPC mainly influenced the expression of GST in the roots and affected their growth. However, how C4-PEPC regulates the expression of GST and the mechanisms involved need further research.
PEPC catalyzes PEP, a central intermediate of glycolysis and HCO3−, to yield OAA and replenish the TCA cycle. When the amount of ME transcripts was raised, the activity of ME and PK increased in transgenic potato plants overexpressing PEPC [33]. In this study, the expression of ME, Lox, α-KGDH, ICDHc, HK and PFK genes were up-regulated in C4-PEPC transgenic rice. However, ME, α-KGDH and PFK activity were higher, but CS activity was lower in C4-PEPC transgenic rice than those in WT. These results indicated that the introduction of C4-PEPC induced changes in genes involved in metabolic reactions including the TCA cycle and glycolysis. In addition, the down-regulation of PEPC expression always resulted in changes of OAA, CA, MA and the other intermediates of the TCA cycle [5, 6]. Overexpression of endogenous PEPC in potato plants resulted in an increase in MA, 2-oxoglutarate (2-OG) and OAA [34]. There was a higher level of OAA in transgenic rice expressing maize PEPC [35]. In our study, C4-PEPC transgenic rice had higher levels of FA, CA, SA, FDP and PA, but had a lower level of OAA, which differed from the results of previous research. This is probably because metabolic reactions are a dynamic process, and the results revealed the difference in metabolic intermediate products between C4-PEPC transgenic rice and WT. Moreover, Li and Wang observed that the total soluble sugar in the leaves and grains of transgenic rice expressing maize PEPC significantly increased compared with those in WT [36]. In our study, sucrose, starch and soluble sugar were significantly higher in C4-PEPC transgenic rice than in WT. Taken together, our study revealed that C4-PEPC altered the TCA cycle and glycolysis, and then induced changes in intermediates and metabolites.
PEPC plays an important role in the carbon-nitrogen coupling metabolism. Carbon and nitrogen metabolism were redirected, and amino acid levels increased in PEPC transgenic plants [9, 34, 37,38,39]. PEPC promotes the accumulation of protein in maturing soybean, and there are higher PEPC activity in high-protein cultivar [40]. Tang et al. indicated that transgenic rice overexpressing maize PEPC were more tolerant to low nitrogen stress than WT [7]. However, the down-regulation of PEPC genes suppressed ammonium assimilation and subsequent amino acid synthesis [5, 6]. In our study, the total nitrogen content of transgenic rice was higher at different growth stages and under different nitrogen source concentrations, which reflected the effects of C4-PEPC on nitrogen metabolism. NiR, a type of oxidoreductase, mainly converts NO2− to NH4+ and plays an important role during NO3− conversion [41]. GOGAT, a key enzyme in the Gln synthetase/GOGAT cycle, plays a critical role in NH4+ assimilation [42]. The expression of the NiR and FD-GOGAT genes significantly increased and GOGAT activity was higher in C4-PEPC transgenic rice in this study. Moreover, the variations of gene expression, enzyme activity, organic acids and hormone content under different nitrogen source concentrations were different between transgenic rice and WT, which suggested there was a distinct reaction to nitrogen in C4-PEPC transgenic rice. Glu plays a critical role in amino acid metabolism and functions in a central signaling and metabolic role in the interface of carbon and nitrogen assimilation [43]. In this study, Glu content was significantly higher in C4-PEPC transgenic rice under total nutrients, which was probably beneficial for ammonium assimilation in the Gln synthetase/GOGAT cycle. In addition, Glu is one of the components for GSH, a substrate of GST. Therefore, it is most likely that the C4-PEPC effect on nitrogen metabolism is related to GSH metabolism, and the mechanism of its regulation is worth further study.
