Abstract
Background
The emergence of carbapenem-resistant Enterobacterales (CRE) continues to threaten public health due to limited therapeutic options. In the current study the incidence of carbapenem resistance among the 104 clinical isolates of Escherichia coli and the genomic features of carbapenem resistant isolates were investigated.
Methods
The susceptibility to imipenem, tigecycline and colistin was tested by broth dilution method. Susceptibility to other classes of antimicrobials was examined by disk diffusion test. The presence of blaOXA-48, blaKPC, blaNDM, and blaVIM carbapenemase genes was examined by PCR. Molecular characteristics of carbapenem resistant isolates were further investigated by whole-genome sequencing (WGS) using Illumina and Nanopore platforms.
Results
Four isolates (3.8%) revealed imipenem MIC of ≥32 mg/L and positive results for modified carbapenem inactivation method and categorized as carbapenem resistant E. coli (CREC). Colistin, nitrofurantoin, fosfomycin, and tigecycline were the most active agents against all isolates (total susceptibility rate of 99, 99, 96 and 95.2% respectively) with the last three compounds being found as the most active antimicrobials for carbapenem resistant isolates (susceptibility rate of 100%). According to Multilocus Sequence Type (MLST) analysis the 4 CREC isolates belonged to ST167 (n = 2), ST361 (n = 1) and ST648 (n = 1). NDM was detected in all CREC isolates (NDM-1 (n = 1) and NMD-5 (n = 3)) among which one isolate co-harbored NDM-5 and OXA-181 carbapenemases. WGS further detected blaCTX-M-15, blaCMY-145, blaCMY-42 and blaTEM-1 (with different frequencies) among CREC isolates. Co-occurrence of NDM-type carbapenemase and 16S rRNA methyltransferase RmtB and RmtC was found in two isolates belonging to ST167 and ST648. A colistin-carbapenem resistant isolate which was mcr-negative, revealed various amino acid substitutions in PmrB, PmrD and PhoPQ proteins.
Conclusion
About 1.9% of E. coli isolates studied here were resistant to imipenem, colistin and/or amikacin which raises the concern about the outbreaks of difficult-to-treat infection by these emerging superbugs in the future.
Similar content being viewed by others
Background
Carbapenems are often one of the last resort options available to clinicians for treatment of serious infections caused by multidrug-resistant (MDR) Gram-negative bacteria including Enterobacterales [1]. However, the clinical overuse of carbapenem agents inevitably resulted in increased drug resistance and emergence of carbapenem resistant Enterobacterales (CRE). Currently, the CRE infections have become an important focus of infection control due to limited therapeutic choices and deleterious outcomes for patients [2, 3]. Treating these infections are considered challenging and often requires the use of older agents, such as tigecycline or colistin, which is frequently associated with unclear efficacy and/or toxicity issues [4, 5]. Carbapenem non-susceptibility in CRE is mainly related to enzymatic hydrolysis of antibiotics by carbapenemase (s), and to lesser extent production of dysfunctional entry routes of carbapenems (e.g. mutated porins) or overexpressed efflux pumps [5]. Clinically relevant carbapenemases belong to Class A Klebsiella pneumoniae carbapenemase [KPC]; Class B metallo-β-lactamases (MBLs) such as New Delhi MBL (NDM), and imipenemase (IMP); Verona integron-encoded MBL (VIM), and Class D oxacillinases [OXA enzymes] such as OXA-48-like carbapenemases. Class A and D β-lactamases each utilize serine at the active site whereas Class B enzymes require divalent cations usually Zn2+ ion as metal cofactor to catalyze the hydrolysis of β-lactams [5, 6]. The MBLs are a source of great concern as they hydrolyze all β-lactams except monobactams including aztreonam, and are not inhibited by the commercially available β-lactamase inhibitors such as avibactam, clavulanate, sulbactam, and tazobactam [7]. Moreover, MBLs are often coproduced with other β-lactamases that can hydrolyze aztreonam or even with other determinants conferring resistance to aminoglycosides and fluoroquinolones resulting in multidrug resistance phenotype [7, 8].
