Abstract
While trimethoprim-sulfamethoxazole (TMP-SMX) is the first-line therapy of Stenotrophomonas maltophilia infections, colistin is one of the therapeutic options in cases of allergy or resistance to TMP-SMX. However, understanding the global status of resistance to colistin amongst S. maltophilia isolates could be helpful for appropriate antibiotic prescription. This study aimed to conduct a systematic review and meta-analysis to examine the prevalence of colistin resistance in clinical S. maltophilia isolates worldwide. According to eligibility criteria, a total of 61 studies were included in the analysis. The pooled prevalence for colistin resistance was 42% (95% CI: 35-49%), ranging from 0.1 to 97%. Subgroups analysis indicated that, the pooled prevalence of colistin resistance was 44% (95% CI: 29-60%) in 15 studies during 2000–2010, and it was estimated to be 41% (95% CI: 33-50%) in 46 articles from 2011 to 2021. It was 46% (95% CI: 35-58%) in the studies that used broth microdilution method, and 39% (95% CI: 30-49%) in the studies with other used methods. The resistance rate in Asian countries was 45% (95% CI: 31-60%), in European countries was 45% (95% CI: 34-56%) and in the countries of North and South America was 33% (95% CI: 20-46%). Our review showed notable resistance to colistin in clinical S. maltophilia isolates. Given the estimated resistance rates, alternative antibiotics could be preferred to treat serious infections due to S. maltophilia.
Similar content being viewed by others
Introduction
Stenotrophomonas maltophilia is a gram-negative non-fermenting bacillus that has been emerged as an important causative agent of severe hospital-acquired infections [1]. It causes several infections, such as bloodstream infection, secondary meningitis, and ventilator-associated pneumonia, predominantly amongst hospitalized patients [2].
Because of intrinsic antimicrobial resistance due to the presence of chromosomally encoded mechanisms, carbapenems and most beta-lactam antibiotics are ineffective against S. maltophilia. Acquired resistance through the horizontal acquisition of resistance genes or mutations, further limits therapeutic options for treating these challenging infections [3, 4]. In general, trimethoprim-sulfamethoxazole (TMP-SMX) is regarded as first-line therapy for S. maltophilia infections, and combination therapies with other antibiotics (e.g. levofloxacin or colistin) are alternative options in case of difficult-to-treat infections [5,6,7,8]. Hence, in the face of emerging resistance in gram-negative bacteria, global trends of colistin use are rising [8,9,10]. However, increased incidence of colistin-resistant S. maltophilia isolates has been recently described [11, 12]. Colistin resistance may occur through several mechanisms in Gram-negative bacteria. Mutations in the genes associated with LPS synthesis and modifications of this molecule are recognized mechanisms of the resistance. The expression of global genes could also be affected by environmental changes such as cations and pH variations. Furthermore, various phenotypic resistance mechanisms including adaptive resistance, heteroresistance and biofilm formation, accelerate the development of resistance [11].
There are various views on the effectiveness of colistin against S. maltophilia in literature [8,9,10,11]. Understanding the current global colistin resistance in this pathogen which is associated with high morbidity and mortality in chronic diseases and immunocompromised patients could be helpful for better perception of this issue, and appropriate prescription of antibiotic. To the best of our knowledge, there is no relevant comprehensive analysis. Therefore, the aim of the present study was to conduct a systematic review and meta-analysis to examine the prevalence of colistin resistance in clinical S. maltophilia isolates worldwide, conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
Study characteristics
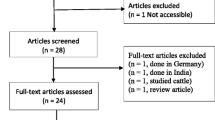
As displayed in Fig. 1, a total of 807 articles were retrieved using the search strategy, 675 were excluded based on index and review of title and abstract, leaving 132 articles for full-text review. Full-text screening caused in exclusion of 71 more studies, resulting in 61 eligible studies. The main characteristics of the included studies and the prevalence of colistin resistance in clinical isolates of S. maltophilia are shown in Table 1. Sixty-one studies investigated the prevalence of colistin resistance in 9082 clinical isolates of S. maltophilia. From those studies, the pooled prevalence for colistin resistance in clinical isolates of S. maltophilia was 42% (95% confidence interval (CI): 35-49%), ranging from 0.1 to 97% (Fig. 2). The symmetric funnel plot showed no evidence of publication bias (Supplementary 1). There was no evidence of publication bias from Begg’s test (p = 0.597).