In addition, hormones are vital for plant growth and development. Auxin plays an important role in plant developmental processes including cell elongation and division, shoot elongation, vascular differentiation, the regulation of tropistic responses and establishment of apical dominance [44,45,46]. In addition, auxin is essential for plant root development including the formation, initiation and emergence of lateral and adventitious roots, as well as root elongation [47, 48]. Rice mutants of genes involved in auxin signaling pathways showed reduced crown roots or defective root development [49,50,51]. Another important phytohormone is cytokinin, which is a group of phytohormones involved in several developmental and growth processes such as circadian rhythms, root development, germination, shoot development, leaf senescence and so on [52,53,54,55]. Auxin and cytokinin interact in the regulation of root meristem activities [56, 57]. The WUSCHEL-related homeobox (WOX) gene was reported as an integrator of auxin and cytokinin signaling that regulates cell proliferation during crown root development [58, 59]. Auxin-induced termed crown rootless5 (CRL5) promotes crown root initiation through the repression of cytokinin signaling [60]. IAA is the most widespread auxin in plants, and ZT and 2ip are spontaneous and predominant cytokinins in higher plants. In this study, C4-PEPC transgenic rice showed lower IAA, ZT and 2ip levels in roots under total nutrients. Shorter primary roots and fewer crown roots in tPEPC-T plants may be owing to a lower level of these hormones in roots. Nutrients including nitrogen are also important factors influencing plant root architecture, so the roots of tPEPC-L did not show a significant difference though they also had relatively lower IAA levels in roots. In addition, it has been proposed that auxin may modulate GST gene expression or enzyme activity resulting in the alteration of redox potential and then alter gene expression and cellular development [61]. Thus, we speculate that there was an inevitable relation between the lower expression level or enzyme activity of GSTs and lower IAA level in C4-PEPC transgenic rice under total nutrients.
Conclusions
PEPC is involved in plant carbohydrate metabolism and supports carbon-nitrogen interactions. In this study, transgenic rice expressing C4-PEPC exhibited characteristics that differed from WT plants, especially shorter primary root and fewer crown roots. Gene expression, enzyme activity, phytohormone content, several metabolic intermediates and metabolites related to the TCA cycle, glycolysis and nitrogen assimilation changed in transgenic rice under different concentrations of nitrogen source. It was remarkable that proteomic analysis revealed “GSH metabolism” as the most enriched pathway in C4-PEPC transgenic rice. The expression of GSTs significantly decreased at the protein and transcriptional levels. Concomitantly, GST enzyme activity was lower with the accumulation of its substrate GSH in C4-PEPC transgenic rice under normal conditions. Notably, the level of Glu that a component of GSH and a critical role in the GOGAT cycle of ammonium assimilation was higher in transgenic rice. Thus, it is most likely that PEPC regulates GST and then affects nitrogen metabolism, which provides new insight into the effect of PEPC on nitrogen metabolism.
Methods
Plant material
tPEPC and WT plants (indica cultivar Hang2) from Rice Research Institute, Fujian Academy of Agricultural Sciences, were cultured in total nutrient solution (Yoshida) containing a normal level of nitrogen (0.11875 g/L NH4NO3), low nitrogen solution containing trace nitrogen (0.0057 g/L NH4NO3) and nitrogen deficiency solution for 20 d, and used in this study.
Protein extraction, identification and bioinformatics analysis
The protein of samples from three biological replicates was extracted using lysis buffer (10 mM/L dithiothreitol, 8 mol/L urea, 1 % Triton-100 and 1 % protease inhibitor cocktail). Then, the protein concentration was determined using a bicinchoninic acid kit following the manufacturer’s instructions. After trypsin digestion, the peptides were labeled using a TMT kit (Thermo) according to the manufacturer’s protocol, and they were fractionated using high pH reverse-phase high-performance liquid chromatography (HPLC). Then, peptides were separated and identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The data of MS/MS result was processed by the Maxquant search engine (v.1.5.2.8), and the tandem mass spectra were searched using the UniProt database (https://www.uniprot.org/) concatenated with a reverse decoy database. The identified proteins were annotated and classified with GO annotation proteome derived from the UniProt-GOA database (http://www.ebi.ac.uk/GOA/). Protein metabolic pathways were annotated using the KEGG database. GO terms or KEGG pathways. P-values < 0.05 were regarded as significantly enriched, and hierarchical cluster were performed. The protein subcellular localization was predicted by WoLF PSORT subcellular localization prediction software.
qRT-PCR
For qRT-PCR, cDNA was generated from the previously collected RNA using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, LTU). The qRT-PCR was performed with the Fast Start Universal SYBR Green Master system (Roche, USA) on a 7500 real-time PCR system (Applied Biosystems). The relative quantification of gene expression was determined using actin gene expression as a reference and the relative quantitative method (ΔΔCT) was used to evaluate the quantitative variation. Primers used in qRT-PCR are listed in Supplementary Table S1.
