Abstract
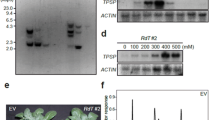
Phosphoenolpyruvate carboxylase (PEPC) genes from Corynebacterium glutamicum (cppc), Escherichia coli (eppc) or Flaveria trinervia (fppc) were transferred to Solanum tuberosum. Plant regenerants producing foreign PEPC were identified by Western blot analysis. Maximum PEPC activities measured in eppc and fppc plants grown in the greenhouse were doubled compared to control plants. For cppc a transgenic plant line could be selected which exhibited a fourfold increase in PEPC activity. In the presence of acetyl-CoA, a known activator of the procaryotic PEPC, a sixfold higher activity level was observed. In cppc plants grown in axenic culture PEPC activities were even higher. There was a 6-fold or 12-fold increase in the PEPC activities compared to the controls measured in the absence or presence of acetyl-CoA, respectively. Comparable results were obtained by transient expression in Nicotiana tabacum protoplasts. PEPC of C. glutamicum (PEPC C.g.) in S. tuberosum leaf extracts displays its characteristic K m(PEP) value. Plant growth was examined with plants showing high expression of PEPC and, moreover, with a plant cell line expressing and antisense S. tuberosum (anti-sppc) gene. In axenic culture the growth rate of a cppc plant cell line was appreciably diminished, whereas growth rates of an anti-sppc line were similar or slightly higher than in controls. Malate levels were increased in cppc plants and decreased in antisense plants. There were no significant differences in photosynthetic electron transport or steady state CO2 assimilation between control plants and transformants overexpressing PEPC C.g. or anti-sppc plants. However, a prolonged dark treatment resulted in a delayed induction of photosynthetic electron transport in plants with less PEPC. Rates of CO2 release in the dark determined after a 45 min illumination period at a high proton flux density were considerably enhanced in cppc plants and slightly diminished in anti-sppc plants. When CO2 assimilation rates were corrected for estimated rates of mitochondrial respiration in the light, the electron requirement for CO2 assimilation determined in low CO2 was slightly lower in transformants with higher PEPC, whereas transformants with decreased PEPC exhibited an appreciably elevated electron requirement. The CO2 compensation point remained unchanged in plants (cppc) with high PEPC activity, but might be increased in an antisense plant cell line. Stomatal opening was delayed in antisense plants, but was accelerated in plants overexpressing PEPC C.g. compared to the controls.
Similar content being viewed by others
Abbreviations
- Г:
-
CO2 compensation point
- ФCO2 :
-
quantum efficiency of CO2 assimilation
- ФPSII :
-
quantum efficiency of photosystem II electron transport
- A:
-
CO2 assimilation rate
- Ci :
-
intercellular CO2 concentration; e, electron
- PFD:
-
photon flux density
- QA :
-
primary quinone electron acceptor of photosystem II
- QN :
-
non-photochemical chlorophyll a fluorescence quenching
- qP :
-
photochemical chlorophyll a fluorescence quenching
References
Andreo CS, Gonzales DH, Iglesias AA: Higher plant phosphoenolpyruvate carboxylase: Structure and regulation. FEBS Lett 213: 1–8 (1987).
Andrews M: The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ 9: 511–519 (1986).
Arnon DJ: Copper enzymes in chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 (1949).
Bakrim N, Echevarria C, Cretin C, Arrio-Dupont M, Pierre JN, Vidal J, Chollet R, Gadal P: Regulatory phosphorylation of Sorghum leaf phosphoenolpyruvate carboxylase. Identification of the protein-serine kinase and some elements of the signal-transduction cascade. Eur J Biochem 204: 821–830 (1992).
Bandurski RS, Greiner CM: The enzymatic synthesis of oxaloacetate from phosphoenolpyruvate and carbon dioxide. J Biol Chem 204: 781–789 (1953).
Bergmeyer HU: Methoden der Enzymatischen Analyse. Verlag 092 Chemie, Weinheim, pp. 1491–1496 (1974).
Champigny ML, Brauer B, Bismuth E, Manh CT, Siegl G, Quy VLV, Stitt M: The short-term effect of NO3 - and NH3 assimilation on sucrose synthesis in leaves. J Plant Physiol 139: 361–368 (1992).
Cooley B, Reiskind JB, Bowes G: Time course for the induction of C4 photosynthetic enzymes in Hydrilla. Plant Physiol (Suppl) 105: 84 (1994).
Demmig B, Winter K, Krüger A, Czygan F-C: Photoinhibition an zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84: 218–224 (1987).
Duff SMG, Chollet R: In vivo regulation of wheat-leaf phosphoenolpyruvate carboxylase by reversible phosphorylation. Plant Physiol 107: 775–782 (1995).