Subgroup analysis
To investigate the prevalence of colistin resistance in clinical isolates of S. maltophilia based on the study period, methods of susceptibility testing, geographical location, sample size, and quality assessment score of the articles, subgroup analysis was used. Based on this, the colistin resistance in clinical isolates of S. maltophilia during 2000–2010 was investigated in 15 studies, and the pooled prevalence was estimated 44% (95% CI: 29-60%) ranging from 0.5 to 96% (Fig. 3). There was a significant heterogeneity among the 15 studies (χ2 = 872.71; P < 0.001; I2 = 98.40). There was no evidence of publication bias from Begg’s test (p = 0.656). We found 46 articles that investigated the prevalence of colistin resistance in clinical isolates of S. maltophilia in 2011–2021. The pooled prevalence of colistin-resistant isolates was estimated 41% (95% CI: 33-50%), ranging from 0.1 to 97% (Fig. 3). Based on Q statistic and the I2 index heterogeneity was significant (χ2 = 4937.65; P < 0.001; I2 = 99.09%). There was no evidence of publication bias according to Begg’s rank correlation analysis (p = 0.925).
The prevalence of colistin resistance in clinical S. maltophilia isolates in the studies with a sample size equal to or less than one hundred samples was equal to 46% (95% CI: 35-57%), in the studies with a sample size of more than one hundred samples was equal to 33% (95% CI: 21-44%), in the studies in American countries was equal to 33% (95% CI: 20-46%), in the studies in Asian countries was equal to 45% (95% CI: 31-60%), in the studies in European countries was equal to 45% (95% CI: 34-56%) and in the studies that samples obtained from different continents was equal to 39% (95% CI: 12-66%) (Table 2).
The prevalence of colistin resistance in the studies that used broth microdilution (BMD) was 46% (95% CI: 35-58%), and in the studies that used other methods, including DDM, agar dilution, E-test, VITEK 2 system and those without exact mentioned method was 39% (95% CI: 30-49%) (Fig. 4).
Meta-regression and sensitivity analysis
In this meta-analysis, it was observed that the heterogeneity between the results of the studies is equal to 98.97%. To investigate the causes of heterogeneity, a meta-regression was performed in variables such as the year of the study, the sample size of the study, the quality evaluation score, the method of susceptibility testing and the geographical location of the study. The results from the meta-regression analysis determined there was no significant source of heterogeneity (P > 0.20). Moreover, sensitivity analysis was performed by excluding each study from the analysis one by one during each run. However, the final estimate of the prevalence of colistin resistance did not change significantly, which indicates the strength of the meta-analysis results (Supplementary 1).
Discussion
S. maltophilia is intrinsically resistant to many antibiotics, such as penicillins, carbapenems and aminoglycosides and occurs in hospitalized patients, particularly in intensive care units (ICUs) [70,71,72]. S. maltophilia is not intrinsically resistant to polymyxins [73], hence, the present study aimed to systematically review the available scientific evidence regarding colistin resistance in clinical S. maltophilia isolates during the years 2000 to 2021. This systematic review is based on the published data spanning the globe.
According to our analysis, the pooled prevalence for colistin resistance among clinical S. maltophilia isolates was 42%. It was 44% from 2000 to 2010 and 41% in 2011–2021. Despite a slight reduction of colistin resistance in 2011–2021 studies compared to 2000–2010, there was no regular trend of colistin resistance among S. maltophilia clinical isolates during the period of 2000–2021.
In this study, the prevalence of colistin resistance in clinical isolates of S. maltophilia based on the antimicrobial susceptibility methods was also investigated. Challenges in the determination of susceptibility to colistin by laboratory testing are described frequently, and the most appropriate method is still controversial [74]. However, only MIC determination using broth microdilution method is recommended by the joint CLSI-EUCAST working group [75], and we believe it is the most appropriate. According to our analysis, the prevalence of colistin resistance in the studies which used the Broth Microdilution (BMD) method was 46% (95% CI: 35-58%), and in those studies which used other methods was 39% (95% CI: 30-49%). The obtained resistance rate by BMD was more than that by other methods. It seems that, determination of MIC and drug resistance by BMD method could lead to more accurate results and prescriptions of the antibiotic should be based on BMD results and not other methods.