Enzyme activity determination
The enzyme activity of GST, GOGAT, PEPC, PDH, ME, CS, α-KGDH, ICDHc, PFK, HK and PK were determined by reagents kits (Suzhou Comin Biotechnology Co., Ltd.) according to the manufacturer’s protocol.
Metabolite assay
Amino acid content was detected by HPLC (Agilent 1100). GSH, sucrose, starch and soluble sugar contents were determined by reagents kits (Suzhou Comin Biotechnology Co., Ltd.) following the manufacturer’s protocol.
IAA, ZT and 2ip assay
IAA, ZT and 2ip contents were determined by HPLC (Agilent 1100).
Statistical analyses
Statistical analyses were performed using one-way ANOVA or Student’s t tests. P-values < 0.05 were considered to indicate statistical significance. Statistical calculations were performed using Microsoft Excel 2019.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PEPC:
-
Phosphoenolpyruvate carboxylase
- WT:
-
Wild type plants
- DEPs:
-
Differentially expressed proteins
- GST:
-
Glutathione S-transferase
- TCA:
-
Tricarboxylic acid
- CS:
-
Citrate synthase
- PFK:
-
6-phosphofructokinase
- PK:
-
Pyruvate kinase
- FD-GOGAT:
-
Ferredoxin-dependent glutamate synthase
- GSH:
-
Glutathione
- Glu:
-
Glutamic acid
- Cys:
-
Cysteine
- Gly:
-
Glycine
- IAA:
-
Indoleacetic acid
- ZT:
-
Zeatin
- 2ip:
-
Isopentenyladenine
- OAA:
-
Oxaloacetate
- Asp:
-
Aspartic acid
- Asn:
-
Asparagine
- Chl a:
-
Chlorophyll a
- FC:
-
Fold changes
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- qRT-PCR:
-
Quantitative real-time PCR
- ME:
-
Malic enzyme
- α-KGDH:
-
α-ketoglutarate dehydrogenase
- ICDHc:
-
Isocitrate dehydrogenase
- HK:
-
Hexokinase
References
Chollet R, Vidal J, O’Leary MH. Phosphoenolpyruvate carboxylase: A ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–98.
O’Leary B, Park J, Plaxton WC. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J. 2011;436:15–34.
Good A, Beatty P. Biotechnological approaches to improving nitrogen use efficiency in plants: alanine aminotransferase as a case study. In: Hawkesford MJ, Barraclough P, editors, The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops. Wiley. 2011; pp. 165–192. https://doi.org/10.1002/9780470960707.ch9.
Champigny ML. Integration of photosynthetic carbon and nitrogen metabolism in higher plants. Photosynth. Res. 1995; 46: 117–127.
Masumoto C, Miyazawa S, Ohkawa H, Fukuda T, Taniguchi Y, Murayama S, Kusano M, Saito K, Fukayama H, Miyao M. Phosphoenolpyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. PNAS. 2010; 107: 5226–5231.
Shi J, Yi K, Liu Y, Xie L, Zhou Z, Chen Y, Hu Z, Zheng T, Liu R, Chen Y, Chen J. Phosphoenolpyruvate carboxylase in Arabidopsis leaves plays a crucial role in carbon and nitrogen metabolism. Plant Physiol. 2015; 167: 671–681.
Tang Y, Li X, Lu W, Wei X, Zhang Q, Lv C, Song N. Enhanced photorespiration in transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase gene contributes to alleviating low nitrogen stress. Plant Physiol Bioch. 2018;130:577–88.
Kandoi D, Mohanty S, Govindjee, Tripathy BC. Towards efficient photosynthesis: overexpression of Zea mays phosphenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth Res. 2016;130:47–72.
