Abstract
Background
Deoxynivalenol (DON) is a type B trichothecene mycotoxin that is commonly found in cereals and grains worldwide. The presence of this fungal secondary-metabolite raises public-health concerns at both the agriculture and food industry level. Recently, we have shown that DON has a negative impact on gut integrity, a feature also noticed for Campylobacter (C.) jejuni. We further demonstrated that DON increased the load of C. jejuni in the gut and inner organs. In contrast, feeding the less toxic DON metabolite deepoxy-deoxynivalenol (DOM-1) to broilers reduced the Campylobacter load in vivo. Consequently, it can be hypothesized that DON and DOM-1 have a direct effect on the growth profile of C. jejuni. The aim of the present study was to further resolve the nature of this interaction in vitro by co-incubation and RNA-sequencing.
Results
The co-incubation of C. jejuni with DON resulted in significantly higher bacterial growth rates from 30 h of incubation onwards. On the contrary, the co-incubation of C. jejuni with DOM-1 reduced the CFU counts, indicating that this DON metabolite might contribute to reduce the burden of C. jejuni in birds, altogether confirming in vivo data. Furthermore, the transcriptomic profile of C. jejuni following incubation with either DON or DOM-1 differed. Co-incubation of C. jejuni with DON significantly increased the expression of multiple genes which are critical for Campylobacter growth, particularly members of the Flagella gene family, frr (ribosome-recycling factor), PBP2 futA-like (Fe3+ periplasmic binding family) and PotA (ATP-binding subunit). Flagella are responsible for motility, biofilm formation and host colonization, which may explain the high Campylobacter load in the gut of DON-fed broiler chickens. On the contrary, DOM-1 downregulated the Flagella gene family and upregulated ribosomal proteins.
Conclusion
The results highlight the adaptive mechanisms involved in the transcriptional response of C. jejuni to DON and its metabolite DOM-1, based on the following effects: (a) ribosomal proteins; (b) flagellar proteins; (c) engagement of different metabolic pathways. The results provide insight into the response of an important intestinal microbial pathogen against DON and lead to a better understanding of the luminal or environmental acclimation mechanisms in chickens.
Similar content being viewed by others
Introduction
Food and feed safety is an important issue worldwide, and Campylobacter (C.) jejuni, being primarily associated with poultry, is the most important foodborne pathogen causing gastroenteritis in humans [1]. A high prevalence of campylobacteriosis in humans together with an increasing level of antimicrobial resistance became a serious problem in recent years [2,3,4]. Understanding how Campylobacter species, especially C. jejuni, establishes successful colonization in chickens remains a foremost research priority as this gastrointestinal pathogen not only overcomes the host’s defense system, but also competes with the microbial community for nutrients and space. Therefore, further research on the pathogenesis of C. jejuni infections in chickens and counteracting strategies are needed. Colonization of the avian gut by C. jejuni is very complex and is influenced by many parameters. Dietary factors have been found to alter the resistance to infection in general and to influence the microbial dynamics of the gut [5,6,7]. It was also demonstrated that feed composition, age, as well as breed of birds, influenced the outcome of C. jejuni colonization, the immune system, and the gut microbiota [8,9,10]. Recently, in various studies, we were able to show that C. jejuni, contrary to the general belief, increases intestinal permeability or “leaky gut” and promotes not only the translocation of C. jejuni itself but also the spread of Escherichia coli to internal organs [10,11,12].
Contamination of animal feed with mycotoxins is a worldwide problem and the presence of mycotoxins in poultry feed compromises the health of birds in a multifaceted way. Deoxynivalenol (DON) is the most common trichothecene mycotoxin detected in feedstuffs worldwide [13, 14]. It is known that DON poses a health risk in livestock and can have consequences on production parameters. The toxicity of this mycotoxin is connected to the epoxide ring in the molecular structure that binds to ribosomes and inhibits protein synthesis [15]. This effect is particularly problematic in rapidly dividing cells of the intestine and the immune system. Beside the effect on protein synthesis, trichothecenes inhibit both RNA and DNA synthesis, presumably as a secondary effect of protein synthesis inhibition [16]. Inhibition of DNA synthesis also results in inhibition of mitosis [16]. In addition, trichothecenes trigger a ribotoxic stress response in various cell lines that stimulates mitogen-activated protein kinase (MAPK) components of a signal transduction pathway, which regulates cell survival and stress response [16,17,18]. Consequently, DON increases the susceptibility to diseases [19,20,21].
So far, few studies have investigated the interaction between DON and enteric pathogens. It was found that the co-exposure of pigs to DON and Salmonella Typhimurium promoted Salmonella invasion and translocation across the intestinal epithelium [19]. It has also been demonstrated that feeding of DON is a predisposing factor for the development of necrotic enteritis in broiler chickens [20]. In agreement with this we found that the co-exposure of broiler chickens to DON and C. jejuni supported C. jejuni colonization in the gut at certain time points post infection, revealing that DON might provide a favourable condition for C. jejuni growth [22].