The geographic distribution of MBL enzymes among CRE is substantially diverse. According to a study on isolates from 40 countries conducted between 2012 and 2014, 44.2% of MBL-positive isolates of Enterobacteriaceae were found to carry blaNDM, 39.3% carried blaVIM, and 16.5% harbored blaIMP [7]. Escherichia coli, as one of the most problematic species of Enterobacterales is an opportunistic pathogen and a common cause of urinary tract, enteric and bloodstream infections worldwide [9]. There is limited information regarding the incidence and the type of carbapenemases mediating resistance to these last-resort agents among E. coli isolates from Iran [10, 11]. Proper monitoring of the drug resistance patterns in the circulating bacteria and unraveling mechanisms conferring carbapenem resistance would provide valuable information for designing appropriate antibiotic prescription guidelines and subsequently better infection control strategies. Therefore, we aimed to investigate the drug susceptibility pattern of E. coli isolates obtained from different clinical samples, to identify prevalence and genotypes of carbapenem resistant isolates.
Results
Bacterial isolates and antimicrobial susceptibility testing
In the current work we studied 104 E. coli isolates obtained from a large referral hospital in an effort to determine the incidence of carbapenem resistance and the genomic features of resistant clones. According to imipenem susceptibility testing results performed by broth macrodiltion, and interpreted by CLSI guidelines, 94% of isolates (n = 98) were characterized with imipenem MICs ≤1 mg/L and were categorized as susceptible. While two (1.9%) and four (3.8%) isolates with imipenem MICs of 2 and ≥ 32 mg/L were categorized as intermediate and resistant respectively (Table 1). A total of 99% (n = 103) and 95.2% (n = 99) of isolates had colistin and tigecycline MICs of ≤2 and ≤ 0.5 mg/L, respectively and were categorized as susceptible to the these antimicrobials (Table 1). The susceptibility rate to other antimicrobials tested by disk diffusion are presented in Table 2. The four imipenem resistant isolates which also showed resistance to meropenem, revealed positive results for Modified Carbapenem Inactivation Method (mCIM) ((supplementary Fig. 1) and were designated as carbapenem resistant E. coli (CREC). Moreover, all imipenem susceptible (n = 98) and intermediate isolates (n = 2) were found to be also susceptible to meropenem. In addition, the two imipenem intermediate-meropenem susceptible isolates as well as 20 randomly selected imipenem-meropenem susceptible isolates showed negative results for mCIM. Since Enterobacterales with imipenem MICs ≤2 mg/L are categorized as susceptible according to EUCAST breakpoints, the above mentioned two isolates (with imipenem MIC = 2 mg/L) were also categorized as carbapenem susceptible E. coli (CSEC) along with other imipenem-meropenem susceptible isolates. Overall, colistin, nitrofurantoin, fosfomycin and tigecycline were the most active agents against all studied isolates (total susceptibility rate of 99, 99, 96, and 95.2% respectively) with the last three compounds being found as the most effective antimicrobials for carbapenem resistant isolates (susceptibility rate of 100%). Among the CSEC, 10% were characterized with no resistance to tested antibiotics and 11, 15, 23, 21 and 20% showed resistance to one, two, three, four and five or more antibiotics respectively. On the other hand, all CREC isolates were found to be resistant to at least three different tested antimicrobial agents (in addition to β-lactams).
Genotypic antimicrobial resistance
PCR screening of 4 carbapenemase genes was performed for all isolates (n = 6) with imipenem MIC ≥2 mg/l. The blaNDM (n = 4 isolates) and blaOXA-48-like (n = 1 isolate) were the only carbapenemase encoding genes detected among CREC isolates and the absence of other carbapenemases in the studied isolates was confirmed by WGS analysis. In two isolates with imipenem MICs of 2 mg/L none of the studied carbapenemase genes were detected by PCR. According to WGS analysis, blaNDM (NDM-1 and NDM-5) was detected in all CREC isolates with blaNDM-5 variant being found in three isolates. The OXA-181 variant was detected in one isolate co-harboring NDM-5 enzyme. Multilocus Sequence Type (MLST) analysis showed that the four CREC isolates belonged to ST167 (n = 2), ST361 (n = 1) and ST648 (n = 1) (Table 3). The CREC isolates harbored one or more β-lactamases such as CTX-M-15, CMY-145, CMY-42 and TEM-1. Two CREC isolates belonging to ST167 and ST648 harbored 16S rRNA methytrnsferaseand (RMTase) RmtB and RmtC conferring resistance to all clinically relevant aminoglycosides. The genes associated with resistance to other antimicrobials including tetracyclines, sulfonamide, aminoglycosides and quinolones are presented in Table 4. Analysis of chromosomally-encoded proteins involved in antimicrobial resistance revealed amino acid substitutions in quinolone resistance-determining regions (QRDR) in GyrA (S83L, D87N) and ParC (S80I) which was consistent with quinolone resistance phenotype in all CREC isolates. In a carbapenem-colistin resistant isolate (colistin MICs of 4 mg/L), no plasmid born colistin resistance mcr-type gene was detected. However, whole genome sequencing revealed various chromosomal point mutations in PmrB (H2R, G19R, D283G, and A360V), PhoQ (L467M), PhoP (I44L) and PmrD (N11D, M20K, A27T, K82Q, V83S) proteins.