Duffus CM, Rosie R: Some enzyme activities associated with the chlorophyll containing layers of the immature barley pericarp. Planta 114: 219–226 (1973).
Eikmanns BJ, Follettie MT, Griot MU, Sinskey AJ: The phosphoenolpyruvate carboxylase gene of Corynebacterium glutamicum: molecular cloning, nucleotide sequence and expression. Mol Gen Genet 218: 330–339 (1989).
Fujita N, Miwa T, Ishijama S, Izuki K, Katzuki H: The primary structure of phosphoenolpyruvate carboxylase of Escherichia coli. Nucleotide sequence of the ppc gene and deduced amino acid sequence. J Biochem 95: 909–916 (1984).
Genty B, Briantais JM, Baker NR: The relationship between the quantum yield of photosynthetic transport and quenching of Chl fluorescence. Biochim Biophys Acta 990: 87–92 (1989).
Graan T, Ort DR: Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem 259: 14003–14010 (1984).
Hedley CL, Harvey DM, Keely RJ: Role of PEP carboxylase during seed development in Pisum sativum. Nature 258: 352–354 (1975).
Hedrich R, Marten I: Malate induced feedback regulation of plasma membrane anion channels would provide a CO2 sensor to guard cells. EMBO J 12: 897–901 (1993).
Henderson PJF: Statistical analysis of enzyme kinetic data. In: Eisenthal R, Danson MJ (eds) Enzyme Assays: A practical approach, pp. 277–316. IRL Press, London (1992).
Höfgen R, Willmitzer L: Storage of competent cells for Agrobacterium transformation. Nucl Acids Res 16: 9877 (1988).
Holaday AS, Salvucci ME, Bowes G: Variable photosynthesis/photorespiration ratios in Hydrilla and other submersed aquatic macrophyte species. Can J Bot 61: 229–236 (1983).
Hudspeth RL, Grula JW, Dai Z, Edwards GE, Ku MSB: Expression of maize phosphoenolpyruvate carboxylase in transgenic tobacco. Plant Physiol 98: 458–464 (1992).
Izui K, Sabe H, Katsuki H: Increased synthesis of phosphoenolpyruvate carboxylase in a strain of Escherichia coli bearing a ColEl-ppc+ hybrid plasmid. FEBS Lett 133: 311–315 (1981).
Jiao JA, Cholett R: Posttranslational regulation of phosphoenolpyruvate carboxylase in C4 and crassulacean acid metabolism plants. Plant Physiol 95: 981–985 (1991).
Kaiser WM, Brendle-Behnisch E: Acid-base-modulation of nitrate reductase in leaf tissues. Planta 196: 1–6 (1995).
Kaiser WM, Förster J: Low CO2 prevents nitrate reduction in leaves. Plant Physiol 91: 970–974 (1989).
Kogami H, Shono M, Koike T, Yanagisawa S, Izui K, Sentoku N, Tanifuji S, Uchimiya H, Toki S: Molecular and physiological evaluation of transgenic tobacco plants expressing a maize phosphoenolpyruvate carboxylase gene under the control of the cauliflower mosaic virus 35S promoter. Transgen Res 3: 287–296 (1994).
Koncz C, Schell J: The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 (1986).
Krall JP, Edwards GE: PEP carboxylases from two C4 species of Panicum with markedly different susceptibilities to cold inactivation. Plant Cell Physiol 34: 1–11 (1993).
Krause GH, Weis E: Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349 (1991).
Krenzer EGJr, Moss DN, Crookstone RK: Carbon dioxide compensation points of flowering plants. Plant Physiol 56: 194–206 (1975).
Ku S-B, Shieh Y-J, Reger BJ, Black CC: Photosynthetic characteristics of Portulaca grandiflora, a succulent C4 dicot. Plant Physiol 68: 1073–1080 (1981).
Latzko E, Kelly GJ: The many-faceted function of phosphoenolpyruvate carboxylase in C3 plants. Physiol Veg 21: 805–815 (1983).
Laval-Martin D, Farineau J, Diamond J: Light versus dark carbon metabolism in cherry tomato fruits. Plant Physiol 60: 872–876 (1977).
Lowry OH, Passoneau JV: in: A Flexible System of Enzymatic Analysis, pp. 201–202. Academic Press, New York (1992).
Martinoia E, Rentsch D: Malate compartmentation: responses to a complex metabolism. Annu Rev Physiol Plant Mol Biol 45: 447–467 (1994).
McNaughton GAL, Fewson CA, Wilkins MB, Nimmo HG: Purification, oligomerization state and malate sensitivity of maize leaf phosphoenolpyruvate carboxylase. Biochem J 261: 349–355 (1989).
