Abstract
Background
Infections affecting neonates caused by Staphylococcus aureus are widespread in healthcare facilities; hence, novel strategies are needed to fight this pathogen. In this study, we aimed to investigate the effectiveness of the FDA-approved medications ascorbic acid, dexamethasone, and sodium bicarbonate to reduce the virulence of the resistant Staphylococcus aureus bacteria that causes neonatal sepsis and seek out suitable alternatives to the problem of multi-drug resistance.
Methods
Tested drugs were assessed phenotypically and genotypically for their effects on virulence factors and virulence-encoding genes in Staphylococcus aureus. Furthermore, drugs were tested in vivo for their ability to reduce Staphylococcus aureus pathogenesis.
Results
Sub-inhibitory concentrations (1/8 MIC) of ascorbic acid, dexamethasone, and sodium bicarbonate reduced the production of Staphylococcus aureus virulence factors, including biofilm formation, staphyloxanthin, proteases, and hemolysin production, as well as resistance to oxidative stress. At the molecular level, qRT-PCR was used to assess the relative expression levels of crtM, sigB, sarA, agrA, hla, fnbA, and icaA genes regulating virulence factors production and showed a significant reduction in the relative expression levels of all the tested genes.
Conclusions
The current findings reveal that ascorbic acid, dexamethasone, and sodium bicarbonate have strong anti-virulence effects against Staphylococcus aureus. Thus, suggesting that they might be used as adjuvants to treat infections caused by Staphylococcus aureus in combination with conventional antimicrobials or as alternative therapies.
Similar content being viewed by others
Introduction
Staphylococcus aureus (S. aureus) is a Gram-positive, human, commensal pathogen that colonizes about 30% of the human population [1]. S. aureus is a common causative pathogen of severe infections in infants, with symptoms ranging from asymptomatic colonization to skin and soft tissue infections, as well as bacteremia, necrotizing pneumonia, and endocarditis [2]. S. aureus is the most common nosocomial pathogen and is heavily associated with high morbidity and mortality [3]. Infections with S. aureus are particularly problematic since antibiotic resistance is common in S. aureus isolates, with methicillin-resistant S. aureus (MRSA) being the most clinically significant [4].
S. aureus is the second most prevalent cause of infection in neonatal intensive care unit (NICU) infants with very low birth weights that cause late-onset septicemia [5]. Sepsis caused by MRSA is a life-threatening medical condition that involves systemic inflammation throughout the body [6]. Preterm infants are also at high risk for S. aureus colonization, a potential risk factor for subsequent infection [7]. Severe S. aureus infections are an important challenge in developing countries [8].
Globally, multidrug-resistant (MDR) bacteria are becoming increasingly resistant to antibiotics [9]. A MDR is an acquired non-susceptibility to at least one agent in three or more antimicrobial categories, while an extensively drug-resistant (XDR) is an acquired non-susceptibility to at least one agent in all but two or fewer antimicrobial categories [10].
S. aureus ability to cause infections is linked to several virulence features that enable it to adhere to surfaces, avoid the immune system, and produce damage and toxic consequences to the host [11]. The virulence factors of S. aureus are numerous and include, for example, bacterial biofilm formation, hemolysins, and staphyloxanthin production. Furthermore, virulence enzymes such as proteases are commonly related to tissue invasion and disease progression [12, 13].
Virulence factors of S. aureus are regulated by several regulatory loci and genes, such as sigma factor (σB) which is encoded by the sigB gene, accessory gene regulator (agr), and staphylococcal accessory regulator (sarA). Also, the hla gene is found to encode hemolysins production in S. aureus. In addition, the icaA and fnbA genes play a key role in biofilm formation. Moreover, the initial process in the synthesis of staphyloxanthin is catalyzed by the dehydrosqualene synthase (CrtM) enzyme, which is encoded by the crtM gene [14,15,16,17,18].
Drugs that target virulence factors may be able to prevent the depletion of the commensal microbiome. As a result, targeting virulence factors may lessen the impact on resistance development [19]. Many previous researches have reported the repurposing of many FDA-approved drugs as promising anti-virulence agents [20]. A promising technique for finding off-label uses for previously approved drugs is drug repurposing that decreases the risk of treatment failure, especially due to safety concerns. It also reduces the cost, effort, and time involved in drug discovery and evaluation [21].
The majority of drugs used in the Neonatal Intensive Care Unit (NICU) are not approved by the Food and Drug Administration (FDA) for use in newborns. When performing neonatal clinical studies, investigators face a variety of obstacles [22]. So, we aimed to investigate the potential inhibitory effects of the FDA-approved drugs (ascorbic acid, dexamethasone, and sodium bicarbonate) on the S. aureus virulence factors by phenotypic, genotypic methods, and in vivo model. Our results might help fighting against lethal infections caused by resistant S. aureus.
Methods
Media and chemicals
Mueller Hinton agar (MHA), Luria-Bertani (LB) broth, and Tryptone Soya Broth (TSB) were purchased from Oxoid, Hampshire, England. The ascorbic acid was obtained from Memphis pharmaceutical company. EIPICO pharmaceutical company provided the dexamethasone, while Sodium bicarbonate was the product of Otsuka Pharmaceutical Industries, Egypt. Other chemicals were of pharmaceutical-grade.
Bacterial isolate and its identification
Ten S. aureus clinical isolates used in the current study were obtained from the stock culture collection of the Microbiology and Immunology Department, Faculty of Pharmacy, Zagazig University. This isolates were recovered from neonates admitted to Nabrouh Central Hospital Intensive Care Unit, Egypt who had a sepsis.