In the present study, no study was included in the analysis from Africa, based on the eligibility criteria. The pooled prevalence of colistin resistance in clinical isolates of S. maltophilia in Europe, Asia and America was 45%, 45% and 33%, respectively. This rate was 39% for the included studies from the countries of different continents (Table 2). Although the same prevalence resistance to colistin in Europe and Asia was more than America, there was no notable difference between their resistance ranges (Table 2). Moreover, low number of studies were included and analyzed from America.
In the recent systematic review and meta-analysis regarding the global prevalence and distribution of levofloxacin, TMP/SMX, and minocycline resistance among clinical isolates of S. maltophilia, the rates of 14.4%, 9.2%, and 1.4% were reported, respectively [76]. These rates were lower than the estimated colistin resistance in the present study (42%). Therefore, these agents had better activity against S. maltophilia compared to colistin.
Conclusions
The prevalence of colistin resistance in clinical isolates of S. maltophilia was estimated to be 42%. According to our analysis, this resistance rate has slightly decreased in the period 2011–2021 compared to 2000–2010. The prevalence of resistance in the studies using BMD method was also higher than that using other methods (46% vs. 39%). Given the toxicity of colistin, and the high prevalence of resistance of S. maltophilia to colistin, alternative antibiotics may be preferred for treating S. maltophilia infections.
Methods
Study details
In the present systematic review and meta-analysis study, all procedures relevant to the papers’ identification were carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Guidelines.
Search strategy
To obtain all studies regarding the prevalence of colistin resistance in clinical isolates of S. maltophilia, a systematic search was done for English-language articles from January 1, 2000, to September 30, 2021, in the international databases PubMed/MEDLINE, Scopus and Web of Science. Records were managed by EndNote X9.0 software to exclude duplicates. The following MeSH terms were used simultaneously to find articles in databases: “Stenotrophomonas maltophilia’’, “Stenotrophomonas’’, “maltophila’’, “drug resistance”, and “antimicrobial resistance”. MeSH terms were combined with other words, including “S. maltophilia”, “colistin’’, “polymyxin’’, “antibiotic(s)” and their synonyms. To identify missing studies, we also searched bibliographies of retrieved articles for additional references.
Eligibility criteria and study selection
Cross-sectional or cohort studies that reported the prevalence of colistin resistance in clinical isolates of S. maltophilia were considered. The titles, abstracts and full texts were screened independently by three reviewers (ADS, EAF and RA) to determine articles that met the inclusion criteria, and any discrepancies were resolved with a fourth investigator (MSSA) or by consensus. The articles published in English, indexed in PubMed/MEDLINE, Scopus and Web of Science with the following characteristics were included: reported the prevalence of colistin resistance in clinical isolates of S. maltophilia with standard laboratory tests. Studies were eligible if they had reported the prevalence of colistin resistance in S. maltophilia. Notably, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI) do not provide breakpoints for colistin and S. maltophilia. Since the clinical isolates of this species possess high genetic diversity, and also the most reliable method to determine the activity of colistin against S. maltophilia is still controversial [11]. The laboratory tests for antibiotic susceptibility tests in the included studies were as follows; disk diffusion methods (DDM), minimum inhibitory concentration (MIC) determination by broth dilution, agar dilution and gradient strips, and the VITEK 2 system (bioMérieux). This study aimed to investigate the prevalence of colistin-resistant S. maltophilia isolates world wide. Studies were excluded if they did not report the prevalence of colistin resistance in clinical isolates of S. maltophilia or comment on the methods of susceptibility used. When the prevalence of colistin resistance from a given study was unavailable, or it was unclear if planned follow-up measurements were published, the authors requested this information via email. If the authors did not respond or did not provide the missing information, and if there was insufficient information available based on the publication, the study was excluded from the meta-analysis. We also excluded studies whose sample size was less than 10 isolates, nonhuman studies, studies published in languages other than English, review articles, meta-analyses or systematic reviews, congress abstracts and duplicate publications of the same study. Case reports were not included in the meta-analysis, as they do not have a denominator for any variables.
Data extraction and definitions
Data collection was performed in parallel by three investigators who performed the literature search and also independently extracted the data from included studies. We extracted the following variables: first author’s name, the study performing time, publication date, the study setting, sample size (numbers of isolated S. maltophilia) and the prevalence of colistin and other antibiotic resistance.