Genomic analysis of virulence genes, revealed presence of genes belonging to several functional categories including those involved in iron acquisition (irp2, fyuA, chuA, iucC, iutA, sitA), adherence (lpfA, yfcV, csgA, fdeC, yeh fimbrial genes, air, fimH), serum survival (iss, traT), tellurite resistance (terC), production of toxin (hlyE), capsular polysaccharide (kpsE), and colicin (colE2, colE8) (Table 4).
Whole-genome assembly and genome visualization of CREC strains
Illumina short read and Oxford Nanopore long read data were used as input of hybrid de novo assembly to reconstruct the complete CREC genomes. The Unicycler-short reads/ Ratatosk corrected long reads hybrid assembly resulted in closed chromosomes for all three isolates. The genomic characteristics of all three assembled genomes are shown in supplementary Table 1.
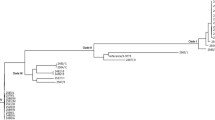
Moreover, to understand the relevance of the assembled genomes of this study in comparison to global genomes, a comparative genome analysis was performed with other complete E. coli genomes of ST648 (GenBank Accessions CP048107, as reference genome), ST167 (GenBank Accessions CANDYB000000000) and ST361 (GenBank Accessions CP103704) (supplementary Fig. 2 & supplementary Table 2).
Gene composition analysis of the plasmids in CREC strains
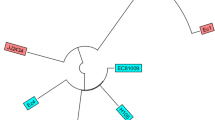
In this study, six closed complete plasmids were detected in three CREC strains and were further analyzed. The CREC-18 strain had an IncF-like plasmid, pCREC-18 (157,125 bp) which co-harbors the blaNDM-5 and genes conferring resistance to other antimicrobials as presented in Fig. 1A. BLASTn of the pCREC-18 sequence showed high similarity to other blaNDM-5 carrying plasmids from clinical isolates i.e. NDM-5- IncFIA plasmid in a clinical E. coli isolate (CP083875.1).
The strain CREC-19 harbored two different plasmids, an IncA/C2-like plasmid (pCREC-19_1, 137,594 bp) co-harboring blaNDM-1, rmtC and additional resistance conferring genes (Fig. 1B) where blaNDM-1 was located in a mobile region with a structure of rmtc-ISKpn14-blaNDM1-bleMBL-trpF-tat-dsbC-groES-groEL as reported in other studies [12, 13]. BLASTn of this plasmid showed high similarity to other blaNDM-1 carrying plasmids found in other Enterobacterales i.e. NDM-1-IncA/C2 plasmid in a clinical Klebsiella pneumoniae isolate (CP050164.1).
The second plasmid in CREC-19 was an IncFII-like plasmid (pCREC-19_2, 96,036 bp) carrying qnrS1, sul1, tetA and other antibiotic resistance genes (ARGs) as indicated in Fig. 1C.
Strain CREC-20 carried three plasmids: The plasmid pCREC-20_1 (128,273 bp) harboring cmlA1, sul3, aadA1 and other ARGs was a type of IncB/O/K/Z plasmid (Fig. 1D). In addition, and similar to CREC-18, this strain also contains a blaNDM-5 containing IncF-like plasmid (104,581 bp) that carries other ARGs including sul1, dfrA12 and aadA2 (Fig. 1E) but, the overall structure of the two NDM-5-producing plasmids were different (Supplementary Fig. 3). Finally, the CREC-17 also contained plasmid(s) carrying blaNDM-5 and other resistance conferring genes. However, the long read sequencing was not successful and no closed plasmid(s) were achieved for this sample relying only on the short reads as input for assembly. The genome information of all detected plasmids are summarized in Table 5.