The identity of the isolates was fully confirmed by using VITEK 2 COMPACT (Biomerieux, France). According to manufacturer’s instructions, the samples were sub-cultured on blood agar and incubated at 37 °C for 24–48 h. A sufficient number of colonies of pure culture were used to suspend the microorganism in a 3.0 ml of a sterile saline test tube. The turbidity of this inoculum was adjusted using normal saline to 0.5 McFarland. The McFarland turbidity was measured using the DensiCHEK Plus equipment. The pure bacterial suspension was added to the device by using the VITEK ID GP identification card for bacterial bio-typing and antibiotic susceptibility testing [23].
Antibiotic susceptibility testing of the tested clinical isolates
The antibiotic susceptibility testing for the clinical isolates were carried out by minimum inhibitory concentration (MIC) determination using the VITEK 2 COMPACT automated machine. The AST-G592 card was used to perform antimicrobial susceptibility tests for gram-positive bacteria by minimum inhibitory concentration estimation using a kit contains 14 antibiotics including, benzylpenicillin (β-lactams), oxacillin (β-lactams), gentamicin (aminoglycosides), ciprofloxacin (quinolones), moxifloxacin (quinolones), erythromycin (macrolides), clindamycin (lincosamides), teicoplanin (glycopeptides), vancomycin (glycopeptides), tetracycline (tetracyclines), tigecycline (glycylcycline), fusidic acid (fusidanes), rifampicin (ansamycins), and Trimethoprim/sulfamethoxazole (sulfonamides).
Minimum inhibitory concentration (MIC) determination of the tested drugs
According to The Clinical and Laboratory Standards Institute (CLSI), the agar dilution method was used to assess the MIC of sodium bicarbonate, dexamethasone, and ascorbic acid [24]. In brief, overnight cultures of the tested isolates were diluted with Mueller-Hinton broth to reach the turbidity of the 0.5 McFarland standard to get a final concentration of 107 CFU/ml. Nutrient agar plates with varying concentrations of each drug (0.25, 0.5, 1, 2, 4, 8, 16, 32, and 64 mg/ml) were prepared in addition to control plates without drugs. An aliquot of 100 μl of the suspensions of the tested isolates was inoculated on the plates’ surfaces and incubated at 37 °C overnight. The MIC of sodium bicarbonate, dexamethasone, and ascorbic acid was determined as the lowest concentration that prevented observable bacterial growth.
Assessment of the impact of sub-MIC of sodium bicarbonate, dexamethasone, and ascorbic acid on S. aureus growth
The impact of 1/8 MIC of the tested drugs on the growth of S. aureus isolates was assessed by inoculating LB broth with an overnight culture of the tested isolates with and without the presence of the tested drugs and incubated overnight at 37 °C. The effect of 1/8 MIC of the three tested drugs on S. aureus isolates growth was determined at 600 nm using a spectrophotometer (Biotek, USA) [25].
For more confirmation to exclude any possible effects of the tested drugs on the growth of the tested bacteria, the growth curve experiment was carried out. Briefly, the isolates were cultured in LB to reach an OD600 of 0.2. After that, 100 ml volumes were aliquoted into four 500-ml flasks. A concentrations of 1/8 MIC of the three potential inhibitors ascorbic acid (12.5 mg/ml), dexamethasone (4 mg/ml), and sodium bicarbonate (2 mg/ml), were added to the three cultures in flasks and the bacteria in the fourth flask were kept without addition of any inhibitors as a control. The flasks were incubated at 37 °C with aeration followed by OD600 measurements every 30 minutes. One ml of each culture was collected immediately after addition of ascorbic acid, dexamethasone, and sodium bicarbonate (t0), respectively. In addition, one ml of each culture was collected at 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 360 min and the optical density was measured each time at OD600 [26].
Evaluation of the effect of ascorbic acid, dexamethasone, and sodium bicarbonate on the virulence factors production in S. aureus using phenotypic tests
Biofilm inhibition assessment
The quantitative assay of biofilm formation was carried out as follows. Briefly, a suspension of the tested isolates in TSB was prepared from overnight culture and adjusted to reach 106 CFU/ml. In a volume of 200 μl of the bacterial suspension was added to the microtiter plate wells in the presence and absence of sub-MIC of the tested drugs and incubated for 48 hours at 37 °C. The TSB was smoothly discarded, and the plates were washed with distilled water to eliminate any planktonic cells, followed by air drying. After 20 minutes of treatment with 200 μl of 99% fixing methanol, the biofilm was dyed for 15 minutes with 200 μl of 1% crystal violet solution. After washing the plate, crystal violet was dissolved in 33% glacial acetic acid, and the absorbance of the solubilized dye was measured at 570 nm using a spectrofluorometer (Biotek, USA) [27].
Staphyloxanthin inhibition assay
The tested isolates were cultured overnight and the suspensions were adjusted to 0.5 McFarland then swabbed on the TSA agar plates containing sodium bicarbonate, dexamethasone, and ascorbic acid and control plates. After 2 days of incubation at 37 °C, the cells were harvested and the agar plates’ surfaces were washed and rinsed 3 times with distilled water. The obtained suspensions were centrifuged to collect the cell pellets at 6000 rpm for 15 min. The pellets were mixed with 3 ml 99% methanol and heated in a water bath at 55 °C for 30 min with gentle stirring and then cooled for 10 min and centrifuged again at 6000 rpm for 15 min. The yellow pigment was measured at 450 nm using a spectrofluorometer (Biotek, USA) [28].
Total protease inhibition assessment
The modified skim milk method was utilized to measure the total proteases in the presence and absence of the tested drugs. S. aureus isolates were grown overnight in LB broth with and without 1/8 MIC of the tested drugs, and supernatants were obtained by centrifugation at 10000 rpm for 10 min. Cell-free supernatants were diluted 10-fold in Tris-HCl buffer (pH 7.4). Next, a volume of 500 μl cell-free supernatant from each of the tested isolates was combined with 1 ml of skim milk solution (1.25% in distilled H2O) and incubated for 30 minutes at 37 °C. As a measure of proteolytic activity, the turbidities of assay solutions were assessed at OD600 using a spectrofluorometer (Biotek, USA) [29].