Quality assessment
The overall quality of studies was assessed using modified Critical Appraisal Checklist recommended by the Joanna Briggs Institute [77] and performed by two reviewers independently, and disagreements were resolved by discussion. The checklist is composed of eight questions that reviewers addressed for each study. The “Yes” answer for each question received a score of 1. Thus, the final scores for each study could range from 0 to 8. Two researchers independently assessed the quality of the articles, and discrepancies were discussed with a third researcher.
Statistical analysis
In studies where the prevalence of colistin resistance in clinical isolates of S. maltophilia was calculated and presented separately for time or seasonal periods, using the meta-analysis method, a total prevalence of colistin resistance in clinical isolates of S. maltophilia was calculated from the presented values and considered in the analysis. Also, in studies where the prevalence of colistin resistance in clinical isolates of S. maltophilia was not reported, but the related data was available in the article text, the prevalence of colistin resistance was estimated. In the studies included in the meta-analysis, the presence of heterogeneity was assessed using graphical methods (forest plot) and statistical tests [chi-square test and I2 (heterogeneity quantification reporting)]. The heterogeneity of study results included in the meta-analysis was investigated using the chi-square test, and the type of design (fixed or random) was determined according to the test results [78, 79]. The most widely used measure of heterogeneity, I2, estimates the proportion of variability in a meta-analysis that is explained by differences between included studies rather than sampling error. Mathematically, I2 is expressed as I2 = τ2/ (σ2 + τ2), where τ2 represents the between-study heterogeneity, σ2 represents the total sampling error between studies, and σ2 + τ2 represents the total variance in the meta-analysis. A meta-regression model was used to identify factors associated with heterogeneity of results, accounting for study year, study sample size, quality score, susceptibility testing method, and geographic location. Sensitivity analysis was also used to assess the effect of omitting each study on the final result. Therefore, to determine the root of heterogeneity in the results of the studies included in the meta-analysis, subgroup analysis, sensitivity analysis, and meta-regression methods were used. Funnel diagrams and Egger’s tests were used for assessing publication bias. All analyses were performed by Stata statistical software (version 14.0, Stata Corp, College Station, TX), and the significance level in this study was considered < 0.05.
Data Availability
The original contributions presented in the study are included in the article/Supplementary Material.
Abbreviations
- MDR:
-
multidrug resistant
- XDR:
-
extensively drug resistant
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- CLSI:
-
Clinical and Laboratory Standards Institute
- DDM:
-
disk diffusion method
- MIC:
-
minimum inhibitory concentration
- CI:
-
confidence interval
- ICUs:
-
intensive care units
References
Insuwanno W, Kiratisin P, Jitmuang A. Stenotrophomonas maltophilia infections: Clinical characteristics and factors Associated with mortality of hospitalized patients. Infect Drug Resist. 2020;13:1559–66.
Tada K, Kurosawa S, Hiramoto N, Okinaka K, Ueno N, Asakura Y, et al. Stenotrophomonas maltophilia infection in hematopoietic SCT recipients: High mortality due to pulmonary hemorrhage. Bone Marrow Transplant. 2013;48(1):74–9.
Sánchez MB. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front Microbiol. 2015;6:658.
Gil-Gil T, Martínez JL, Blanco P. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: A review of current knowledge. Expert Rev Anti Infect Ther. 2020;18(4):335–47.
Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21.
Gajdács M, Urbán E. Prevalence and antibiotic resistance of Stenotrophomonas maltophilia in respiratory tract samples: A 10-Year epidemiological snapshot. Health Serv Res Manag Epidemiol. 2019;6:2333392819870774.
Agarwal S, Kakati B, Khanduri S, Gupta S. Emergence of Carbapenem resistant non-fermenting gram-negative Bacilli isolated in an ICU of a Tertiary Care Hospital. J Clin Diagn Res. 2017;11(1):Dc04–dc7.
Doi Y. Treatment Options for Carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):565–s75.
Ciacci N, Boncompagni S, Valzano F, Cariani L, Aliberti S, Blasi F et al. In Vitro Synergism of Colistin and N-acetylcysteine against Stenotrophomonas maltophilia. Antibiot (Basel). 2019; 8(3).