Discussion
MDR Gram-negative bacteria are a major global public health threat, with an increasing incidence of CRE infections, for which therapeutic choices are limited. The most common mechanism of carbapenem resistance in CRE is production of carbapenemase enzymes which are encoded by genes located on plasmids, making them readily transferable [14]. In the current study the rate of carbapenem resistance among the studied isolates was found to be 3.84% (n = 4). MLST showed that the two CREC isolates (CREC-18 and 19) belonged to ST167, and the remaining 2 isolates were characterized with ST361 (CREC-20) and ST648 (CREC-17). The E. coli ST167 clone is emerging throughout the world, being frequently recognized for having a link with carbapenem resistance [15, 16] and for being involved in the transmission of the blaNDM-1 and blaNDM-5 genes in hospitals [17]. It has been demonstrated that the ST167 has peculiar virulence characteristics which facilitate its evolution to a high-risk clone with widespread colonization and infection capabilities [18]. Moreover, ST361 clone has been recently reported as the most frequent lineage of NDM-5 producing E. coli from Korea [19].
NDM was found in all CREC isolates with the NDM-5 being the most prevalent variant (n = 3). This is consistent with previous reports identifying the highest prevalence of NDM-positive Enterobacterales in the Indian subcontinent and the Middle East region [20]. Overall the prevalence of carbapenem resistant E. coli isolates in Iran is relatively low and a handful of studies have reported NDM-1 (the most prevalent), NDM-7 or OXA-181 producing E. coli isolates from the country [10, 11]. Our study would be the first report of clinical isolates of carbapenem-resistant E. coli carrying NDM-5 from Iran. Recently we reported NDM-5 producing K. pneumoniae isolates of clinical origin for the first time from Iran [21]. While NDM-1 is the most prevalent variant with widespread distribution among several members of Enterobacterales and other Gram-negative bacilli, NDM-5 appears to be more confined to E. coli isolates [22]. It has been described that NDM-producing strains are frequently resistant to a wide range of antimicrobial agents, due to co-harboring additional resistance determinants [22]. According to antimicrobial susceptibility testing results tigecycline, nitrofurantoin and fosfomycin were found as the most active agents against the CREC isolates. Colistin and tigecycline have been recognized as promising alternatives for treatment of CRE infections [23]. However, one CREC isolate (CREC17) in this study was characterized with colistin MIC of 4 mg/l and categorized as colistin resistant. Colistin resistance in Enterobacterales is found to be mainly mediated by acquisition of a plasmid-borne mobile colistin resistance (mcr) gene (encoding phosphoethanolamine transferase,) and/or chromosomal mutations within PhoPQ, PmrAB two component system or their regulatory gene mgrB, all of which result in LPS modifications [24]. The colistin-carbapenem resistant isolate identified in this study lacked an mcr-type gene but revealed chromosomal mutations within pmrB, phoP, phoQ and pmrD genes. Some of the detected amino acid variations have previously been reported from colistin resistant E. coli isolates including PmrB D283G [25], PmrB G19R [26], PmrB H2R/ A360V, PhoP I44L, PhoQ L467M and PmrD N11D/M20K/A27T /K82Q/V83S [27]. More importantly we found that two CRE isolates belonging to ST167 and ST648 co-harbored 16S rRNA methyltransferase RmtB/RmtC and NDM-type carbapenemases. The RMTases have been found to confer high-level and broad spectrum resistance to all clinically-relevant aminoglycosides (MIC > 256 mg/L) and to date ten 16S RMTases have been identified (namely ArmA, RmtA-H and NpmA)) [28]. Co-occurrence of RMTases and NDM-type carbapenemase has recently been reported in Enterobacterales from Europe [29, 30] and Latin America [31]. The emergences and spread of RMTase producing Enterobacterales is of great concern as the genes encoding RMTases are frequently located on plasmids along with those coding for ESBLs or carbapenemases rendering these bacteria resistant to multiple classes of antimicrobials used to treat MDR Gram-negative infections [30].
Conclusion
Our study reports emergence of carbapenem resistant E. coli isolates harboring NDM-1, NDM-5 and OXA-181 variants. Although the prevalence of the CREC isolates were relatively low (< 4%) about 1.9% of E. coli isolates studied here were resistant to imipenem, quinolones, and colistin and/or amikacin which raises concerns about the future outbreak by highly resistant Enterobacterales. Considering the importance of aminoglycosides and polymyxins in the treatment of MDR Enterobacterales, resistance to these agents is considered as another emerging issue in addition to carbapenem resistance. Therefore, it is necessary to implement appropriate surveillance studies and infection control measures, to carefully monitor the prevalence of these pan- β-lactam-aminoglycoside-polymyxin resistant isolates in order to prevent the occurrence of outbreaks caused by these emerging superbugs.