Hemolysin inhibition assay
The hemolysins assay was evaluated by mixing 600 μl of cell-free supernatants prepared as mentioned in the proteases assay in the presence and absence of 1/8MIC of the tested drugs with 600 μl of fresh 2% v/v defibrinated rabbit blood cells in saline. This mixture was incubated at 37 °C for 2 h and then centrifuged at 10000 rpm for 8 min at 4 °C. Released hemoglobin was measured at 540 nm with a spectrofluorometer and was compared to both a negative and positive control (erythrocytes incubated in LB broth and erythrocytes lysed completely with 0.1% SDS, respectively). The formula [X-B/T-B] × 100 was used to calculate hemolysis percentage, where X represents the tested drug-treated or untreated isolates, B represents the negative control, and T represents the positive control. The hemolysis by the treated cultures was expressed as a percentage compared to the hemolysis by untreated ones [30].
Sensitivity to oxidative stress assessment
The ability of ascorbic acid, dexamethasone, and sodium bicarbonate to interfere with S. aureus resistance to oxidative stress was examined using the modified disk method. The tested isolates in the presence and absence of the tested drugs was cultured in TSB and incubated overnight. Aliquots of 0.1 ml were put on the surface of TSA plates containing 1/8 MIC of the tested drugs. On the surface of TSA plates, sterile paper discs (6 mm) were inserted and 10 μl of hydrogen peroxide (1.5%) was added. After incubating the plates for 24 hours at 37 °C, the inhibition zones were measured [31].
RNA extraction and relative gene expression determination using qRT-PCR
The most resistant isolate (isolate 1) was chosen to perform the genotypic experiment as a representative example. S. aureus was cultured overnight at 37 °C in TSB with and without 1/8 MIC of sodium bicarbonate, dexamethasone, and ascorbic acid until the bacteria reached the middle log phase (OD600 of 0.5–0.6). The pellets were obtained by centrifugation at 6000 g for 15 min, and RNA was extracted using the GeneJET RNA Purification Kit (Thermo Fisher Scientific Inc., Germany) according to manufacture instructions. Reverse transcription followed by qRT-PCR of virulence factors genes crtM, sigB, sarA, agrA, hla, fnbA, and icaA was performed followed the protocol described in SensiFAST™ SYBR® Hi-ROX One-Step Kit (Bioline, UK). RT-qPCR analysis was performed using a StepOne RT-PCR thermal cycler (Applied Biosystem, USA) using primers described in Table 1. The housekeeping gene 16S rRNA was used to standardize the relative expression values of each gene. The 2−∆∆CT approach was used to compare the relative gene expression in the treated and untreated isolates [36].
The mice survival model
The protective activities of ascorbic acid, dexamethasone, and sodium bicarbonate against S. aureus pathogenesis were investigated by the mice survival model [37]. The S. aureus cultures with or without sub-MIC of the tested drugs were adjusted to 1 × 108 CFU/ml in phosphate-buffered saline (PBS). The study was conducted in six groups, each consisting of 10 albino mice (Mus musculus) of similar weight and health characteristics. There are two negative control groups; one group was administered intraperitoneally with 100 μl of sterile PBS and the other group was left un-inoculated. A positive control group was injected with 100 μl of untreated S. aureus. The three other groups were injected with 100 μl of overnight bacterial cultures in LB broth in presence of sub-MIC of ascorbic acid, dexamethasone, and sodium bicarbonate, respectively. Three consecutive days were observed to determine the survival rate of experimental mice.
Statistical analysis
The data in the current study were analyzed with the help of the GraphPad Prism 8 software package. The effect of the tested drugs on S. aureus virulence factors was compared using One Way ANOVA at P > 0.05 and > 0.001 for significance. The means and standard errors of three biological experiments with three technical replicates were used to calculate the results.
Results
Clinical isolates identification
The clinical isolates in the current study were confirmed as S. aureus using VITEK 2 COMPACT system (BIOMERIEUX) for bacterial bio-typing and antibiotic susceptibility patterns.
Antibiotic susceptibility and resistance profile of the tested isolates
The tested isolates showed a high level of resistance against the antibiotics tested. From the 10 isolates, 100% resistance was found against each of benzylpenicillin, and fusidic acid. While the resistance rates were 80% against oxacillin, erythromycin, and vancomycin, and clindamycin (8 isolates), 60% against ciprofloxacin, tiecoplanin, and tetracycline (6 isolates), 50% against trimethoprim/sulfamethoxazole (5 isolates) and 40% against gentamicin, and rifampicin (4 isolates). Tigecycline was the most effective antibiotic with a resistance rate of 10% (one isolate). The complete results of antibiotic susceptibility testing are illustrated in Table 2.
Data from antibiotic sensitivity tests revealed that 20% of the isolates (2 out of 10) were XDR. 80% of the isolates were MDR (8 out of 10).
Determination of MIC of sodium bicarbonate, dexamethasone, and ascorbic acid
The MIC for the 10 isolates was determined using the broth micro-dilution method. Ascorbic acid, dexamethasone, and sodium bicarbonate inhibited the growth of the tested S. aureus isolates at 100 mg/ml, 32 mg/ml, and 16 mg/ml, respectively. The inhibitory activities against S. aureus virulence factors were tested at concentrations equivalent to 1/8 MIC of the tested drugs, (2 mg/ml) for sodium bicarbonate, (4 mg/ml) for dexamethasone, and (12.5 mg/ml) for ascorbic acid as other sub-inhibitory concentrations did not show any inhibitory impact on virulence factors production by phenotypic tests.