Rolletschek H, Borisjuk L, Radchuk R, Miranda M, Heim U, Wobus U, Weber H. Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in vicianarbonensis increases protein content and improves carbon economy. Plant Biotechnol J. 2004;2:211–9.
Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T. Metabolic engineering with dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. PNAS. 2004; 101: 7833–7838.
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J. 2011;9:826–37.
Bao A, Zhao Z, Ding G, Shi L, Xu F, Cai H. Accumulated expression level of cytosolic, glutamine synthetase 1 gene (OsGS1;1 or OsGS1;2) alter plant development and the carbon-nitrogen metabolic status in rice. PLos One. 2014;9:e95581
Bao A, Zhao Z, Ding G, Shi L, Xu F, Cai H. The stable level of glutamine synthetase 2 plays an important role in rice growth and in carbon-nitrogen metabolic balance. Int J Mol Sci. 2015;16:12713–36.
Lian L, Wan X, Zhu Y, He W, Cai Q, Xie H, Zhang M, Zhang J. Physiological and photosynthetic characteristics of indica Hang2 expressing the sugarcane PEPC gene. Mol Biol Rep. 2014;41:2189–97.
Xu N, Chu Y, Chen H, Li X, Wu Q, Liang J, Wang G, Huang J. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging. PLoS Genet. 2018;14:e1007662.
Edwards R, Dixon D, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000; 5(5): 193–198.
Sharma R, Sahoo A, Devendran R, Jain M. Over-expression of a rice tau class glutathione s-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PLoS One. 2014;9:e92900.
Roxas V, Smith R, Allen E, Allen R. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol. 1997;15:988–91.
Toshikazu T, Minako I, Hiroyuki K, Nanako K, Ikuo N. Over-expression of ζ glutathione S-transferase in transgenic rice enhances germination and growth at low temperature. Mol Breed. 2002;9:93–101.
Yu T, Li Y, Chen X, Hu J, Chang X, Zhu Y. Transgenic tobacco plants over expressing cotton glutathione S-transferase (GST) show enhanced resistance to methyl viologen. J Plant Physiol. 2003;160:1305–11.
Zhao F, Zhang H. Salt and paraquat stress tolerance results from co-expression of the Suaeda salsa glutathione S-transferase and catalase in transgenic rice. Plant Cell Tiss Org. 2006; 86: 349–358.
Ding N, Wang A, Zhang X, Wu Y, Cui H, Huang R, Luo Y. Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol. 2017; 17: 225.
Jiao D, Huang X, Li X, Chi W, Kuang T, Zhang Q, Ku M, Cho D. Photosynthetic characteristics and tolerance to photo-oxidation of transgenic rice expressing C4 photosynthesis enzymes. Photosynth Res. 2002;72:85–93.
Jiao D, Li X, Ji B. Photoprotective effects of high level expression of C4 phosphoenolpyruvate carboxylase in transgenic rice during photoinhibition. Photosynthetica. 2005; 43: 501–508.
Gu J, Qiu M, Yang J. Enhanced tolerance to drought in transgenic rice plants overexpressing C4 photosynthesis enzymes. CROP J. 2013; 1: 105–114.
Qian B, Li X, Liu X, Wang M. Improved oxidative tolerance in suspension-cultured cells of C4-pepctransgenic rice by H2O2 and Ca2+under PEG-6000. J Integr Plant Biol. 2014;57:534–49.
Qian B, Li X, Liu X, Chen P, Ren C, Dai C. Enhanced drought tolerance in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene via NO and Ca2+. J Plant Physiol. 2015;175:9–20.
Zhang C, Li X, He Y, Zhang J, Yan T, Liu X. Physiological investigation of C4-phosphoenolpyruvate-carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance. Plant Physiol Biochem. 2017;115:328–42.
Liu X, Li X, Zhang C, Dai C, Zhou J, Ren C, Zhang J. Phosphoenolpyruvate carboxylase regulation in C4-PEPC-expressing transgenic rice during early responses to drought stress. Physiol Plant. 2017;159:178–200.