Materials and methods
Study design and bacterial isolates
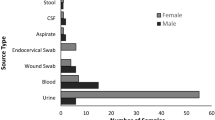
A total of 104 isolates of E. coli obtained from patients hospitalized at Imam Reza hospital, an 800-bed training hospital in Tabriz city (in period of June to September 2021) were studied. The clinical samples from which bacterial strains were isolated included urine, wound, blood and tracheal aspirates. Bacterial isolates were identified to species level using conventional biochemical methods including IMViC tests (indole test, methyl red test, Voges-Proskauer reaction, citrate utilization test), urease test, motility, ONPG (O-nitrophenyl-beta-D-galactopyranoside), reactions observed on Triple Sugar Iron (TSI) agar (H2S and gas production, carbohydrate utilization pattern) [32].
Antimicrobial susceptibility testing
Susceptibility of the bacterial isolates to imipenem, colistin and tigecycline was performed by reference broth macrodilution methodology using imipenem, tigecycline hydrae and colistin sulphate powders from Glentham Life Sciences (Corsham, United Kingdom) and freshly prepared (less than 12-h-old) Mueller-Hinton broth from Difco (BD Diagnostic Systems, Sparks, MD, United States). Testing susceptibility to other antibiotics was performed by disc diffusion method (Kirby– Bauer) using the following antibiotics: meropenem, gentamicin, amikacin, ceftazidime, ciprofloxacin, tetracycline, doxycycline, chloramphenicol, nitrofurantoin and fosfomycin (BBL Sensi-DiscTM, Becton-Dickinson, Sparks, MD, United States). Clinical and Laboratory Standards Institute (CLSI) guidelines was used for data interpretation of all tested antimicrobials except for tigecycline and colistin. The European committee on antimicrobial susceptibility testing (EUCAST) criteria issued for Enterobacterales were applied for interpretation of tigecycline and colistin susceptibility results (tigecycline MIC > 0.5 mg/l, resistant; colistin MIC > 2 mg/l, resistant). Escherichia coli ATCC 25922 was used as a quality-control strain for antimicrobial susceptibility testing.
Phenotypic detection of carbapenemase production by mCIM
The mCIM test was performed for all isolates with imipenem MIC≥2 mg/L or meropenem resistant isolates and 20 imipenem-meropenem susceptible isolates, which were selected randomly as negative controls. For each isolate to be tested a 1-μL loopful of bacteria from an overnight blood agar plate was resuspended in 2-mL tube of tryptic soy broth (TSB). A 10-μg meropenem disk was then placed in the suspension, and incubated at 35 °C for 4 h ± 15 min. Subsequently, the disks were removed and placed on MHA plates freshly inoculated with a 0.5 McFarland suspension of E. coli ATCC 25922 strain. The plates were incubated at 35 °C for 18 to 24 h. An inhibition zone diameter of 6–15 mm or colonies within a 16–18 mm zone was considered to be a positive result, and a zone of inhibition ≥19 mm was considered to be a negative result [33].
Detection of carbapenemase encoding genes
The genomic DNA was extracted from the bacterial strains by boiling the lysates prepared from the strains as described previously [34]. The presence of four major carbapenemase-encoding genes, blaKPC, blaVIM, blaNDM, and blaOXA-48-like, was examined by PCR using the primers and amplification conditions described previously [35]. Clinical isolates of Klebsiella pneumoniae harboring 4 different carbapenemases (obtained from our microbial collection and confirmed for having carbapenemase gene by PCR and sequencing) were used as positive controls.
Whole genome sequencing
All four CREC strains were shipped to the Emerging Bacterial Pathogens Unit, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy in order to preform illumina short-read and Nanopore long-read whole genome sequencing.
Illumina short-read DNA sequencing and analysis
In brief, genomic DNA from the CREC strains were extracted from MHA plate cultures using the Maxwell 16 Cell DNA Purification kit (Promega, US). Concentration of extracted DNA was determined using the fluorescent based assays on the Thermo Fisher® Scientific Qubit® fluorometer. Libraries of genome fragments were prepared for sequencing using the Nextera XT v2 set A kit (Illumina, CA, US) as per manufacturer’s instruction. Sequencing was performed on an Illumina NextSeq 500 platform with 150-bp paired-end reading.
The sequenced raw reads were trimmed with Trimmomatic [36] and reads shorter than 20 bp were discarded. The resulting reads were mapped using BWA-MEM to the complete genome of Escherichia coli ATCC 8739 and also the two colistin susceptible strains of this study (CREC18, 19) (for identification of altered loci involved in colistin resistance in CREC17). Variant calling process and filtering for high-quality variants were performed as described previously [37].