Melzer E, O'Leary H: Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol 84: 58–60 (1987).
Merkelbach S, Gehlen J, Denecke M, Hirsch HJ, Kreuzaler F: Cloning, sequence analysis and expression of a cDNA encoding active phosphoenolpyruvate carboxylase of the C3 plant Solanum tuberosum. Plant Mol Biol 23: 881–888 (1993).
Nakamoto H, Ku MSB, Edwards GE: Photosynthetic characteristics of C3–C4 intermediate Flaveria species. II. Kinetic properties of phosphoenolpyruvate carboxylase from C3, C4 and C3–C4 intermediate species. Plant Cell Physiol 24: 1387–1393 (1983).
Negruitu I, Shillito R, Potrykus I, Biasini G, Sala F: Hybrid genes in the analysis of transformation conditions: I. Setting up a simple method for direct gene transfer in plant protoplasts. Plant Mol Biol 8: 363–373 (1987).
Nishikido T, Wanda T: Comparative studies of NADP-ME-enzyme from C3 and C4 plants. Biochem Biophys Res Com 61: 243–249 (1979).
Oberhuber W, Dai Z, Edwards E: Light dependence of quantum yield of photosystem II and CO2 fixation in C3 and C4 plants. Photosyn Res 35: 265–274 (1993).
Poetsch W, Hermans J, Westhoff P: Multiple cDNAs of phosphoenolpyruvate carboxylase in the C4 dicot Flaveria trinervia. FEBS Lett 292: 133–136 (1991).
Potrykus I, Shillito R: Protoplasts: Isolation, culture, plant regeneration. Meth Enzymol 118: 549–578 (1986).
Raschke K, Hedrich R, Reckmann U, Schroeder JI: Exploring biophysical and biochemical components of the osmotic motor that drives stomatal movement. Bot Acta 101: 283–294 (1988).
Reimold U, Kröger M, Kreuzaler F, Hahlbrock K: Coding and 3′ non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J 2: 1801–1805 (1983).
Sabe H, Miwa T, Kodaki T, Izui K, Hiraga S, Katsuki H: Molecular cloning of the phosphoenolpyruvate carboxylase gene, ppc, of Escherichia coli. Gene 31: 279–283 (1984).
Salvucci ME, Bowes G: Two photosynthetic mechanisms mediating the low photorespiratory state in submersed aquatic angiosperms. Plant Physiol 73: 488–496 (1983).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sayre RT, Kennedy RA: Photosynthetic enzymatic activities and localization in Mollugo verticillata populations differing in the levels of C3 and C4 cycle operation. Plant Physiol 64: 293–299 (1979).
Schreiber U, Schliwa U, Bilger B: Continuous recording of photochemical and non-photochemical Chl fluorescence quenching with a new type of modulation fluorometer. Photosyn Res 10: 51–62 (1986).
Teraoka H, Izui K, Katsuki H: Phosphoenolpyruvate carboxylate of Escherichia coli. Multiple conformational states elicited by allosteric effectors. Biochemistry 13: 5121–5128 (1974).
Thi Manh C, Bismuth E, Boutin J-P, Provot M, Champigny M-L: Metabolic effectors for short-term nitrogen-dependent enhancement of phosphoenolpyruvate carboxylase activity and decrease of net sucrose synthesis in wheat leaves. Physiol Plant 89: 460–466 (1993).
Van Quy L, Foyer C, Champigny M-J: Effect of light and NO- 3 on wheat leaf phosphoenolpyruvate carboxylase activity. Evidence for covalent modulation of the C3 enzyme. Plant Physiol 97: 1476–1482 (1991).
Van Quy L, Lamaze T, Champigny M-L: Short term effects of nitrate on sucrose synthesis in wheat leaves. Planta 185: 53–57 (1991).
Vanlerberghe GC, Schuller KA, Smith RG, Feil R, Plaxton WC, Turpin DH: Relationship between NH+ 4 assimilation rate and in vivo phosphoenolpyruvate carboxylase activity. Regulation of anaplerotic carbon flow in the green alga Selenastrum minutum. Plant Physiol 94: 284–290 (1990).
vanCaemmerer S, Farquhar GD: Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 (1981).
Wirth E, Kelly GJ, Fischbeck G, Latzko E: Enzyme activities and products of CO2 fixation in various photosynthetic organs of wheat and oat. Z Pflanzenphysiol 82: 78–87 (1977).
Zhang XQ, Li B, Chollet B: In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol 108: 1561–1568 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gehlen, J., Panstruga, R., Smets, H. et al. Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum . Plant Mol Biol 32, 831–848 (1996). https://doi.org/10.1007/BF00020481
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020481