To mitigate the toxicity of DON, various approaches have been formulated, with one of them being bacterial biotransformation which relies on the ability of microorganisms to produce metabolites of DON with decreased toxicity [23]. Biotransforming of DON occurs mainly through de-epoxidation, oxidation or isomerization. The de-epoxidation process consists of a reductive chemical transformation that breaks open the 12,13-epoxy ring, leading to the conversion of DON into its deepoxide derivative known as de-epoxy-deoxynivalenol (deepoxy-DON or DOM-1) [23]. Incubating DON with contents from the large intestine of hens showed complete transformation to deepoxy-DON [24]. Earlier investigations indicated that the deepoxidation of DON results in a loss of toxicity [25]. It has also been demonstrated in various studies that a microbial feed additive with de-epoxidation activity could neutralize the toxic effects induced by DON in chickens [26,27,28,29,30]. Formation of deepoxy-DON (DOM-1) in pigs, rats, mice, chickens, and cows has been shown in different studies [31,32,33,34]. Pierron [35] showed that administration of pure DOM-1 by gavage for 21 days in pigs does not have toxic effects on zootechnical parameters, intestinal histology, intestinal and inflammatory response and liver histology. The implications of co-exposure to DOM-1 in combination with a pathogen like C. jejuni or other species have not been studied. To our knowledge, we have shown for the first time that the feeding of DOM-1 reduced the colonization of C. jejuni NCTC 12744 in the intestine by approximately 1.5–3.0 log10 (CFU/g) within the first two weeks post infection compared with co-exposure of C. jejuni to DON [36]. Such findings highlight the need for further investigations of DON and DOM-1 effects on C. jejuni at the transcriptome level as a model prokaryote. Therefore, co-incubation during in vitro studies followed by RNA sequencing were implemented to resolve the mycotoxin-bacteria interaction. Hence, a better understanding of the pathogen response to DON at the transcriptome level may also lead to the identification of novel detoxification mechanisms, which can be applied to overcome or reduce DON contamination.
Materials and methods
Campylobacter jejuni strains and growth curve analysis
Different C. jejuni (reference strains NCTC 12744 (Campylobacter jejuni subsp. jejuni, isolated from contaminated milk, Public Health England, UK) and ATCC 700819 (Campylobacter jejuni subsp. jejuni (Jones et al.) Veron and Chatelain ATCC 700819, isolated from human faeces, LGC Standards ATCC, UK), and field isolates (1303, isolated from broiler chicken flock, pooled faecal samples), and 969 (isolated from environmental sample, swab from anteroom). Both strains were identified as C. jejuni by PCR and PCR-RFLP at our clinic [37, 38], and were cultivated at 41.5 °C for 48 h under microaerophilic conditions (Genbox microaer, BioMerieux, Vienna, Austria) on Campylobacter Selective Agar (Campylosel agar, BioMerieux), which was used to determine bacterial counts in the samples.
In the first set of experiments (3 technical replicates in 3 biological replicates), the interaction of different C. jejuni strains with DON and DOM-1 was investigated in vitro by measuring the optical density at a wavelength of 690 nm using a microplate photometer (Micronaut-S Microdilution system, Merlin Diagnostika GmbH, Kleinstraße 14, Barnheim-Hersel, Germany) and determining the bacterial count at 48 h. Different strains of C. jejuni at 105 CFU/mL were grown in 100 µl of Mueller-Hinton-Broth (Merck KGaA, Darmstadt, Germany), 95 µl of bacterial suspension were inoculated into 96-well plates (Sarstedt AG&Co KG, Sarstedtstr.1, Nümbrecht, Germany). DON or DOM-1 was dissolved in PBS (10 mg/mL, stock solutions). In the 96-well plates, 5µl of PBS was added to the bacterial suspension as a control sample. The 96-well plates were then inoculated with either DON (5µl) or DOM-1 (5µl) (a total volume of 100 µl in one well) and incubated under microaerophilic conditions over 48 h. The DON concentration used in the in vitro model is relevant to the field situation and is also of practical relevance as the current guidance for the tolerated value of DON in poultry diets is set at 5 mg/kg feed [39]. In the second series of experiments, to confirm the results, the bacterial growth curve was measured with different DON concentrations 5 and 20 ppm (low and high levels) to find out whether there is a difference in the bacterial response when exposed to high DON concentrations. For this, we used only two strains (a reference strain and a field isolate as a representative) for characterizing the bacterial activity based on CFU counts over the period of time (24 h, 30 h, 36 h, 42 h, 48 h).