The impact of sub-inhibitory concentrations of sodium bicarbonate, dexamethasone, and ascorbic acid on S. aureus viable growth
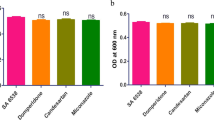
The impact of ascorbic acid, dexamethasone, and sodium bicarbonate on S. aureus virulence factors may be connected to their inhibitory effect on bacterial growth. To avoid this possibility, the effect of sub-inhibitory concentrations on bacterial growth was evaluated by measuring the optical density at OD600 of the overnight cultures with and without the sub-MIC of the three tested drugs. Notably, there was no significant difference observed in the growth of both treated and untreated isolates, proposing that treating S. aureus with sub-inhibitory concentrations of the tested drugs has no adverse effect on bacterial growth (Fig. 1A, B, C).
A-ascorbic acid, B-dexamethasone, and C-sodium bicarbonate exhibited no effect on S. aureus growth. The OD600 of bacteria was measured after culturing overnight in LB broth both in the presence and absence of 1/8 MIC of sodium bicarbonate, dexamethasone, and ascorbic acid. D- The growth curve showed no effect of ascorbic acid, dexamethasone, or sodium bicarbonate on S. aureus growth. After culturing bacteria in LB broth, OD600 was measured every 30 minutes with 1/8 MIC of ascorbic acid, dexamethasone, and sodium bicarbonate when compared to that of untreated bacteria. The tests were performed in triplicate. Values are the averages of three independent experiments. The data shown are the means ± standard errors
In addition, a bacterial growth curve assay was conducted to confirm the non-lethality of the tested drugs. The growth curves of S. aureus that was cultured at sub-inhibitory concentrations of ascorbic acid, dexamethasone, and sodium bicarbonate are depicted in (Fig. 1D). Importantly, at 1/8MIC, neither of the tested drugs significantly affected the growth of S. aureus.
Impact of ascorbic acid, dexamethasone, and sodium bicarbonate on S. aureus virulence factors production
Biofilm inhibition assessment
The crystal violet technique was used to assess the prevention of biofilm development. It’s interesting to note that S. aureus ability to produce biofilms was greatly reduced by ascorbic acid, dexamethasone, and sodium bicarbonate. The biofilm formation were reduced by a percentage ranging between (1.35–41.77%), (22.29–63.17%), and (8.13–76.26%) for ascorbic acid, dexamethasone, and sodium bicarbonate, respectively in treated isolates in comparison to untreated isolates (Fig. 2).
Inhibition of biofilm formation in S. aureus by A-ascorbic acid, B-dexamethasone, and C-sodium bicarbonate. A significant reduction of biofilm formation was found with 1/8 MIC of ascorbic acid, dexamethasone, and sodium bicarbonate in untreated isolate compared to treated isolate. The data shown represent the means ± standard errors. One WAY ANOVA test followed by Dunnett’s Multiple Comparison test was used for statistical analysis. *, Significant P < 0.05 was considered significant
Staphyloxanthin inhibition assay
Ascorbic acid, dexamethasone, and sodium bicarbonate treated isolates displayed a striking decrease in staphyloxanthin production (Fig. 3). The production in treated isolates was reduced to (10–60%) by ascorbic acid (55–71%) by dexamethasone, and, (19–60%) by sodium bicarbonate, respectively in comparison to control untreated isolate.
Staphyloxanthin production was significantly reduced in A-ascorbic acid, B-dexamethasone, and C-sodium bicarbonate-treated isolates compared to control untreated bacteria. The absorbance of staphyloxanthin was measured at 560 nm after overnight incubation. The results shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P < 0.05 (following One Way ANOVA) was considered significant
Total protease inhibition assessment
The proteolytic activity was examined using the modified skim milk assay method with and without the presence of sub-MICs of the tested drugs. The inhibitory effect of proteases activity obtained with ascorbic acid was (44–75%). Dexamethasone, and sodium bicarbonate also showed a significant decrease in proteases activity by (20–87%), and (78–89%) respectively (Fig. 4).
Significant reduction of protease production was found with A-ascorbic acid, B-dexamethasone, and C-sodium bicarbonate-treated isolates. OD600 was measured following overnight culturing of bacteria in LB broth in the presence and absence of 1/8 MIC of tested isolates followed by incubation of supernatants with skim milk for 30 min at 37 °C. The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P < 0.05
Hemolysin inhibition assay
To assess the tested drugs’ ability to suppress S. aureus hemolytic activity, the hemolytic activity of untreated and treated isolates was assessed. Notably, ascorbic acid, dexamethasone, and sodium bicarbonate-treated isolates exhibited a significant decrease in hemolysin activity compared to untreated isolate. The inhibition of hemolysin activity under the effect of ascorbic acid, dexamethasone, and sodium bicarbonate was reduced by a percentage ranging between (27–71%), (8–55%), and (19–56%) respectively at 1/8 MIC as shown in Fig. 5.
a significant decrease in hemolytic activity was found with A-ascorbic acid, B-dexamethasone, and C-sodium bicarbonate-treated isolates. The hemolytic activity of the drug-free supernatant was considered as 100% hemolysis (control), and the percentage of hemolysis in presence of 1/8 MIC of sodium bicarbonate, dexamethasone, and ascorbic acid were calculated as compared to the control. The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P < 0.05
Sensitivity to oxidative stress assessment
The impact of ascorbic acid, dexamethasone, and sodium bicarbonate reducing the tolerance of S. aureus to oxidative stress was investigated by testing the increasing hydrogen peroxide lethal effect on the growth of S. aureus by the tested drugs. Ascorbic acid, dexamethasone, and sodium bicarbonate showed a significant impact in decreasing the tolerance of S. aureus tested isolate to oxidative stress by a percentage of (25–40%), (7–45%), and (30–48%), respectively (Fig. 6).