Binsker U, Käsbohrer A, Hammerl JA. Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol Rev 2022; 46(1).
Martínez-Servat S, Yero D, Huedo P, Marquez R, Molina G, Daura X, et al. Heterogeneous colistin-resistance phenotypes coexisting in Stenotrophomonas maltophilia isolates influence Colistin susceptibility testing. Front Microbiol. 2018;9:2871.
Juhász E, Iván M, Pintér E, Pongrácz J, Kristóf K. Colistin resistance among blood culture isolates at a tertiary care centre in Hungary. J Glob Antimicrob Resist. 2017;11:167–70.
Naas T, Lina G, Santerre Henriksen A, Longshaw C, Jehl F. In vitro activity of cefiderocol and comparators against isolates of Gram-negative pathogens from a range of infection sources: SIDERO-WT-2014-2018 studies in France. JAC Antimicrob Resist. 2021;3(2):dlab081.
Ibn Saied W, Merceron S, Schwebel C, Le Monnier A, Oziel J, Garrouste-Orgeas M, et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: Risk factors and outcome. J Infect. 2020;80(3):279–85.
Abat C, Fournier PE, Jimeno MT, Rolain JM, Raoult D. Extremely and pandrug-resistant bacteria extra-deaths: myth or reality? Eur J Clin Microbiol Infect Dis. 2018;37(9):1687–97.
Corlouer C, Lamy B, Desroches M, Ramos-Vivas J, Mehiri-Zghal E, Lemenand O, et al. Stenotrophomonas maltophilia healthcare-associated infections: Identification of two main pathogenic genetic backgrounds. J Hosp Infect. 2017;96(2):183–8.
Biswas S, Dubus JC, Reynaud-Gaubert M, Stremler N, Rolain JM. Evaluation of colistin susceptibility in multidrug-resistant clinical isolates from cystic fibrosis, France. Eur J Clin Microbiol Infect Dis. 2013;32(11):1461–4.
Jacquier H, Le Monnier A, Carbonnelle E, Corvec S, Illiaquer M, Bille E, et al. In vitro antimicrobial activity of “last-resort” antibiotics against unusual nonfermenting gram-negative bacilli clinical isolates. Microb drug Resist. 2012;18(4):396–401.
Cercenado E, Cardenoso L, Penin R, Longshaw C, Henriksen AS, Pascual A. In vitro activity of cefiderocol and comparators against isolates of Gram-negative bacterial pathogens from a range of infection sources: SIDERO–WT–2014–2018 studies in Spain. J Glob Antimicrob Resist. 2021;26:292–300.
Gómez-Garcés JL, Aracil B, Gil Y, Burillo A. Susceptibility of 228 non-fermenting gram-negative rods to tigecycline and six other antimicrobial drugs. J Chemother. 2009;21(3):267–71.
Hrbacek J, Cermak P, Zachoval R. Current antibiotic resistance patterns of rare uropathogens: survey from central european Urology Department 2011–2019. BMC Urol. 2021;21(1):61.
Yero D, Huedo P, Conchillo-Solé O, Martínez-Servat S, Mamat U, Coves X, et al. Genetic variants of the DSF Quorum sensing system in Stenotrophomonas maltophilia influence virulence and resistance phenotypes among Genotypically Diverse Clinical isolates. Front Microbiol. 2020;11:1160.
Gajdács M, Urbán E. A 10-year single-center experience on Stenotrophomonas maltophilia resistotyping in Szeged, Hungary. Eur J Microbiol Immunol (Bp). 2020;10(2):91–7.
Gajdács M, Burián K, Terhes G. Resistance levels and epidemiology of non-fermenting Gram-Negative Bacteria in urinary tract infections of inpatients and outpatients (RENFUTI): a 10-Year epidemiological snapshot. Antibiot (Basel). 2019;8(3):143.
Emese J, Andrea K, Júlia P, Miklós I, Katalin K. In vitro activity of colistin and trimethoprim/sulfamethoxazole against consortia of multidrug resistant non-fermenting gram-negative bacilli isolated from lower respiratory tract. 2017; 10(7): e14034.
Juhász E, Pongrácz J, Iván M, Kristóf K. Antibiotic susceptibility of sulfamethoxazole-trimethoprim resistant Stenotrophomonas maltophilia strains isolated at a tertiary care centre in Hungary. Acta Microbiol Immunol Hung. 2015;62(3):295–305.