For RNA sequencing, we proceeded only with the reference strain C. jejuni NCTC 12744, which we used in the in vivo experiments. The optical density was determined at 30 h and 48 h and the experiment was repeated three times for each bacterial strain (3 biological replicates). For each biological replicate, we had three technical replicates for each treatment and time point. Bacteria were harvested at 30 h and 48 h, 3 wells per plate (3 × 100 µL) and pooled together. The bacterial cells were then centrifuged at 16,000 x g for 2 min for immediate RNA extraction as described below for RNA-sequencing analysis. For each time point, four pooled biological replicates (12 samples) were used as control for C. jejuni, and due to the workload and the availability of the mycotoxin metabolite (DOM-1), three pooled biological replicates (9 samples) each for C. jejuni + DON (5 µg/mL) and for C. jejuni + DOM-1 (5 µg/mL), respectively.
In parallel to the determination of the optical density, CFU counts (at 24 h and 48 h) were performed from each well as described by Ruhnau et al. [36]. For bacterial enumeration, serial 10-fold dilutions were made from each sample and 100 µL from each dilution were direct-plated in duplicate onto Campylosel agar (BioMérieux, Vienna, Austria). The plates were incubated under microaerobic conditions at 41.5ºC for 48 h and typical Campylobacter colonies were enumerated by plate colony counter (ThermoFisher Scientific, USA) as colony-forming units. CFU counts were determined by calculating the mean value of both plates.
RNA extraction and bacterial RNA sequencing
Total RNA extractions were conducted with the RNAsnap method with slight modifications [40]. In short, the cell pellet was resuspended in 300 µL RNAsnap solution by shaking at 5000 rpm (2 × 20 s). Cells were lysed by incubating at 95 °C for 3 min, vortexed for 30 s, and incubation for 4 min at 95 °C. After pelleting the cell debris at 16,000 x g for 5 min, the supernatant was carefully transferred to a new tube for RNA cleanup. Cleanup was achieved with the Monarch RNA Cleanup kit (New England Biolabs, Frankfurt am Main, Germany) according to the manufacturer’s instructions. Total RNA concentration was determined using a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific, USA). Afterwards, the RNA samples were qualified for sequencing, and shipped to the sequencing platform provider (LGC Standards GmbH, Wesel, Germany). The provider further treated the RNA samples with DNase and performed rRNA depletion using the MICROBExpress™ bacterial mRNA enrichment kit (ThermoFisher Scientific, USA). The RNA-Seq libraries were pooled and sequenced on Illumina NextSeq 500/550 V2 with 75 bp single reads. In total, approximately 100 million reads were obtained across all 20 samples, with an average of 5.4 million of reads per sample (genome size of C. jejuni is 1.6 Mbp). The resulting reads were demultiplexed (Illumina bcl2fastq 2.17.1.14 software), trimmed, and further filtered for rRNA sequences using RiboPicker 0.4.3. Alignment of RNA-Seq reads was performed with Bowtie 2, and against the annotated genome of C. jejuni 81–176 (NC_008787.1) showing 98.41% homology with our C. jejuni strain NCTC 12744 (microbial nucleotide BLAST, database prok_complete_genomes, NCBI). HTSeq was used for counting of TopHat-aligned reads, and gene expression levels were normalized as reads per kilobase of transcript per million mapped reads (RPKM).
Statistical analysis
For bacterial cell counts, the data are presented as means with standard error of mean (SEM). To evaluate the normality Kolmogorov-Smirnov´s test was utilized. A multivariate general linear model, ANOVA, Duncan´s multiple range test and LSD were applied to analyze differences between groups. Differences were considered significant at a level of P ≤ 0.05. Data were analyzed by IBM SPSS Statistics 24 software for Windows (Chicago, IL, USA). For RNA-seq analysis, RPKM were used for fold change calculation between control and treatments (DON or DOM-1) at each time point (30–48 h). The mixed effects model approach was applied on the individual RPKM (reads per kilobase of transcript per million mapped reads) with treatment and time as two factors (Two-way ANOVA), followed by Sidak’s multiple comparison test with P value. The principal component analysis (PCA) and heatmaps were conducted with the online tool ClustVis (https://biit.cs.ut.ee/clustvis/) and using the normalized gene expression levels (RPKM). The PCA was implemented to measure the strength of association between Campylobacter genes at both time points (30 h and 48 h). In addition, two-way ANOVA was done on the pooled normalized counts for flagellar genes (treatment and time factors), followed by Dunnett’s multiple comparison test.
