Abstract
Background
Hematoma expansion can be related to increased mortality and poor clinical outcomes in patients with intracerebral hemorrhage (ICH). So, early identification and prevention of hematoma expansion can be considered as an important therapeutic aim. This study aimed to evaluate the hypothesis that the neutrophil to lymphocyte ratio (NLR) is associated with hematoma expansion in ICH patients.
Methods
We retrospectively evaluated the clinical data of a total of 221 patients with ICH who were treated in our department between April 2018 and April 2021. The demographic, clinical, radiological, and laboratory test data including the NLR upon admission were investigated. A binary logistic regression analysis was used to assess the independent associations between different variables and hematoma expansion.
Results
A total of 221 patients with ICH were included. There were 122 (55.2%) males and 99 (44.8%) females. The mean age (years) at admission was 66.43 ± 8.28.
The hematoma expansion occurred in 57 (25.8%) cases. The results of the multivariate analysis showed that hematoma volume at baseline (OR, 3.12; 95% CI 1.78–5.02; P < 0.001), admission systolic blood pressure (OR, 2.87; 95% CI 1.79–4.34; P = 0.013), Glasgow Coma Scale (GCS) (OR, 1.94; 95% CI 1.45–2.93; P = 0.020), and NLR (OR, 1.74; 95% CI 1.16–2.60; P = 0.032) were correlated with hematoma expansion in these patients.
Conclusions
Our findings suggest that NLR can be a predictor of hematoma expansion in patients with ICH. This cost-effective and easily available biomarker could be used to early prediction of hematoma expansion in these patients.
Similar content being viewed by others
Introduction
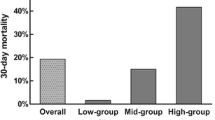
Intracerebral hemorrhage (ICH) is one of the most common causes of morbidity and mortality throughout the world with an estimated 35–52% rate of 30-day mortality [1, 2].
Hematoma expansion occurs in approximately 30% of ICH cases within the first 24 h [1, 3]. It has been demonstrated that hematoma expansion is related to increased mortality and poor clinical outcomes [4, 5]. As a result, early identification and prevention of hematoma expansion can be considered an important therapeutic aim.
Several factors have been known as the predictors of hematoma expansion including the size and location of the hematoma, elevated systolic blood pressure, the presence of coagulopathy, and the presence of systemic inflammatory response syndrome (SIRS) during hospitalization [6,7,8].
Some studies have suggested absolute and differential leukocyte counts as a marker for central nervous system inflammation [9]. The inflammatory response can lead to a series of neurochemical cascade events including alteration in cerebral blood flow, breakdown of the blood–brain barrier, dysfunction of brain tissue metabolism, and cell damage [10, 11]. In a retrospective study, Chen et.al investigated the predictive value of NLR for the prognosis of patients with severe traumatic brain injury (TBI). Their results showed that the baseline NLR was significantly higher in the unfavorable outcome group than in the favorable outcome group and the higher NLR was related to an unfavorable outcome. It has been shown that elevated neutrophil–lymphocyte ratio (NLR) in patients with ICH can be associated with subsequent neurologic deterioration, higher 30-day mortality, and stroke severity [9, 12].
The present study aimed to evaluate the relationship between NLR and hematoma expansion.
Methods
All consecutive patients with spontaneous ICH presenting to Imam Reza hospital, Kermanshah, Iran from April 2018 and April 2021 were investigated retrospectively. We included all ICH patients who had a primary spontaneous ICH and at least two head CTs obtained within the first 24 h of admission. The exclusion criteria of the study were: age less than 18 at admission, secondary causes of ICH (i.e., trauma, aneurysms, tumors, and arteriovenous malformations), history of anticoagulant medications, conditions with associated leukocytosis, such as infection and hematologic malignancies. This study was approved by the Scientific Research Board of the Kermanshah University of Medical Sciences.
The demographic, clinical, radiological, and laboratory test data were extracted from hospital medical records.
We determined the location of hematoma according to the initial brain CT scan of all patients and divided the location of the hematoma into four categories including lobar, deep, cerebellar, and brain stem.
The hematoma volume was calculated according to the ellipsoid formula (4/3 π a × b × c), where a, b, and c represents the respective radii in 3-dimensional neuroimaging [13].
Hematoma expansion was defined as relative enlargement > 33% or absolute growth > 6 mL [4].
We evaluated the clinical outcome at the time of hospital discharge using the Glasgow Outcome Scale (GOS) [14]. The GOS measures global functioning with five outcome categories: (1) death, (2) persistent vegetative state, (3) severe disability, (4) moderate disability, and (5) good recovery. We classified the GOS groups in binary categories: favorable (GOS 4, 5) and unfavorable (GOS 1, 2, 3).
Blood sampling was attended on admission. Neutrophil and lymphocyte counts were collected based on the peripheral hemogram which was evaluated using venous blood samples by an automated blood counter (XN-10, Sysmex Inc., Japan).
We calculated NLR by dividing the absolute neutrophil count by the lymphocyte count.