Nanopore long-read DNA sequencing and analysis
In order to recover large-size DNA, genomic DNA of CREC strains were purified from liquid culture using the Genomic DNA Clean & Concentrator kit (Zymo Research, Irvine, CA, US), according to the standard protocol (https://files.zymoresearch.com/protocols/). In the next step, rapid barcoding sequencing kit (SQK-RBK004 version 6, Oxford Nanopore) was used according to the manufacturer’s protocol for genomic DNA. The sequencing was carried out on a portable MinION device using a flow cell R9.4.1(Oxford Nanopore). Local basecalling was performed using the Guppy (version 3.1.5) (Oxford Nanopore) with the option enabled to trim the sequencing adapters. NanoFilt program (version 2.0.0) was used to filter out the reads with Phred-score < 7 and a length < 1000 and the statistics of the reads and quality scores were extracted with NanoStat (version 0.8.0). Finally, NanoLyse software (version 0.5.0) was used to remove the control DNA [38].
Hybrid whole-genome assembly and annotation
In order to reconstruct a high-quality assembled genome and plasmids based on the Illumina short reads and ONT long reads, a hybrid genome assembly was exploited through the following steps. Nanopore long reads were corrected using the Ratatosk de novo error correction tool [39] using Illumina paired-end short reads. In the next step the corrected short and long reads were assembled using Unicycler (version 0.4.8), a widely-used algorithm based on short-long reads hybrid approach [40]. The assemblies were initially visualized by Bandage (version 0.4.8) [41], a program for visualising de novo assembly graphs. For each isolate the complete and closed plasmids were exported for annotation using prokka [42] and the General Feature Format (GFF) file of each plasmid were imported to ApE software (A plasmid Editor, version 3.1.2) to draw the plasmid maps.
Plasmid replicon typing was also performed using the PlasmidFinder (Database version November/2021) with recommended parameters (https://cge.cbs.dtu.dk/services/PlasmidFinder/). The pMLST type of the plasmids were assigned using pMLST 2.0 (https://cge.food.dtu.dk/services/pMLST/). Moreover, MobileElementFinder (https://cge.food.dtu.dk/services/MobileElementFinder/, Software version: v1.0.3 (2020-10-09), Database version: v1.0.2 (2020-06-09)) was used to identify the mobile genetic elements and their relation to antimicrobial resistance genes and virulence factors. In parallel to our variant calling using only the short reads, WGS-based antimicrobial susceptibility testing was performed on hybrid assemblies using the ResFinder 4.1 with default parameters (https://cge.food.dtu.dk/services/ResFinder/) to detect chromosomal point mutations and acquired antimicrobial resistance.
The sequence reads of all strains were submitted to the NCBI sequence read archive with Project number PRJNA809646. The accession numbers for the publicly available illumina short reads and ONT long reads are as follows respectively: CREC-17(SRR21721852), CREC-18(SRR21721851 and SRR21721848), CREC-19 (SRR21721850 and SRR21721847) and CREC-20 (SRR21721849 and SRR21721846).
Availability of data and materials
The sequence reads of all strains can be accessed in GenBank under accession numbers SRR21721846 to SRR21721852.
References
Sheu C-C, Chang Y-T, Lin S-Y, Chen Y-H, Hsueh P-R. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol. 2019:80.
Tzouvelekis L, Markogiannakis A, Piperaki E, Souli M, Daikos G. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2014;20(9):862–72.
Cerqueira GC, Earl AM, Ernst CM, Grad YH, Dekker JP, Feldgarden M, et al. Multi-institute analysis of carbapenem resistance reveals remarkable diversity, unexplained mechanisms, and limited clonal outbreaks. Proc Natl Acad Sci. 2017;114(5):1135–40.
El Chakhtoura NG, Saade E, Iovleva A, Yasmin M, Wilson B, Perez F, et al. Therapies for multidrug resistant and extensively drug-resistant non-fermenting gram-negative bacteria causing nosocomial infections: a perilous journey toward ‘molecularly targeted’therapy. Expert Rev Anti-Infect Ther. 2018;16(2):89–110.
Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Supplement_7):S521–S8.
Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–76.
Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(2):1067–78.
Zhao W-H, Hu Z-Q. IMP-type metallo-β-lactamases in gram-negative bacilli: distribution, phylogeny, and association with integrons. Crit Rev Microbiol. 2011;37(3):214–26.
Kaper JB, Nataro JP, Mobley HL. Pathogenic escherichia coli. Nat Rev Microbiol. 2004;2(2):123–40.