Results
Bacterial growth analysis
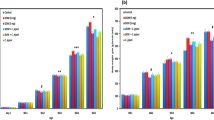
In the first set of experiments, to investigate a possible interference of DON with different C. jejuni strains, the in vitro co-incubation of C. jejuni NCTC 12744, C. jejuni ATCC 700,918, C. jejuni 1303, and C. jejuni 969 with DON or DOM-1 was investigated applying the Micronaut-Microdilution system. The results showed that that the presence of DON resulted in higher growth rates in all strains compared to the results when C. jejuni was grown alone (Fig. 1a, b). However, these findings were only significant for the reference strains NCTC 12744 and ATCC 700,819 at 48 h and not for C. jejuni 969 and C. jejuni 1303 (Fig. 1b). For confirming the results, a bacterial growth curves were established to characterize the bacterial activity based on CFU counts over this period (Fig. 2a, b). This effect was statistically consistent and had good predictability, indicating a growth stimulation effect of the bacteria in the presence of DON (1.4 × 1010 CFU, Fig. 1a). On the contrary, co-incubation with DOM-1 resulted in a significant decrease in CFU counts for all C. jejuni strains investigated (2.3 × 108 CFU, Fig. 3b).
Strain-specific differences (a) (Reference strains C. jejuni NCTC 12744 and C. jejuni ATCC 700819; (b) Field isolates (C. jejuni 969 and C. jejuni 1303) in Campylobacter proliferation in the presence of DON. Results are presented as mean values and standard error of mean (SEM) (n = 9). Asterisks mark differences with P ≤ 0.05 (*), or P ≤ 0.01 (**)
Strain-specific differences (a) Reference strains (C. jejuni NCTC 12744 and C. jejuni ATCC 700819; (b) Field isolates (C. jejuni 969 and C. jejuni 1303) in Campylobacter proliferation in the presence of DOM-1. Results are presented as mean values and standard error of mean (SEM) (n = 9). Asterisks mark differences with P ≤ 0.05 (*)
Effect of DON on the transcriptome of C. Jejuni NCTC 12744 over time
Bacterial genes that were affected by DON at both time points, 30 h and 48 h, are presented in Fig. 4. A fold change of at least 1.5 was seen for those genes when DON was present compared to C. jejuni alone, either upregulating or downregulating their expressions. Interestingly, most of the genes showing lower expression were related to ribosomal RNAs, with 19 out of 32 downregulated genes mapping to the ribosome pathway (i.e. subunit rpl, 50 S ribosomal protein, and subunit rps, 30 S ribosomal protein, Fig. 5). Regarding the upregulated genes, the effects were more pronounced after 48 h of exposure to DON than after 30 h. This is for instance the case for the bacterial gene Acfc, accessory colonization factor, involved in infectivity, and exhibiting a significant 3.42-fold increase at 48 h. Furthermore, the expression of 6 genes (flgD, flgH, flgI, flgB, flgG, flgE), encoding bacterial motility proteins was increased in the C. jejuni samples treated with DON at 30 h (Fig. 6). However, after 48 h, the mRNA levels of those genes were either unchanged or reduced, showing a different profiling depending on the duration of DON exposure.
Summary of the expression of genes of C. jejuni NCTC 12744 affected by DON and DOM-1 at both time points, 30 h and 48 h. The Y-axis represents the functional genes, and the X-axis represents the fold change with genes at least 1.5-fold decreased or increased compared to C. jejuni alone. (a) The blue color of bar represents the fold-change of up- or down-regulated genes for DON treatment at 30 h, and the grey color of bar represent the fold-change of up-or down-regulated genes for DON treatment at 48 h. P-values from the treatment effect (i.e. DON) following 2-way ANOVA (with treatment and time factors) were plotted as grey dots, and their values are seen through the extra X-axis on top of the bar chart. (b) Differences in gene transcription profiles between DON and DOM-1 on the genes affected by DON at 48 h. The blue color of bar represents the fold-change of up- or down-regulated genes for DON treatment, and the green color of bar represent the fold-change of the same genes for DOM-1 treatment. (c) The bright green color of bar represents the fold-change of up- or down-regulated genes for DOM-1 treatment at 30 h, and the grey color of bar represent the fold-change of up-or down-regulated genes for DOM-1 treatment at 48 h. P-values from the treatment effect (i.e. DOM-1) following 2-way ANOVA (with treatment and time factors) were plotted as grey dots, and their values are seen through the extra X-axis on top of the bar chart
Expression of C. jejuni NCTC 12744 ribosomal genes compared with the expression of the same genes of C. jejuni in the presence of DON at the two sampling points (30 h and 48 h) determined by RNA-seq. The functional analysis was done with KEGG pathway. The pathway enrichment analysis showed that many down-regulated genes mapped to the cje03010 ribosome pathway (i.e. cje03010 in KEGG pathway analysis corresponding to subunit rpl, 50S ribosomal proteins, and subunit rps, 30S ribosomal proteins)
Expression of C. jejuni NCTC 12744 flagella genes compared with the expression of the same genes of C. jejuni in the presence of DON or DOM-1 at the two sampling points (30 h and 48 h) determined by RNA-seq. Data are presented as the mean values and SD. The functional analysis was done with KEGG pathway (i.e. cje02035 in KEGG pathway analysis corresponding to bacterial motility proteins)
Effect of DOM-1 on the transcriptome of C. Jejuni over time
Like the approach with DON, the genes showing either a 1.5 fold increase or decrease at both time points were selected. Only 20 bacterial genes met these criteria at 30 h and 48 h. Interestingly, most of the genes showing lower expression were related to ribosomal RNAs, with 19 out of 32 downregulated genes mapping to the ribosome pathway (i.e. cje03010 in KEGG pathway analysis corresponding to subunit rpl, 50 S ribosomal proteins, and subunit rps, 30 S ribosomal proteins; Fig. 5 presents the expression of some of those ribosomal proteins). Furthermore, the expression of 6 genes, encoding bacterial motility proteins, was also decreased in the C. jejuni samples treated with DOM-1. These genes (flgD, flgH, flgI, flgB, flgG, flgE; not polycistronic mRNAs) are related to flagellar assembly in bacteria (i.e. cje02035 in KEGG pathway analysis corresponding to bacterial motility proteins), and their expressions are presented in Fig. 6. Since these genes belong to the same pathway, their normalized counts were pooled to increase the number of data points. A significant effect of DON treatment on flagellar genes (p = 0.0031) and, in contrast, a significant decrease in expression with DOM-1 as revealed by the multiple comparisons test (p = 0.084 at 30 h, and p = 0.0021 at 48 h).