Juhász E, Krizsán G, Lengyel G, Grósz G, Pongrácz J, Kristóf K. Infection and colonization by Stenotrophomonas maltophilia: Antimicrobial susceptibility and clinical background of strains isolated at a tertiary care centre in Hungary. Ann Clin Microbiol Antimicrob. 2014;13:333.
Vincenti S, Quaranta G, De Meo C, Bruno S, Ficarra MG, Carovillano S, et al. Non-fermentative gram-negative bacteria in hospital tap water and water used for haemodialysis and bronchoscope flushing: prevalence and distribution of antibiotic resistant strains. Sci Total Environ. 2014;499:47–54.
Lambiase A, Raia V, Del Pezzo M, Sepe A, Carnovale V, Rossano F. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis. 2006;6:4.
Turan Özden H, Togan T, Azap Ö. In Vitro Susceptibility of Tigecycline and Colistin Against Stenotrophomonas maltophilia. 2018; 23(1):25–30.
Küçükates E, Gültekin N. Antimicrobial susceptibility and microorganisms isolated from blood cultures of hospitalized patients in intensive care units. Haseki Tip Bulteni. 2016;54(2):97–102.
Gülmez D, Cakar A, Sener B, Karakaya J, Hasçelik G. Comparison of different antimicrobial susceptibility testing methods for Stenotrophomonas maltophilia and results of synergy testing. J Infect Chemother. 2010;16(5):322–8.
Vidigal PG, Dittmer S, Steinmann E, Buer J, Rath PM, Steinmann J. Adaptation of Stenotrophomonas maltophilia in cystic fibrosis: molecular diversity, mutation frequency and antibiotic resistance. Int J Med Microbiol. 2014;304(5–6):613–9.
Goncalves-Vidigal P, Grosse-Onnebrink J, Mellies U, Buer J, Rath PM, Steinmann J. Stenotrophomonas maltophilia in cystic fibrosis: Improved detection by the use of selective agar and evaluation of antimicrobial resistance. J Cyst Fibros. 2011;10(6):422–7.
Hogardt M, Schmoldt S, Götzfried M, Adler K, Heesemann J. Pitfalls of polymyxin antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2004;54(6):1057–61.
Milne KE, Gould IM. Combination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patients. Antimicrob Agents Chemother. 2012;56(8):4071–7.
Samonis G, Karageorgopoulos DE, Maraki S, Levis P, Dimopoulou D, Spernovasilis NA, et al. Stenotrophomonas maltophilia infections in a general hospital: patient characteristics, antimicrobial susceptibility, and treatment outcome. PLoS ONE. 2012;7(5):e37375.
Samonis G, Maraki S, Rafailidis PI, Kapaskelis A, Kastoris AC, Falagas ME. Antimicrobial susceptibility of Gram-negative nonurinary bacteria to fosfomycin and other antimicrobials. Future Microbiol. 2010;5(6):961–70.
Galani I, Kontopidou F, Souli M, Rekatsina PD, Koratzanis E, Deliolanis J, et al. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Agents. 2008;31(5):434–9.
Marchac V, Equi A, Le Bihan-Benjamin C, Hodson M, Bush A. Case-control study of Stenotrophomonas maltophilia acquisition in cystic fibrosis patients. Eur Respir J. 2004;23(1):98–102.
Laffineur K, Janssens M, Charlier J, Avesani V, Wauters G, Delmée M. Biochemical and susceptibility tests useful for identification of nonfermenting gram-negative rods. J Clin Microbiol. 2002;40(3):1085–7.
Kuo SC, Liu CE, Lu PL, Chen YS, Lu MC, Ko WC, et al. Activity of ceftolozane-tazobactam against Gram-negative pathogens isolated from lower respiratory tract infections in the Asia-Pacific region: SMART 2015–2016. Int J Antimicrob Agents. 2020;55(3):105883.
Wu RX, Yu CM, Hsu ST, Wang CH. Emergence of concurrent levofloxacin- and trimethoprim/sulfamethoxazole-resistant Stenotrophomonas maltophilia: Risk factors and antimicrobial sensitivity pattern analysis from a single medical center in Taiwan. J Microbiol Immunol Infect. 2022;55(1):107–13.