Eyvazi S, Hakemi-Vala M, Hashemi A, Bejestani FB, Elahi N. Emergence of NDM-1-producing Escherichia coli in Iran. Archives of. Clin Infect Dis. 2018;13(4).
Solgi H, Giske CG, Badmasti F, Aghamohammad S, Havaei SA, Sabeti S, et al. Emergence of carbapenem resistant Escherichia coli isolates producing blaNDM and blaOXA-48-like carried on IncA/C and IncL/M plasmids at two Iranian university hospitals. Infect Genet Evol. 2017;55:318–23.
Wailan AM, Sidjabat HE, Yam WK, Alikhan N-F, Petty NK, Sartor AL, et al. Mechanisms involved in acquisition of Bla NDM genes by IncA/C2 and IncFIIY plasmids. Antimicrob Agents Chemother. 2016;60(7):4082–8.
Gruber TM, Göttig S, Mark L, Christ S, Kempf VA, Wichelhaus TA, et al. Pathogenicity of pan-drug-resistant Serratia marcescens harbouring Bla NDM-1. J Antimicrob Chemother. 2015;70(4):1026–30.
Aguilera-Alonso D, Escosa-García L, Saavedra-Lozano J, Cercenado E, Baquero-Artigao F. Carbapenem-resistant gram-negative bacterial infections in children. Antimicrob Agents Chemother. 2020;64(3):e02183–19.
Li F, Ye K, Li X, Ye L, Guo L, Wang L, et al. Genetic characterization of Carbapenem-resistant Escherichia coli from China, 2015–2017. BMC Microbiol. 2021;21(1):1–7.
Manyahi J, Moyo SJ, Kibwana U, Goodman RN, Allman E, Hubbard AT, et al. First identification of blaNDM-5 producing Escherichia coli from neonates and a HIV infected adult in Tanzania. J Med Microbiol. 2022;71(2):001513.
Huang Y, Yu X, Xie M, Wang X, Liao K, Xue W, et al. Widespread dissemination of carbapenem-resistant Escherichia coli sequence type 167 strains harboring Bla NDM-5 in clinical settings in China. Antimicrob Agents Chemother. 2016;60(7):4364–8.
Garcia-Fernandez A, Villa L, Bibbolino G, Bressan A, Trancassini M, Pietropaolo V, et al. Novel insights and features of the NDM-5-producing Escherichia coli sequence type 167 high-risk clone. MSphere. 2020;5(2):e00269–20.
Park Y, Choi Q, Kwon GC, Koo SH. Emergence and transmission of New Delhi metallo-beta-lactamase-5-producing Escherichia coli sequence type 361 in a tertiary Hospital in South Korea. J Clin Lab Anal. 2020;34(2):e23041.
Khan AU, Maryam L, Zarrilli R. Structure, genetics and worldwide spread of New Delhi metallo-β-lactamase (NDM): a threat to public health. BMC Microbiol. 2017;17(1):1–12.
Moghimi M, Haeili M, Mohajjel SH. Characterization of Tigecycline resistance among Tigecycline non-susceptible Klebsiella pneumoniae isolates from humans, food-producing animals, and in vitro selection assay. Front Microbiol. 2021;2165.
Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev. 2019;32(2):e00115–8.
Zhou Y-F, Liu P, Zhang C-J, Liao X-P, Sun J, Liu Y-H. Colistin combined with Tigecycline: a promising alternative strategy to combat Escherichia coli harboring Bla NDM–5 and mcr-1. Front Microbiol. 2020;10:2957.
Haeili M, Javani A, Moradi J, Jafari Z, Feizabadi MM, Babaei E. MgrB alterations mediate colistin resistance in Klebsiella pneumoniae isolates from Iran. Front Microbiol. 2017:8.
Li F, Cheng P, Li X, Liu R, Liu H, Zhang X. Molecular epidemiology and Colistin-resistant mechanism of mcr-positive and mcr-negative Escherichia coli isolated from animal in Sichuan Province, China. Front Microbiol. 2022;13:818548.
Huang J, Dai X, Ge L, Shafiq M, Shah JM, Sun J, et al. Sequence duplication within pmrB gene contribute to high-level Colistin resistance in avian pathogenic Escherichia coli. Microb Drug Resist. 2020;26(12):1442–51.
Han S, Kim JS, Hong C-K, Park S-H, Kim HS, Yu JK, et al. Identification of an extensively drug-resistant Escherichia coli clinical strain harboring mcr-1 and blaNDM-1 in Korea. J Antibio. 2020;73(12):852–8.