Relationships among treatments were examined by Principal component analysis (PCA). The PCA showed no clear clustering of Campylobacter genes between control and DOM-1 treatment, especially at 48 h (Fig. 7). PCA plots also demonstrate that Campylobacter genes were more separated with DON treatment at both time points (Fig. 7). In addition, the fold changes of the genes sorted by time points of control, DON, and DOM-1 treatment of each biological replicate are shown in the heatmaps (Fig. 7). The gene similarity among all samples showed clear differences between treatments at both time points, indicating strong shifts in Campylobacter genes as a result of DON treatment.
The principal component analysis (PCA) and heatmaps were generated with the online tool ClustVis (https://biit.cs.ut.ee/clustvis/), and using the normalized gene expression levels (reads per kilobase of transcript per million mapped reads, RPKM). PCA were analysed for all samples at 30 h (a), and at 48 h (b). Green (control), dark orange (DOM-1), and purple (DON), with each dot indicating a biological replicate. Heatmaps were analysed for all samples at 30 h (c), and at 48 h (d)
Discussion
Chickens serve as a major source of human infections with C. jejuni, and therefore, infected birds remain a substantial problem for poultry production [41]. Likewise, contamination of food and feed with mycotoxins is a global problem and the presence of mycotoxins in poultry feeds is a significant economical factor. DON is the most common trichothecene mycotoxin detected in feedstuffs worldwide [14].
The effect of DON on prokaryote remains unclear, although the mechanisms and physiological disruption of this toxin in eukaryotes have been well characterized [42, 43]. It was reported that DON can alter the gut microbiota in humans and animals [44, 45]. Recently, we demonstrated that the co-exposure of broiler chickens to DON and C. jejuni increased the intestinal C. jejuni load, indicating that DON may represent a favorable prerequisite for Campylobacter multiplication [22]. Furthermore, the DON impact on Campylobacter growth can also be explained by the fact that this bacterium can rely on DON as a sole source of carbon [46]. This raises questions about the synergism between food contaminants and Campylobacter with regard to food-borne gastroenteritis. This, in turn, has led to a greater interest in understanding bacterial responses toward DON. Johnson et al. [47] reported that prokaryotic RNA-Seq analysis is challenging because most available RNA-Seq packages assume the input data reflect eukaryotic gene structures, which in many aspects differ from those of prokaryotes. Hence, RNA-Seq technology has been extensively used in studies of pathogenic bacteria to identify and quantify changes in gene expression among different samples from bacteria exposed to various conditions.
To resolve the nature of the influence of DON on the infection profile of C. jejuni, the direct interactions between C. jejuni and DON or a less-toxic DON-metabolite were investigated. Overall, the actual study demonstrated that the presence of DON resulted in significantly higher growth rates of C. jejuni from 30 h incubation onwards, confirming the in-vivo data using the same concentration, indicating growth-stimulating effects of the bacteria. However, none of the field strains showed stronger growth in the presence of DON. One explanation for this might be that the presence of DON could alter the activity of the reference strains and thus has an impact on growth. Thus, we employed RNA-seq technology to explore the changes in bacterial mechanisms in response to DON. Co-incubation of C. jejuni with DON increased the expression of Flagella gene family, frr (ribosome-recycling factor), PBP2 futA-like (Fe3+ periplasmic binding family), biosynthesis of amino acid and PotA (ATP-binding subunit), which are required for motility, biofilm formation, host cell interactions, and host colonization. The C. jejuni multiplication can be also explained by the fact that the bacterium evades DON toxicity by upregulating several ABC-dependent membrane transporters and efflux pumps that remove many undesirable toxins/ chemicals (including DON) from the environment [48]. In line with that, we also found that DON increased the expression of efflux transporters (e.g. ATP-binding subunit). Together, co-incubation of C. jejuni with DON led to the development of a variety of mechanisms to compete environmental challenges.