Liu X, Li X, Dai C, Zhou J, Yan J. Improved short-term drought response of transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase via calcium signal cascade. J Plant Physiol. 2017;218:206–21.
Vaish S, Gupta D, Mehrotra R, Mehrotra S, Basantani MK. Glutathione S-transferase: a versatile protein family. 3 Biotech 2020;10: 321.
Chen I, Huang I, Liu M, Wang Z, Chung S, Hsien H. Glutathione S-transferase interacting with far-red insensitive 219 is involved in phytochrome A-mediated signaling in Arabidopsis. Plant Physiol. 2007; 143: 1189–1202.
Häusler R, Rademacher T, Li J, Lipka V, Fischer K, Schubert S, Kreuzaler F, Hirsch H. Single and double overexpression of C4-cycle genes had differential effects on the pattern of endogenous enzymes, attenuation of photorespiration and on contents of UV protectants in transgenic potato and tobacco plants. J Exp Bot. 2001;52:1785–803.
Rademacher T, Häusler R, Hirsch H, Zhang L, Lipka V, Weier D, Kreuzaler F, Peterhänsel C. An engineered phosphoenolpyruvate carboxylase redirects carbon and nitrogen flow in transgenic potato plants. Plant J. 2002; 32: 25–39.
Fukayama H, Hatch M, Tamai T, Tsuchida H, Sudoh S, Furbank R, Mitsue M. Activity regulation and physiological impacts of maize C4-specific phosphoenolpyruvate carboxylase overproduced in transgenic rice plants. Photosynth Res. 2003;77:227–39.
Li X, Wang C. Physiological and metabolic enzymes activity changes in transgenic rice plants with increased phosphoenolpyruvate carboxylase activity during the flowering stage. Acta Physiol Plant. 2013;35:1503–12.
Gehlen J, Panstruga R, Smets H, Merkelbach S, Kleines M, Porsch P, Fladung M, Becker I, Rademacher T, Häusler E, Hirsch HJ. Effects of altered PEPC on transgenic C3 plant Solanum tuberosum. Plant Mol Biol. 1996;32:831–48.
Häusler R, Kleines M, Uhrig H, Hirsch H, Smets H. Overexpression of PEPC from Corynebacterium glutamicum lowers the CO2 compensation point (г*) and enhances dark and light respiration in transgenic potato. J Exp Bot. 1999;50:1231–42.
Wang L, He M, Chen S, Wang K, Cui D, Huang X, Liu L. Overexpression of PtPEPCK1 gene promotes nitrogen metabolism in poplar. J For Res. 2019;30:2289–303.
Yamamoto N, Masumura T, Kentaro Y, Suginoto T. Pattern analysis suggests that phosphoenolpyruvate carboxylase in maturing soybean seeds promotes the accumulation of protein. Biosci Biotech Bioch. 2019;83:2238–43.
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105:1141–57.
Suzuki A, Knaff D. Glutamate synthase: structural, mechanistic and regulatory properties, and role in the amino acid metabolism. Photosyn Res. 2005;83:191–217.
Forde B, Lea P. Glutamate in plants: metabolism, regulation, and signalling. J Exp Bot. 2007;58:2339–58.
Walker L, Estelle M. Molecular mechanisms of auxin action. Curr Opin Plant Biol. 1998;1:434–9.
Gallei M, Luschnig C, Friml J. Auxin signalling in growth: Schrödinger’s cat out of the bag. Curr Opin Plant Biol. 2020;53:43–9.
Du M, Spalding EP, Gray WM. Rapid auxin-mediated cell expansion. Annu Rev Plant Biol. 2020; 71: 379–402.
Yu J, Zhang Y, Liu W, Wang H, Wen S, Zhang Y, Xu L. Molecular evolution of auxin-mediated root initiation in plants. Mol Biol Evol. 2020; 37: 1387–1393.
Guan L, Tayengwa R, Cheng Z, Peer W, Murphy A, Zhao M. Auxin regulates adventitious root formation in tomato cuttings. BMC Plant Biol. 2019; 19: 435.
Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. Crown rootless1, which is essential for crown root formation in rice, is a target of an auxin response factor in auxin signaling. Plant Cell. 2005; 17: 1387–1396.
Wang X, He F, Ma X, Mao C, Hodgman C, Lu C, Wu P. OsCAND1 is required for crown root emergence in rice. Mol Plant. 2011;4:289–99.
Wang S, Xu, Y, Li Z, Zhang S, Lim J, Lee K, Li C, Qian Q, Jiang D, Qi Y. OsMOGS is required for N-glycan formation and auxin-mediated root development in rice (Oryza sativa L.). Plant J. 2014; 78: 632–645.
Riefler M, Novak O, Strnad M, Schmullin T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. 2006; Plant Cell 18: 40–54.
Hanano S, Domagalska M, Nagy F, Davis S. Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells. 2006;11:1381–92.
Tessi TM, Brumm S, Winklbauer E, Schumacher B, Pettinari G, Lescano I, González CA, Wanke D, Maurino VG, Harter K, Desimone M. Arabidopsis AZG2 transports cytokinins in vivo and regulates lateral root emergence. New Phytol. 2021; 229: 979–993.
Li S, Zheng H, Zhang X, Sui N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021; 40: 271–282.
Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008; 453: 1094–1097.
Su Y, Liu Y, Zhang X. Auxin-cytokinin interaction regulates meristem development. Mol Plant. 2011;4:616–25.
Zhao Y, Hu Y, Dai M, Huang L, Zhou D. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009; 21: 736–748.
Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou D. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell. 2015; 27: 2469–2483.
Kitomi Y, Ito H, Hobo T, Aya K, Kitano H, Inukai Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OSRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011; 67: 472–484.
Jones A. Auxin-binding proteins. Annu. Rev. Plant Physiol. Plant Mol Biol. 1994;45:393–420.
Acknowledgements
Not applicable.
Statement
The study protocol complies with relevant institutional, national, and international guidelines and legislation. We have permission to collect “Oryza sativa” materials in this study.
Funding
This work was funded by the National Key Research and Development Program of China (2017YFD0100100), the Open Research Fund of State Key Laboratory of Hybrid Rice (Hunan Hybrid Rice Research Center) (2019KF10) and the Cultivation Project for National Natural Science Foundation of Fujian Academy of Agricultural Sciences (GJPY2019005).
Author information
Authors and Affiliations
Contributions
JFZ and LL conceived and designed the research. LL, YLL, YDW, WH1, QHC, WH2, YMZ, FXW, YSZ and XL performed all the experiments. HBX analyzed the data. HAX guided and supported the research. LL wrote the manuscript. JFZ revised the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Determination of total nitrogen content. a Total nitrogen content at seeding stage. b Total nitrogen content at the tillering stage.
Additional file 2: Figure S2.
Chlorophyll (Chl) and carotenoid (Car x) content, and root dehydrogenase activity in 6-day-old seedlings. a Phenotype of 6-day-old seedings, b Root dehydrogenase activity. c Chl and Car x content of leaf.
Additional file 3: Figure S3.
GO classifications of DEPs in a tPEPC-T/WT-T, b tPEPC-L/WT-L and c tPEPC-D/WT-D.
Additional file 4: Figure S4.
Subcellular localization map of DEPs in a tPEPC-T/WT-T, b tPEPC-L/WT-L and c tPEPC-D/WT-D.
Additional file 5: Figure S5.
GO enrichment analysis of DEPs in a tPEPC-T/WT-T, b tPEPC-L/WT-L and c tPEPC-D/WT-D.
Additional file 6: Figure S6.
KEGG enrichment analysis of DEPs in a tPEPC-T/WT-T, b tPEPC-L/WT-L and c tPEPC-D/WT-D.
Additional file 7: Table S1.
Primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lian, L., Lin, Y., Wei, Y. et al. PEPC of sugarcane regulated glutathione S-transferase and altered carbon–nitrogen metabolism under different N source concentrations in Oryza sativa. BMC Plant Biol 21, 287 (2021). https://doi.org/10.1186/s12870-021-03071-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-021-03071-w