Doi Y, Wachino J-i, Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin. 2016;30(2):523–37.
Taylor E, Sriskandan S, Woodford N, Hopkins KL. High prevalence of 16S rRNA methyltransferases among carbapenemase-producing Enterobacteriaceae in the UK and Ireland. Int J Antimicrob Agents. 2018;52(2):278–82.
Fournier C, Poirel L, Despont S, Kessler J, Nordmann P. Increasing trends of association of 16S rRNA Methylases and Carbapenemases in Enterobacterales clinical isolates from Switzerland, 2017–2020. Microorgan. 2022;10(3):615.
Costa A, Figueroa-Espinosa R, Gaudenzi F, Lincopan N, Fuga B, Ghiglione B, et al. Co-occurrence of NDM-5 and RmtB in a clinical isolate of Escherichia coli belonging to CC354 in Latin America. Frontiers in cellular and infection. Microbiol. 2021:364.
Procop GW, Church DL, Hall GS, Janda WM. Koneman's color atlas and textbook of diagnostic microbiology: Jones & Bartlett Publishers; 2020.
Weinstein MP. Performance standards for antimicrobial susceptibility testing: Clinical and Laboratory Standards Institute; 2021.
Pishnian Z, Haeili M, Feizi A. Prevalence and molecular determinants of colistin resistance among commensal Enterobacteriaceae isolated from poultry in northwest of Iran. Gut pathogens 2019;11(1):1-8.
Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–23.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Haeili M, Shoghi Y, Moghimi M, Ghodousi A, Omrani M, Cirillo DM. Genomic features of in vitro selected mutants of Escherichia coli with decreased susceptibility to tigecycline. Journal of Global Antimicrobial Resistance. 2022;31:32–7.
Berbers B, Saltykova A, Garcia-Graells C, Philipp P, Arella F, Marchal K, et al. Combining short and long read sequencing to characterize antimicrobial resistance genes on plasmids applied to an unauthorized genetically modified Bacillus. Sci Rep. 2020;10(1):1–13.
Holley G, Beyter D, Ingimundardottir H, Møller PL, Kristmundsdottir S, Eggertsson HP, et al. Ratatosk: hybrid error correction of long reads enables accurate variant calling and assembly. Genome Biol. 2021;22(1):1–22.
Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595.
Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinform. 2015;31(20):3350–2.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinform. 2014;30(14):2068–9.
Acknowledgments
This study was supported by the University of Tabriz.
Funding
None.
Author information
Authors and Affiliations
Contributions
SB and VB preformed the antimicrobial susceptibility testing + gene detection experiments and WGS respectively. MH, AG and DMC conceived the idea, designed the study and analyzed and interpreted the data. AG and MO analyzed the WGS data. HSK and NZ was involved in study design, manuscript editing and data interpretation. All authors contributed to data interpretation and final draft of the paper and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of University of Tabriz. All isolates in this study were granted from the bacterial collection of hospital for research purposes and no human samples or data were used in this study. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest in relation to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
mCIM results for four carbapenem resistance E .coli isolates (CREC-17 to CREC-20) (positive results) and one carbapenem susceptible E. coli (CSEC-85) (negative result).
Additional file 2: Supplementary Table1.
General characteristics of assembled genomes obtained from CREC-18, CREC-19 and CREC-20.
Additional file 3: Supplementary Figure 2.
Circular visualization of the comparative genome analysis of CREC-18, CREC-19, CREC-20 with E. coli ST648 (Genbank Accessions CP048107, as reference genome), E. coli ST167 (Genbank Accessions CANDYB000000000) and E. coli ST361 (Genbank Accessions CP103704).
Additional file 4: Supplementary Table 2.
Comparative genome analysis of CREC-18, CREC-19, CREC-20 with E. coli ST648 (Genbank Accessions CP048107, as reference genome), E. coli ST167, E. coli ST167 (Genbank Accessions CANDYB000000000) and E. coli ST361 (Genbank Accessions CP103704).
Additional file 5: Supplementary Figure 3.
Alignment results of two NDM-5 carrying plasmids pCREC-18 and pCREC-20_2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Haeili, M., Barmudeh, S., Omrani, M. et al. Whole-genome sequence analysis of clinically isolated carbapenem resistant Escherichia coli from Iran. BMC Microbiol 23, 49 (2023). https://doi.org/10.1186/s12866-023-02796-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02796-y