It has been reported that mycotoxin deactivators can convert DON into the non-toxic metabolite DOM-1 through enzymatic biotransformation, thereby reducing DON burden in chickens [49]. The mechanisms of action of DOM-1 are activation of metabolic enzyme activity and feed digestibility, activation of the liver function as well as strengthening of the immune system [50]. Recently, we found that feeding of DOM-1 reduced intestinal C. jejuni load by 1.5-3.0 log10 (CFU/g) [36]. Furthermore, we hypothesized that DOM-1 might create a different intestinal environment to which C. jejuni could adapt [35, 51]. Therefore, based on these findings, the current study was conducted to explain how DOM-1 might directly affect a prokaryote such as Campylobacter in chickens. In the current experiments, we found that the presence of DOM-1 leads to a significant decrease in C. jejuni CFU counts of all strains. These results confirm our previous in vivo data, as we found that the dietary inclusion of DOM-1 reduced the intestinal load of C. jejuni at 7- and 14-days post infection [36]. The results showed that, in contrast to DON, DOM-1 downregulated bacterial motility genes (Flagella gene family) and upregulated ribosomal proteins, implying reduced proliferation activity of this bacteria. The results showed that DOM-1 could threaten the survival of Campylobacter, indicating a specific response to the influx of DON and DOM-1 into bacterial cells through changes in the bacteria’s metabolic pathways. However, the bacteria could be mitigating the effects of a metabolite targeting its ribosomal genes, allowing it to continue with protein synthesis. Overall, through a combination of our recent in vivo trial with the current results, we demonstrated that that mycotoxin-bacteria interactions can alter the virulence of Campylobacter in its hosts. The results may provide new insights into targets that can be used for future studies of molecular mechanisms underlying the Campylobacter response to DON and DOM-1. However, the specific functions of the identified genes in Campylobacter metabolic pathways should be confirmed by further molecular biological investigations.
Conclusion
The results of the present studies confirmed that DON interfered with the ribosomal proteins and upregulated the flagellar proteins. On contrast, it demonstrated that deepoxy-DON (DOM-1) did not activate these signaling pathways. These results expand the current knowledge of the toxicity of DON and beneficial effects of deepoxy-DON (DOM-1) and contribute to the evaluation of the efficacy of the microbial biotransformation strategies in the fight against mycotoxins. Finally, the study also demonstrated that DOM-1 has a substantial impact on the C. jejuni propagation and by this also on the colonization.
Data availability
Sequencing data are available in the European Nucleotide Archive (ENA) database under the accession number GSE248277.
Abbreviations
- DON:
-
Deoxynivalenol
- DOM-1:
-
Deepoxy-deoxynivalenol
- C. :
-
Campylobacter
- Frr:
-
Ribosome-recycling factor
- PBP2:
-
futA-like Fe3 + periplasmic binding family
- PotA:
-
ATP-binding subunit
- MAPK:
-
Mitogen-activated protein kinase
- CFU:
-
Colony-forming units
- RPKM:
-
Reads per kilobase of transcript per Million mapped reads
- PCA:
-
Principal component analysis
- FDR:
-
False discovery rate correction
References
European Food Safety Authority (EFSA). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021;19:e06406.
Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24:718–33.
Tang Y, Fang L, Xu C, Zhang Q. Antibiotic resistance trends and Mecha-Nisms in the Foodborne Pathogen. Campylobacter Anim Health Res Rev. 2017;18:87–98.
García-Fernández A, Dionisi AM, Arena S, Iglesias-Torrens Y, Carattoli A, Luzzi I. Human campylobacteriosis in Italy: emergence of Multi-drug Resistance to Ciprofloxacin, Tetracycline, and Erythromycin. Front Microbiol. 2018;9:1906.
Klasing KC. Nutritional modulation of resistance to infectious diseases. Poult Sci. 1998;77:1119–25.
Molnár A, Hess C, Pál L, Wágner L, Awad WA, Husvéth F, Hess M, Dublecz K. Composition of diet modifies colonization dynamics of Campylobacter jejuni in broiler chickens. J Appl Microbiol. 2015;118:245–54.
Sahin O, Kassem II, Shen Z, Lin J, Rajashekara G, Zhang Q. Campylobacter in poultry: ecology and potential interventions. Avian Dis. 2015;59:185–200.
Han Z, Willer T, Li L, Pielsticker C, Rychlik I, Velge P, Velgec P, Kaspersd B, Rautenschlein S. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect Immun. 2017;85:e00380–00317.
Awad WA, Dublecz F, Hess C, Dublecz K, Khayal B, Aschenbach JR, Hess M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult Sci. 2016;95:2259–65.