Inhibition of resistance to H2O2 in S. aureus by A-ascorbic acid, B-dexamethasone, and C-sodium bicarbonate. A significant reduction of resistance to H2O2 was found with 1/8 MIC of ascorbic acid, dexamethasone, and sodium bicarbonate-treated isolates as compared to control untreated bacteria. The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P < 0.05
RT-qPCR estimation of relative gene expression of virulence factors encoding genes in S. aureus
To demonstrate that the virulence factors in S. aureus may be inhibited by ascorbic acid, dexamethasone, and sodium bicarbonate at the molecular level, we selected the most resistant isolate (isolate 1) for estimating the relative expression of the genes regulating the production of virulence factors in S. aureus in treated and untreated isolate using qRT-PCR and was analyzed using the 2−∆∆Ct method.
The relative expression levels of crtM, sigB, sarA, agrA, hla, fnbA, and icaA were significantly decreased in ascorbic acid, dexamethasone, and sodium bicarbonate treated isolates in comparison to control untreated isolate. At sub-MIC, ascorbic acid was found to be more potent than sodium bicarbonate and dexamethasone against S. aureus virulence factors genes, decreasing expression levels of crtM, sigB, sarA, agrA, hla, fnbA, and icaA genes by a percentage ranging between (88.24–95%), in comparison to dexamethasone (30.77–76%), and (65.38–85%), by sodium bicarbonate.
In the current study, ascorbic acid decreased crtM relative expression level by 88.24%, while dexamethasone and sodium bicarbonate had 52.94, and 76.47% inhibition effect, respectively; while, sigB gene relative expression was suppressed by 84, 76, and 92%in ascorbic acid-, dexamethasone-, and sodium bicarbonate treated-isolates, respectively. In addition, ascorbic acid, dexamethasone, and sodium bicarbonate suppressed the sarA gene relative expression by 93.33, 43.33%, and, 76.67% respectively. Also, ascorbic acid inhibited the relative expression level of the agrA gene by 88.46% and by 30.77, and 65.38% under the effect of dexamethasone, and, sodium bicarbonate respectively. Moreover, ascorbic acid, dexamethasone, and sodium bicarbonate significantly lowered hla relative expression levels by 89.47, 47.37, and73.68%, respectively, while fnbA relative expression level was reduced by 86.96% in ascorbic acid-treated isolate, 47.37% in dexamethasone-treated isolate, and 82.61% in sodium bicarbonate-treated isolate. Finally, ascorbic acid, dexamethasone, and sodium bicarbonate reduced the level of relative gene expression of icaA by 95, 60, and 85% respectively (Fig. 7).
Down-regulation of virulence genes of S. aureus by sodium bicarbonate, dexamethasone, and ascorbic acid at 1/8 MIC produced a significant reduction in the expression levels of all the tested virulence genes. A- crtM gene, B- sigB gene, C- sarA gene, D- agrA gene, E- hla gene, F- fnbA gene, G- icaA gene. The data shown represent the means ± standard errors. One WAY ANOVA test was used for statistical analysis. *, significant P < 0.05
The mice survival model
The in vivo protective activities of ascorbic acid, dexamethasone, and sodium bicarbonate from S. aureus pathogenesis were assessed using six groups of mice. The mice survival was reported for 3 successive days and plotted using the Kaplan-Meier method and significance (P < 0.05) was calculated using the Log-rank test, GraphPad Prism 8 (Fig. 8). All mice in negative control groups (uninoculated or PBS injected) totally survived (100%). In the positive control group that was injected with untreated bacteria, only 40% (4 out of 10) of mice were survived. Interestingly, mice injected with ascorbic acid-treated bacteria in sub-MIC showed a significant increase in survival rate of with an increased protection percentage of 60% as compared to those mice injected with untreated bacteria. Additionally, dexamethasone, and sodium bicarbonate also showed a significant rise in survival rate with an increased protection percentage of 20% as compared to those mice injected with untreated bacteria in both drugs.
In vivo survival test of S. aureus. 6 groups of healthy mice comprising 10 mice each were used. Two negative control groups either injected with sterile PBS or kept uninfected and a positive control group injected with untreated S. aureus. 3 test groups were injected with ascorbic acid, dexamethasone, and sodium bicarbonate-treated S. aureus. Mice survival in each group was observed every day for 3 days, plotted using the Kaplan-Meier method, GraphPad Prism 8. All mice in the negative control groups survived, while only 40% of mice survived in the positive control group. Ascorbic acid in sub-MIC (12.5 mg/ml) protected all mice, conferring 60% increased protection in comparison with the group inoculated with untreated bacteria. Meanwhile, dexamethasone, and sodium bicarbonate in sub-MIC (2 mg and 4 mg/ml); (6 mice survived) confer 20% increased protection in comparison with the group inoculated with untreated bacteria
Discussion
The antibiotic resistance is one of the greatest threats to public health [38]. The emergence of antibiotic resistance has resulted in the development of S. aureus strains that are resistant to nearly all available antibiotics, making treatment a clinical challenge [39]. S. aureus infections are a significant clinical burden for newborns around the world, and they were found to be the second most common cause of late-onset sepsis among very low birth weight infants referred to NICUs [7]. Among the most fatal infections for infants is neonatal sepsis, caused mainly by S. aureus [40].
To combat the problem of antibiotic resistance, it is now crucial to develop novel treatment approaches, either on their own or in combination with antibiotics; in this context, the term “repurposing” has resurfaced [41]. Several publications have discussed the concept of targeting bacterial virulence rather than viability [42]. The goal of this study is to repurpose the tested drugs in such serious infections caused by MDR S. aureus, especially in neonates.
The FDA-approved drugs, ascorbic acid, dexamethasone, and sodium bicarbonate have been extensively used for the treatment of many conventional medical conditions in neonates. Previously, ascorbic acid is also an important physiological antioxidant [43]. Low levels of plasma vitamins C and E are associated with significant hyperbilirubinemia in full-term neonates [44]. Dexamethasone has also been extensively studied in neonatal medicine, where it helps improve pulmonary function and facilitate extubation [45]. In addition, dexamethasone has become widely accepted in standard practice for the postnatal treatment or prevention of chronic lung disease in preterm infants over the last two decades [46]. On the other hand, it was reported that intravenous sodium bicarbonate has been traditionally used to correct metabolic acidosis in neonates [47]. Also, it is used as a therapy designed to prevent azotemia, hypoglycemia, and elevations in serum potassium levels in neonates [48].