Statistical analysis
Data are presented as mean ± standard deviation. The independent t-test, the Chi-square test, and Fisher’s exact test were used to compare different variables between the groups. A binary logistic regression analysis was used to assess the independent associations between different variables and hematoma expansion. The data analysis was performed using the SPSS 21 software (SPSS Inc. Chicago, Illinois). P values < 0.05 were considered as the significant level.
Results
We investigated a total of 221 patients with ICH. There were 122 (55.2%) males and 99 (44.8%) females. The mean age (years) at admission was 66.43 ± 8.28. The hematoma expansion occurred in 57 (25.8%) cases. The descriptive characteristics of the sample are presented in Tables 1 and 2.
Patients with hematoma expansion had a worse outcome in comparison with those without hematoma expansion (p < 0.05; Table 3). As shown in Table 3 the need for surgery was higher in the patients in the hematoma expansion group compared to cases in the non-hematoma expansion group (p < 0.05; Table 3).
According to the univariate analysis, GCS, hematoma volume at baseline, admission systolic blood pressure, the baseline neutrophil count, and the baseline NLR were associated with hematoma expansion in patients with ICH (p < 0.05; Tables 3, 4).
The results of the multivariate analysis showed that hematoma volume at baseline (OR, 3.12; 95% CI 1.78–5.02; P < 0.001), admission systolic blood pressure (OR, 2.87; 95% CI 1.79–4.34; P = 0.013), GCS (OR, 1.94; 95% CI 1.45–2.93; P = 0.020), and NLR (OR, 1.74; 95% CI 1.16–2.60; P = 0.032) were correlated with hematoma expansion in these patients (Table 5).
Discussion
The results of the present study show that baseline NLR can be correlated with 24-h hematoma expansion after ICH. It has been demonstrated that the inflammatory response after ICH can lead to peripheral leukocytosis. The hemorrhage leads to microglial activation and as a result secrete cytokines and chemokines that can promote leukocyte infiltration within hours.
Some studies showed that astrocytes shed extracellular vesicles which regulate peripheral leukocyte response in response to brain inflammation.
The inflammatory response can result in a series of neurochemical cascade events including alteration in cerebral blood flow, breakdown of the blood–brain barrier, dysfunction of brain tissue metabolism, and cell damage [10, 11]. The NLR is considered as a nonspecific marker of systemic inflammation [15]. Elevated NLR has been found to be related to poor prognosis in patients with ICH and those with traumatic brain injury [16,17,18]. Jamali et al. in their retrospective study found that an NLR > 12.5 at admission can predict higher mortality in patients with aneurysmal subarachnoid hemorrhage [19]. In another study, Chen et al. evaluated the relationship between peak NLR and clinical outcomes of patients with severe TBI. They reported that peak NLR can be a predictor for unfavorable outcomes after severe TBI [16].
Neutrophils are the major component of the innate immune system that play a major role in mediating inflammation-induced injury [20,21,22]. It has been demonstrated that, more than inflammation-related cytokines, neutrophils also contain angiogenic and neurotrophic factors [11, 23, 24].
Moreover, neutrophils are associated with vascular dysfunction that leads to cerebral hypoperfusion [25]. The hypoperfusion may lead to an increase in the interactions of neutrophils with blood vessels by inducing the expression of l-selectin and intercellular adhesion molecule 1 in endothelial cells [26]. So, neutrophils may affect microcirculation rheology as well as the sustained pressure of the microvasculature [27]. Neutrophils could alter cerebral blood flow by forming pseudopods and adhering to the endothelium and platelets [28].
As mentioned above, the inflammatory response could be associated with hematoma expansion. So, the peripheral leukocyte counts may help in predicting hematoma expansion after ICH.
Limitations
The present study has several limitations. It is a retrospective single-center study with relatively small sample size. Bias in terms of data selection and analysis due to the retrospective nature of the study may be considered as another limitation of our study.
Meanwhile, we evaluated hematoma expansion only up to the first 24 h, whereas it is known that hematoma expansion may evolve beyond this time frame [29]. Finally, we did not have data on body temperature and osmotherapy, both may be related to hematoma expansion [30].
Conclusions
Our findings suggest that NLR can be a predictor of hematoma expansion in patients with ICH. Further studies are warranted to understand the association between NLR and hematoma expansion. However, this cost-effective and easily available biomarker, along with previously established variables, could be used to early prediction of hematoma expansion in patients with ICH.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due them containing information that could compromise research participant privacy/consent but are available from the corresponding author on reasonable request.
Abbreviations
- NLR:
-
Neutrophil to lymphocyte ratio
- ICH:
-
Intracerebral hemorrhage
- GOS:
-
Glasgow Outcome Scale
- SIRS:
-
Systemic inflammatory response syndrome
References
Davis S, Broderick J, Hennerici M, Brun N, Diringer M, Mayer S, et al. Recombinant activated factor VII intracerebral hemorrhage trial investigators: hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8):1175–81.