Awad WA, Hess C, Hess M. Re-thinking the chicken-Campylobacter jejuni interaction: a review. Avian Pathol. 2018;47:352–63.
Awad WA, Ruhnau D, Hess C, Hess M. Campylobacter jejuni increases the paracellular permeability of broiler chickens in a dose-dependent manner. Poult Sci. 2020;99:5407–14.
von Buchholz JS, Ruhnau D, Hess C, Aschenbach JR, Hess M, Awad WA. Paracellular intestinal permeability of chickens induced by DON and/or C. Jejuni is associated with alterations in tight junction mRNA expression. Microb Pathog. 2022;168:105509.
European Food Safety Authority (EFSA). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017;15:4718.
Awad WA, Zentek J. The feed contaminant deoxynivalenol affects the intestinal barrier permeability through inhibition of protein synthesis. Arch Toxicol. 2015;89:961–5.
Payros D, Alassane-Kpembi I, Pierron A, Loiseau N, Pinton P, Oswald IP. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch Toxicol. 2016;90:2931–57.
Rocha O, Ansari K, Doohan FM. Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit Contaminants. 2005;22:369–78.
Pestka JJ. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contaminants Part A. 2008;25:1128–40.
Escrivá L, Font G, Manyes L. In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem Toxicol. 2015;78:185–206.
Vandenbroucke V, Croubels S, Martel A, Verbrugghe E, Goossens J, Van Deun K, Boyen F, Thompson A, Shearer N, De Backer P, Haesebrouck F, Pasmans F. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella Typhimurium in porcine ileal loops. PLoS ONE. 2011;6:e23871.
Antonissen G, Van Immerseel F, Pasmans F, Ducatelle R, Haesebrouck F, Timbermont L, Verlinden M, Janssens GP, Eeckhaut V, Eeckhout M, De Saeger S, Hessenberger S, Martel A, Croubels S. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE. 2014;309:e108775.
Awad WA, Ruhnau D, Hess C, Doupovec B, Schatzmayr D, Hess M. Feeding of deoxynivalenol increases the intestinal paracellular permeability of broiler chickens. Arch Toxicol. 2019;93:2057–64.
Ruhnau D, Hess C, Grenier B, Doupovec B, Schatzmayr D, Hess M, Awad WA. The mycotoxin deoxynivalenol (DON) promotes Campylobacter jejuni multiplication in the intestine of broiler chickens with consequences on bacterial translocation and gut integrity. Front Vet Sci. 2020;7:573894.
Awad WA, Ghareeb K, Böhm J, Zentek J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit Contam A. 2010;27:510–20.
He P, Young LG, Forsberg C. Microbial transformation of deoxynivalenol (vomitoxin). Appl Environ Microbiol. 1992;58:3857–63.
Sundstøl Eriksen G, Pettersson H, Lundh T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem Toxicol. 2004;42:619–24.
Awad WA, Böhm J, Razzazi-Fazeli E, Hulan HW, Zentek J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult Sci. 2004;83:1964–72.
Awad WA, Böhm J, Razzazi-Fazeli E, Ghareeb K, Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. 2006;85:974–9.
Ghareeb K, Awad WA, Soodoi C, Sasgary S, Strasser A, Böhm J. Effects of feed contaminant deoxynivalenol on plasma cytokines and mRNA expression of immune genes in the intestine of broiler chickens. PLoS ONE. 2013;8:e71492.
Ghareeb K, Awad WA, Sid-Ahmed OE, Böhm J. Insights on the host stress, fear and growth responses to the deoxynivalenol feed contaminant in broiler chickens. PLoS ONE. 2014;9:e87727.
Ghareeb K, Awad WA, Böhm J, Zebeli Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J Appl Toxicol. 2015;35:327–37.
Fuchs E, Binder EM, Heidler D, Krska R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by bacterial strain BBSH 797. Food Addit Contaminants. 2002;4:379–86.
Awad WA, Ghareeb K, Böhm J. Effect of addition of a probiotic microorganism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J Anim Physiol Anim Nutrit. 2010;94:486–94.
Awad WA, Hess M, Twarużek M, Grajewski J, Kosicki R, Böhm J, Zentek J. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int J Mol Sci. 2011;12:7996–8012.
Mayer E, Novak B, Springler A, et al. Effects of deoxynivalenol (DON) and its microbial biotransformation product deepoxy-deoxynivalenol (DOM-1) on a trout, pig, mouse, and human cell line. Mycotoxin Res. 2017;33:297–308.
Pierron A. Toxicity of three biological derivatives of deoxynivalenol: deepoxy-deoxynivalenol, 3-epideoxynivalenol and deoxynivalenol-3-glucoside on pigs. Agricultural sciences. Université Paul Sabatier- Toulouse III; 2016.