Based on previous data that reporting the effectiveness of ascorbic acid, dexamethasone, and sodium bicarbonate as anti-virulence agents in some other pathogens and studies reported the possibility and safety of using them in some health problems in neonates, we tested their effect as anti-virulence agents in 2 XDR, and 8 MDR S. aureus isolates from a neonate.
In the current study, the antibacterial activity of the tested drugs ascorbic acid, dexamethasone, sodium bicarbonate, and was determined through MIC assessment. Ascorbic acid was able to inhibit the growth at 100 mg/ml, while dexamethasone inhibited the growth at 32 mg/ml, and Sodium bicarbonate inhibited S. aureus growth at 16 mg/ml.
S. aureus is a biofilm-forming bacterium that makes therapy difficult. In light of this, focusing on biofilm and other virulence factors may prove to be a viable method for eradicating S. aureus infection [49]. S. aureus biofilm structure includes polysaccharides and proteins and the bacterial cells compose persistent resistant cells that exhibit multidrug resistance [50]. Other important virulence factors include staphyloxanthin pigment, hemolysins, and proteases that help bacteria to cause infection [35]. Staphyloxanthin is an orange-red triterpenoid carotenoid. It has been suggested that staphyloxanthin can protect S. aureus against oxidative stress [51]. Staphyloxanthin has been identified as one of the striking targets of anti-virulent therapy [52]. S. aureus also produces extracellular proteases with proposed roles in virulence [53]. S. aureus extracellular proteases are recognized to play a key role in pathogenesis; they have been found to compromise the integrity of the airway epithelial barrier, resulting in lung injury [54]. Meanwhile, hemolysins lyse red blood cells. Meanwhile, hemolysins lyse red blood cells, and the most well-known toxin of S. aureus is alpha-toxin that can destroy red blood cells and certain types of leukocytes [3].
Five virulence factors in S. aureus, including biofilm formation, staphyloxanthin production, proteases, and hemolysins production, and resistance to oxidative stress, were evaluated in the presence of 1/8 MIC (12.5 mg/ml, 4 mg/ml, and 2 mg/ml) of the tested drugs ascorbic acid, dexamethasone, and, sodium bicarbonate respectively. It is important to note that all tested virulence factors were evidently suppressed phenotypically by the tested drugs.
Many previous studies showed results similar to our phenotypic results. For example, in a previous study, ascorbate inhibits proteases and hemolysins’ activities and attenuates the biofilm production of P. aeruginosa [55]. Also, in a previous study, ascorbate significantly decreased biofilm production and hemolysins in Vibrio campbellii which is similar to our findings [56]. Moreover, in a report following the current results, ascorbic acid exhibited strong dose-dependent bactericidal, anti-biofilm, and virulence-suppressing effects in carbapenem-resistant hypervirulent Klebsiella pneumonia [57]. In another report, vitamin C inhibited quorum sensing and other stationary phase regulatory mechanisms that support biofilm development in E. coli and P. aeruginosa [58].
Moreover, in a previous report, dexamethasone showed anti-biofilm activity against fluconazole-resistant Candida albicans, which is in accordance with the present results of the anti-biofilm activity of dexamethasone [59]. In parallel with our results, PYED-1 (pregnadiene-11-hydroxy-16,17-epoxy-3,20-dione-1), which is a synthetic heterocyclic corticosteroid that inhibited the production of S. maltophilia biofilms at sub-inhibitory doses [60]. Supporting our results, a previous study showed that a combination of dexamethasone and cloxacillin was more effective than cloxacillin alone in treating bacterial arthritis caused by S. aureus in mice [61].
In accordance with the current results, a previous study revealed that sodium bicarbonate impedes the growth and biofilm formation of several pulmonary bacterial pathogens including S. aureus, Streptococcus agalactiae, P. aeruginosa, and E. coli [62]. In another study matching our results, delafloxacin efficacy against MDR S. aureus is modulated and increased by the effect of bicarbonate [63].
Additionally, the anti-virulence activity of ascorbic acid, dexamethasone, and sodium bicarbonate in the current study against biofilm formation, staphyloxanthin, proteases, hemolysin production, and tolerance to oxidative stress against S. aureus is proportional to earlier studies that tested some potential anti-virulence agents. For example, in a previous study, candesartan, domperidone, and miconazole inhibited biofilm formation, proteases, hemolysins, and staphyloxanthin production in S. aureus [64]. Furthermore, a recent study showed that hesperidin treatment significantly impedes hemolysin and staphyloxanthin production, which possibly increases the MRSA susceptibility rate to H2O2 oxidative stress condition which matches to some extent the current results [65]. In another study in accordance with the present study, diclofenac has a remarkable inhibition effect on biofilm formation, hemolysin activity, and staphyloxanthin production against MDR-MRSA [66]. Moreover, glyceryl trinitrate showed significant inhibition of biofilm, staphyloxanthin production, and tolerance to oxidative stress, which is similar to our findings [67]. Also, thymol treatment inhibited staphyloxanthin by 90% and made MRSA cells more susceptible to membrane-targeting antibiotic polymyxin B, which matches the current results to some extent [68].
The molecular understanding of S. aureus pathogenesis is critical in the fight against this important human pathogen, since it may aid in the development of new therapeutic techniques [69]. At the molecular level in S. aureus, it was found that the crtM gene controls the first step in the biosynthesis of staphyloxanthin [70], while the alternative sigma B (sigB) gene regulates S. aureus response to changing conditions and allows it to adapt to various environments, including those involved in general stress response, virulence, capsule formation, and biofilm formation [14].