Wang CH, Yu CM, Hsu ST, Wu RX. Levofloxacin-resistant Stenotrophomonas maltophilia: Risk factors and antibiotic susceptibility patterns in hospitalized patients. J Hosp Infect. 2020;104(1):46–52.
Azimi A, Aslanimehr M, Yaseri M, Shadkam M, Douraghi M. Distribution of smf-1, rmlA, spgM and rpfF genes among Stenotrophomonas maltophilia isolates in relation to biofilm-forming capacity. J Glob Antimicrob Resist. 2020;23:321–6.
Motamedifar M, Heidari H, Yasemi M, Sedigh Ebrahim-Saraie H. Molecular epidemiology and characteristics of 16 cases with Stenotrophomonas maltophilia bacteraemia in pediatric intensive care units. Ann Ig. 2017;29(4):264–72.
Averbuch D, Avaky C, Harit M, Stepensky P, Fried I, Ben-Ami T, et al. Non-fermentative gram-negative rods bacteremia in children with cancer: a 14-year single-center experience. Infection. 2017;45(3):327–34.
Paopradit P, Srinitiwarawong K, Ingviya N, Singkhamanan K, Vuddhakul V. Distribution and characterization of Stenotrophomonas maltophilia isolates from environmental and clinical samples in Thailand. J Hosp Infect. 2017;97(2):185–91.
Wei C, Ni W, Cai X, Zhao J, Cui J. Evaluation of Trimethoprim/Sulfamethoxazole (SXT), Minocycline, Tigecycline, Moxifloxacin, and Ceftazidime alone and in combinations for SXT-Susceptible and SXT-Resistant Stenotrophomonas maltophilia by in Vitro Time-Kill experiments. PLoS ONE. 2016;11(3):e0152132.
Ni W, Li Y, Guan J, Zhao J, Cui J, Wang R, et al. Effects of Efflux Pump inhibitors on Colistin Resistance in Multidrug-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother. 2016;60(5):3215–8.
Asaad AM, Al-Ayed MS, Qureshi MA. Emergence of unusual nonfermenting gram-negative nosocomial pathogens in a saudi hospital. Jpn J Infect Dis. 2013;66(6):507–11.
Somily AM. Comparison of E-test and disc diffusion methods for the in vitro evaluation of the antimicrobial activity of colistin in multi-drug resistant gram-negative Bacilli. Saudi Med J. 2010;31(5):507–11.
Tan TY, Ng SY. The in-vitro activity of colistin in gram-negative bacteria. Singap Med J. 2006;47(7):621–4.
Deslouches B, Steckbeck JD, Craigo JK, Doi Y, Burns JL, Montelaro RC. Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob Agents Chemother. 2015;59(2):1329–33.
Church D, Lloyd T, Peirano G, Pitout J. Antimicrobial susceptibility and combination testing of invasive Stenotrophomonas maltophilia isolates. Scand J Infect Dis. 2013;45(4):265–70.
Moskowitz SM, Garber E, Chen Y, Clock SA, Tabibi S, Miller AK, et al. Colistin susceptibility testing: evaluation of reliability for cystic fibrosis isolates of Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother. 2010;65(7):1416–23.
San Gabriel P, Zhou J, Tabibi S, Chen Y, Trauzzi M, Saiman L. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2004;48(1):168–71.
Wu K, Yau YC, Matukas L, Waters V. Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 2013;57(3):1546–8.
Rodríguez CH, Nastro M, Calvo JL, Fariña ME, Dabos L, Famiglietti A. In vitro activity of colistin against Stenotrophomonas maltophilia. J Glob Antimicrob Resist. 2014;2(4):316–7.
Nicodemo AC, Araujo MR, Ruiz AS, Gales AC. In vitro susceptibility of Stenotrophomonas maltophilia isolates: Comparison of disc diffusion, etest and agar dilution methods. J Antimicrob Chemother. 2004;53(4):604–8.
Gales AC, Reis AO, Jones RN. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J Clin Microbiol. 2001;39(1):183–90.
Kidd TJ, Ramsay KA, Hu H, Bye PT, Elkins MR, Grimwood K, et al. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J Clin Microbiol. 2009;47(5):1503–9.