Roh D, Sun C-H, Murthy S, Elkind MS, Bruce SS, Melmed K, et al. Hematoma expansion differences in lobar and deep primary intracerebral hemorrhage. Neurocrit Care. 2019;31(1):40–5.
Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Opin Crit Care. 2004;10(2):94–100.
Dowlatshahi D, Demchuk A, Flaherty M, Ali M, Lyden P, Smith E. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238–44.
Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38(4):1257–62.
Melmed KR, Carroll E, Lord AS, Boehme AK, Ishida K, Zhang C, et al. Systemic inflammatory response syndrome is associated with hematoma expansion in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(8): 105870.
Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158–64.
Melmed KR, Lyden P, Gellada N, Moheet A. Intracerebral hemorrhagic expansion occurs in patients using non-vitamin K antagonist oral anticoagulants comparable with patients using warfarin. J Stroke Cerebrovasc Dis. 2017;26(8):1874–82.
Walsh KB, Sekar P, Langefeld CD, Moomaw CJ, Elkind MS, Boehme AK, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. 2015;46(8):2302–4.
Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Investig. 2010;120(5):1368–79.
Liu Y-W, Li S, Dai S-S. Neutrophils in traumatic brain injury (TBI): friend or foe? J Neuroinflamm. 2018;15(1):1–18.
Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47(6):1654–7.
Togha M, Bakhtavar K. Factors associated with in-hospital mortality following intracerebral hemorrhage: a three-year study in Tehran, Iran. BMC Neurol. 2004;4(1):9.
Jennett B, Snoek J, Bond M, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285–93.
Ashizawa N, Furuya S, Katsutoshi S, Sudo M, Akaike H, Hosomura N, et al. Clinical significance of dynamic neutrophil–lymphocyte ratio changes in patients with colorectal cancer. Anticancer Res. 2020;40(4):2311–7.
Chen J, Qu X, Li Z, Zhang D, Hou L. Peak neutrophil-to-lymphocyte ratio correlates with clinical outcomes in patients with severe traumatic brain injury. Neurocrit Care. 2019;30(2):334–9.
Sabouri E, Jangjui P, Rahigh-Aghasan S, Alavi SAN. Neutrophil-to-lymphocyte ratio and traumatic brain injury: a review study. World Neurosurg. 2020;140:142–7.
Chen W, Yang J, Li B, Peng G, Li T, Li L, et al. Neutrophil to lymphocyte ratio as a novel predictor of outcome in patients with severe traumatic brain injury. J Head Trauma Rehabilit. 2018;33(1):E53–9.
Jamali SA, Turnbull MT, Kanekiyo T, Vishnu P, Zubair AC, Raper CC, et al. Elevated neutrophil–lymphocyte ratio is predictive of poor outcomes following aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(4): 104631.
Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–88.
Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–31.
Mortaz E, Zadian SS, Shahir M, Folkerts G, Garssen J, Mumby S, et al. Does neutrophil phenotype predict the survival of trauma patients? Front Immunol. 2019;10:2122.
Werner JK, Stevens RD. Traumatic brain injury: recent advances in plasticity and regeneration. Curr Opin Neurol. 2015;28(6):565–73.
Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8(2):101–5.
Palmer C, Roberts RL, Young PI. Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic-ischemic brain injury. Pediatr Res. 2004;55(4):549–56.
Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood. 2005;106(2):584–92.
Roca-Cusachs P, Almendros I, Sunyer R, Gavara N, Farré R, Navajas D. Rheology of passive and adhesion-activated neutrophils probed by atomic force microscopy. Biophys J. 2006;91(9):3508–18.
Zhang X, Cheng R, Rowe D, Sethu P, Daugherty A, Yu G, et al. Shear-sensitive regulation of neutrophil flow behavior and its potential impact on microvascular blood flow dysregulation in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2014;34(3):587–93.
Gusdon AM, Gialdini G, Kone G, Baradaran H, Merkler AE, Mangat HS, et al. Neutrophil–lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. 2017;48(9):2589–92.
Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, et al. Targeting secondary injury in intracerebral haemorrhage—perihaematomal oedema. Nat Rev Neurol. 2015;11(2):111–22.
Acknowledgements
We appreciate the Clinical Research Development Center of Imam Reza Hospital for their wise advice.
Funding
There was no external source of funding.
Author information
Authors and Affiliations
Contributions
EA, FA, PM, and SRB had the idea for this study. EA, FA, and SRB participated in outlining the concept and design. PM, and FM did the data acquisition. EA, NE, and FM did the statistical analysis and wrote the first draft of the manuscript. EA, SRB, and NE revised the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received ethics approval by the Kermanshah University of Medical Science Ethics Committee. Written informed consent to participate was obtained from all patients. All methods were carried out in accordance with relevant guidelines and regulations. The patient's data included in this manuscript has not been previously reported.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alimohammadi, E., Bagheri, S.R., Mardanpour, P. et al. Baseline neutrophil–lymphocyte ratio can be associated with hematoma expansion in patients with intracerebral hemorrhage: a retrospective observational study. BMC Neurosci 23, 18 (2022). https://doi.org/10.1186/s12868-022-00705-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-022-00705-z