In the late stages of growth, the quorum regulator SarA of S. aureus upregulates the expression of many virulence factors, including biofilm formation, to mediate pathogenesis and immune evasion and has been shown in multiple studies to be a potent regulator of proteases synthesis [71, 72].
Furthermore, S. aureus’ accessory gene regulator (agr) system regulates the expression of virulence factors in response to cell density [73] while the hla gene encodes a key virulence factor called hemolysin [74]. S. aureus adheres to fibrinogen, elastin, and fibronectin of the host via fibronectin-binding proteins A and B (FnBPA and FnBPB), which are encoded by two genes, fnbA and fnbB, that are closely related [75].
In this study, the inhibitory activity of sodium bicarbonate, dexamethasone, and ascorbic acid against the regulatory genes agrA, sarA, sigB, and the virulence genes crtM, icaA, hla, and fnbA was examined using qRT-PCR. In general, ascorbic acid was found to be the most powerful inhibiting drug against the tested genes, followed by sodium bicarbonate, and finally dexamethasone.
Similarly, hesperidin treatment down-regulated the relative expression of the biofilm-associated gene (sarA), the polysaccharide intracellular adhesion regulating gene (icaA), the fibronectin-binding protein regulating gene (fnbA) and the staphyloxanthin production regulating gene (crtM) in S. aureus [65]. In proportional with our findings, a previous study that used the three FDA drugs (candesartan, miconazole, and domperidone) reported that the three mentioned drugs decreased the relative expression of crtM, sigB, sarA, agrA, hla, and icaA in S. aureus. Additionally, diclofenac had a significant down-regulation effect on the relative expression of virulence regulating genes sarA, agrA, hla, fnbA, icaA, sigB, and crtM in S. aureus [66]. In another study, parallel with the current results, azan-7 (a novel aza-derivative) significantly reduced hla and agrA gene expression in MRSA [76]. Moreover, luteolin (a flavonoid of plant origin) and alpha-cyperone (a Chinese medicinal herb) inhibit hla and agrA gene expression in S. aureus [77].
Considering that current data show that S. aureus can produce less virulence factors when treated with ascorbic acid, dexamethasone, and sodium bicarbonate, it was crucial to characterize its pathogenesis in vivo. Therefore, mice survival in vivo model was used to evaluate the protective activities of the tested drugs in sub-MIC against S. aureus pathogenesis. Importantly, all the tested mice were protected by ascorbic acid, with 60% protection rate more than the mice group inoculated with untreated bacteria. These results are in accordance with our phenotypic and genotypic results. In addition, dexamethasone and sodium bicarbonate were able to give protection rate of 20% more than mice group inoculated with untreated bacteria. Similar to our findings a previous study reported that quercetin was found to be an effective anti-virulence agent and protected mice against lethal pneumonia caused by S. aureus [78].
Conclusions
In light of our results, we could suggest that ascorbic acid, dexamethasone, and sodium bicarbonate represent effective inhibitors of the production of virulence factors in S. aureus. This suggests the possibility of using them as an adjuvant therapeutic approach to treat XDR, and MDR S. aureus as alternatives or in combination with traditional antibiotics, especially in neonatal sepsis in developing countries. Results such as these can support the repurposing of FDA-approved drugs as a novel strategy to overcome antimicrobial resistance.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
References
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–61.
Kalu IC, Kao CM, Fritz SA. Management and prevention of Staphylococcus aureus infections in children. Infect Dis Clin N Am. 2022;36:5520.
Otto M. Staphylococcus aureus toxins. Curr Opin Microbiol. 2014;17:32–7.
Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12:547–69.
Sharma P, Kaur P, Aggarwal A. Staphylococcus aureus- the predominant pathogen in the neonatal ICU of a tertiary care hospital in Amritsar. India J Clin Diagn Res. 2013;7:66–9.
Yang Y, Li H, Sun H, Gong L, Guo L, Shi Y, et al. A novel nitro-dexamethasone inhibits agr system activity and improves therapeutic effects in MRSA sepsis models without antibiotics. Sci Rep. 2016;6:20307.
Geng W, Qi Y, Li W, McConville TH, Hill-Ricciuti A, Grohs EC, et al. Epidemiology of Staphylococcus aureus in neonates on admission to a Chinese neonatal intensive care unit. PLoS One. 2020;15:e0211845.
Kwiecinski JM, Horswill AR. Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms. Curr Opin Microbiol. 2020;53:51–60.
Rello J, Bunsow E, Perez A. What if there were no new antibiotics? A look at alternatives. Expert Rev Clin Pharmacol. 2016;9:1547–55.
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Holmes A, Ganner M, McGuane S, Pitt TL, Cookson BD, Kearns AM. Staphylococcus aureus isolates carrying Panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384–90.
Selvaraj A, Jayasree T, Valliammai A, Pandian SK. Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism. Front Microbiol 2019;10 September:1–15.
Algammal AM, Hetta HF, Elkelish A, Alkhalifah DHH, Hozzein WN, Batiha GE-S, et al. Methicillin-resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect Drug Resist. 2020;13:3255–65.
Tuchscherr L, Bischoff M, Lattar SM, Noto Llana M, Pförtner H, Niemann S, et al. Sigma factor SigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog. 2015;11:e1004870.
Burnside K, Lembo A, de Los RM, Iliuk A, Binhtran N-T, Connelly JE, et al. Regulation of hemolysin expression and virulence of Staphylococcus aureus by a serine/threonine kinase and phosphatase. PLoS One. 2010;5:e11071.
Yeswanth S, Chaudhury A, Sarma PVGK. Quantitative expression analysis of SpA, FnbA and Rsp genes in Staphylococcus aureus: actively associated in the formation of biofilms. Curr Microbiol. 2017;74:1394–403.
Otto M. MRSA virulence and spread. Cell Microbiol. 2012;14:1513–21.