Sader HS, Duncan LR, Arends SJR, Carvalhaes CG, Castanheira M. Antimicrobial activity of Aztreonam-Avibactam and Comparator Agents when tested against a large Collection of Contemporary Stenotrophomonas maltophilia isolates from Medical Centers Worldwide. Antimicrob Agents Chemother. 2020;64(11):e01433–20.
Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, Against Gram-Negative Bacilli isolated by Clinical Laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int J Antimicrob Agents. 2019;53(4):456–66.
Jacobs MR, Abdelhamed AM, Good CE, Rhoads DD, Hujer KM, Hujer AM, et al. ARGONAUT-I: activity of Cefiderocol (S-649266), a Siderophore Cephalosporin, against Gram-Negative Bacteria, including carbapenem-resistant nonfermenters and Enterobacteriaceae with defined extended-spectrum β-Lactamases and Carbapenemases. Antimicrob Agents Chemother. 2019;63(1):e01801–18.
Jayol A, Nordmann P, André C, Poirel L, Dubois V. Evaluation of three broth microdilution systems to determine colistin susceptibility of Gram-negative bacilli. J Antimicrob Chemother. 2018;73(5):1272–8.
Averbuch D, Tridello G, Hoek J, Mikulska M, Akan H, Yanez San Segundo L, et al. Antimicrobial Resistance in Gram-Negative rods causing bacteremia in hematopoietic stem cell transplant recipients: Intercontinental prospective study of the infectious Diseases Working Party of the european bone marrow Transplantation Group. Clin Infect Dis. 2017;65(11):1819–28.
Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: Results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int J Antimicrob Agents. 2014;43(4):328–34.
Sader HS, Flamm RK, Jones RN. Tigecycline activity tested against antimicrobial resistant surveillance subsets of clinical bacteria collected worldwide (2011). Diagn Microbiol Infect Dis. 2013;76(2):217–21.
Guan X, He L, Hu B, Hu J, Huang X, Lai G, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):15–25.
Jean SS, Chang YC, Lin WC, Lee WS, Hsueh PR, Hsu CW. Epidemiology, treatment, and Prevention of Nosocomial bacterial pneumonia. J Clin Med 2020; 9(1).
Vijay S, Bansal N, Rao BK, Veeraraghavan B, Rodrigues C, Wattal C, et al. Secondary infections in hospitalized COVID-19 patients: indian experience. Infect Drug Resist. 2021;14:1893–903.
Mojica MF, Humphries R, Lipuma JJ, Mathers AJ, Rao GG, Shelburne SA, et al. Clinical challenges treating Stenotrophomonas maltophilia infections: An update. JAC Antimicrob Resist. 2022;4(3):dlac040.
Rhoads DD. Stenotrophomonas maltophilia Susceptibility Testing Challenges and strategies. J Clin Microbiol. 2021;59(9):e0109421.
Chew KL, La MV, Lin RTP, Teo JWP. Colistin and Polymyxin B susceptibility testing for Carbapenem-Resistant and mcr-positive Enterobacteriaceae: comparison of Sensititre, MicroScan, Vitek 2, and Etest with Broth Microdilution. J Clin Microbiol. 2017;55(9):2609–16.
Dadashi M, Hajikhani B, Nazarinejad N, Nourisepehr N, Yazdani S, Hashemi A et al. Global prevalence and distribution of antibiotic resistance among clinical isolates of Stenotrophomonas maltophilia: A systematic review and meta-analysis. J Glob Antimicrob Resist. 2023; S2213-7165(23)00039 – 5.
Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–8.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol methods. 2006; 11(2): 193–206.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Acknowledgements
This is to acknowledge that the project leading to the publication of this paper was supported by the research deputy of Shahrekord University of Medical Sciences (SKUMS), with project number 4027.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Work design and task assignment: MSSA, HH and AM-H. Data collection and analysis: AM-H, ADS, RA and EAF. Manuscript writing: AT, MSSA. Manuscript revision: D L.P, MSSA and HH. Manuscript approval: all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shahrekord University of Medical Sciences (Register code: IR.SKUMS.REC.1401.174).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Delgarm Shams-Abadi, A., Mohammadian-Hafshejani, A., Paterson, D.L. et al. The prevalence of colistin resistance in clinical Stenotrophomonas maltophilia isolates worldwide: a systematic review and meta-analysis. BMC Microbiol 23, 200 (2023). https://doi.org/10.1186/s12866-023-02950-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02950-6