Ruhnau D, Hess C, Doupovec B, Grenier B, Schatzmayr D, Hess M, Awad WA. Deepoxy-deoxynivalenol (DOM-1), a derivate of deoxynivalenol (DON), exhibits less toxicity on intestinal barrier function, Campylobacter jejuni colonization and translocation in broiler chickens. Gut Pathog. 2021;13:44.
Neubauer C, Szölgyényi W, Jauk V, Vasicek L. Polymerase chain reaction versus culture technique for detection of thermophilic Campylobacter in chicken faecal samples. Wien Tierärztl Mschr. 2004;91:287–91.
Jauk V, Neubauer C, Szölgyenyi W, Vasicek L. Phenotypic and genotypic differentiation of Campylobacter spp. isolated from Austrian broiler farms: a comparison. Avian Pathol. 2003;32:33–7.
European Commission. Commission regulation (EC) 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official J EU. 2006;364:5–24. Available online at:. http://data.europa.eu/eli/reg/2006/1881/oj.
Stead MB, Agrawal A, Bowden KE, Nasir R, Mohanty BK, Meagher RB, Kushner SR. RNAsnap™: a rapid, quantitative and inexpensive, method for isolating total RNA from bacteria. Nucleic Acids Res. 2012;40:e156.
Wassenaar TM. Following an imaginary Campylobacter population from farm to fork and beyond: a bacterial perspective. Lett Appl Microbiol. 2011;53:253–63.
Payros D, Dobrindt U, Martin P, Secher T, Bracarense AP, Boury M, Laffitte J, Pinton P, Oswald E, Oswald IP. The Food Contaminant Deoxynivalenol exacerbates the genotoxicity of gut microbiota. mBio. 2017;8:e00007–17.
Hassan YI, He JW, Lepp D, Zhou T. Understanding the bacterial response to mycotoxins: the transcriptomic analysis of Deoxynivalenol-Induced changes in Devosia mutans 17-2-E-8. Front Pharmacol. 2019;10:1098.
Robert H, Payros D, Pinton P, Théodorou V, Mercier-Bonin M, Oswald IP. Impact of mycotoxins on the intestine: are mucus and microbiota new targets? J Toxicol Environ Health B Crit Rev. 2017;20:249–75.
Lucke A, Böhm J, Zebeli Q, Metzler-Zebeli BU. Dietary deoxynivalenol contamination and oral lipopolysaccharide challenge alters the cecal microbiota of broiler chickens. Front Microbiol. 2018;9:804.
Hassan YI, Zhou T. Addressing the mycotoxin deoxynivalenol contamination with soil-derived bacterial and enzymatic transformations targeting the C3 carbon. World Mycotoxin J. 2018;11:101–11.
Johnson BK, Scholz MB, Teal TK, Abramovitch RB. SPARTA: simple program for automated reference-based bacterial RNA-seq transcriptome analysis. BMC Bioinformatics. 2016;17:66.
Barabote RD, Thekkiniath J, Strauss RE, Vediyappan G, Fralick JA, San Francisco MJ. Xenobiotic efflux in bacteria and fungi: a genomics update. Adv Enzymol Relat Areas Mol Biol. 2011;77:237–306.
Vanhoutte I, De Mets L, De Boevre M, Uka V, Di Mavungu JD, De Saeger S, De Gelder L, Audenaert K. Microbial Detoxification of Deoxynivalenol (DON), assessed via a Lemna minor L. Bioassay, through Biotransformation to 3-epi-DON and 3-epi-DOM-1. Toxins. 2017;13:9:63.
Kiyothong K, Rowlinson P, Wanapat M, Khampa S. Effect of mycotoxin deactivator product supplementation on dairy cows. Anim Prod Sci. 2012;52:832–41.
Guerre P. Mycotoxin and gut microbiota interactions. Toxins (Basel). 2020;12:769.
Acknowledgements
We would like to thank B. Doupovec (DSM Animal Nutrition and Health, Research Center Tulln) for her contribution in the animal trials and D. Jandreski-Cvetkovic (Clinic for Poultry and Fish Medicine) for her technical assistance.
Funding
This work was financed by the Austrian Research Promotion Agency (FFG; 858543) and DSM Animal Nutrition and Health (Research Center Tulln, Technopark 1, 3430 Tulln, Austria).
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.WA and MH conceived and designed the project. DR performed the in vitro experiments and collected the data. BG performed the RNA-sequencing data analysis. WA, DR, CH, BG, DS, and MH discussed the results. WA and BG wrote the manuscript. All the authors contributed to manuscript revision and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Awad, W.A., Grenier, B., Ruhnau, D. et al. Diametral influence of deoxynivalenol (DON) and deepoxy-deoxynivalenol (DOM-1) on the growth of Campylobacter jejuni with consequences on the bacterial transcriptome. BMC Microbiol 24, 306 (2024). https://doi.org/10.1186/s12866-024-03452-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03452-9