Yehia FAA, Yousef N, Askoura M. Celastrol mitigates staphyloxanthin biosynthesis and biofilm formation in Staphylococcus aureus via targeting key regulators of virulence; in vitro and in vivo approach. BMC Microbiol. 2022;22:106.
Lee MH, Nuccio S-P, Raffatellu M. Pathogen interference: targeting virulence factors to tackle intracellular microbes. Cell Chem Biol. 2020;27:765–7.
D’Angelo F, Baldelli V, Halliday N, Pantalone P, Polticelli F, Fiscarelli E, et al. Identification of FDA-approved drugs as Antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2018;62:e01296–18.
Mostafa A, Kandeil A, Elshaier YAMM, Kutkat O, Moatasim Y, Rashad AA, et al. Fda-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals. 2020;13:1–24.
Smith AM, Davis JM. Challenges and opportunities to enhance global drug development in neonates. Curr Opin Pediatr. 2017;29:149–52.
de Cueto M, Ceballos E, Martinez-Martinez L, Perea EJ, Pascual A. Use of positive blood cultures for direct identification and susceptibility testing with the Vitek 2 system. J Clin Microbiol. 2004;42:3734–8.
CLSI. Clinical and laboratory standards institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed: CLSI supplement M100; 2020.
Nalca Y, Jänsch L, Bredenbruch F, Geffers R, Buer J, Häussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother. 2006;50:1680–8.
Qiu J, Wang D, Xiang H, Feng H, Jiang Y, Xia L, et al. Subinhibitory concentrations of Thymol reduce enterotoxins a and B and α-Hemolysin production in Staphylococcus aureus isolates. PLoS One. 2010;5:e9736.
Stepanovic S, Vukovic D, Hola V, Giovanni Di Bonaventura SD, And ICI, Ruzicka Fi. Quantification of biofilm in microtiter plates : overview of testing conditions and practical recommendations for assessment of biofilm ... Quantification of biofilm in microtiter plates : overview of testing conditions and practical recommendations for a. Apmis 2007;115:231–241.
Al-kazaz EJ, Melconian AK, Kandela NJ. Extraction of Staphyloxanthin from Staphylococcus aureus isolated from clinical sources to determine its antibacterial activity against other Bacteria. Iraqi J Sci. 2014;55:1823–32.
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog. 2014;74:25–32.
Xiang H, Qiu J, Wang D, Jiang Y, Xia L, Deng X. Influence of Magnolol on the secretion of α-toxin by Staphylococcus aureus. Molecules. 2010;15:1679–89.
Hassett DJ, Schweizer HP, Ohman DE. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol. 1995;177:6330–7.
Antonic V, Stojadinovic A, Zhang B, Izadjoo MJ, Alavi M. Pseudomonas aeruginosa induces pigment production and enhances virulence in a white phenotypic variant of Staphylococcus aureus. Infect Drug Resist. 2013;6:175–86.
Sambanthamoorthy K, Smeltzer MS, Elasri MO. Identification and characterization of msa (SA 1233), a gene involved in expression of SarA and several virulence factors in Staphylococcus aureus. Microbiology (N Y). 2006;152:2559–72.
Kot B, Sytykiewicz H, Sprawka I. Expression of the biofilm-associated genes in methicillin-resistant Staphylococcus aureus in biofilm and planktonic conditions. Int J Mol Sci. 2018;19.
Lee JH, Cho HS, Kim Y, Kim JA, Banskota S, Cho MH, et al. Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl Microbiol Biotechnol. 2013;97:4543–52.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8.
Kim H-S, Lee S-H, Byun Y, Park H-D. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep. 2015;5:8656.
Ferrara P, Albano L. Azithromycin Has Been Flying Off the Shelves : The Italian Lesson Learnt from Improper Use of Antibiotics against COVID-19. Medicina (Kaunas). 2022;58:4–6.
Newstead LL, Varjonen K, Nuttall T, Paterson GK. Staphylococcal-produced Bacteriocins and antimicrobial peptides: their potential as alternative treatments for Staphylococcus aureus infections. Antibiotics (Basel). 2020;9:40.
Darlow CA, Hope W. Flomoxef for neonates: extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales. J Antimicrob Chemother. 2022;77:711–8.
Miró-canturri A, Ayerbe-algaba R, Smani Y. Drug Repurposing for the Treatment of Bacterial and Fungal Infections. Front Microbiol. 2019;10:41.
Muñoz-Cazares N, García-Contreras R, Pérez-López M, Castillo-Juárez I. Phenolic compounds with anti-virulence properties. In: Phenolic Compounds - Biological Activity; 2017. p. 140–67.
Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci. 1989;86:6377–81.
Abdul-Razzak KK, Nusier MK, Obediat AD, Salim AM. Antioxidant vitamins and hyperbilirubinemia in neonates. Ger. Med Sci. 2007;5:Doc03.
Gupta S, Prasanth K, Chen C-M, Yeh TF. Postnatal corticosteroids for prevention and treatment of chronic lung disease in the preterm newborn. Int J Pediatr. 2012;2012:315642.
Tarnow-Mordi W, Mitra A. Postnatal dexamethasone in preterm infants is potentially lifesaving, but follow up studies are urgently needed. BMJ. 1999;319:1385–6.
Massenzi L, Aufieri R, Donno S, Agostino R, Dotta A, (SIN) NPSG of the IS of N. Use of intravenous sodium bicarbonate in neonatal intensive care units in Italy: a nationwide survey. Ital. J Pediatr. 2021;47:63.
Hein HA. The use of sodium bicarbonate in neonatal resuscitation: help or harm? Pediatrics. 1993;91:496–7.
Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti-Infect Ther. 2015;13:1499–516.
Parastan R, Kargar M, Solhjoo K, Kafilzadeh F. Staphylococcus aureus biofilms: structures, antibiotic resistance, inhibition, and vaccines. Gene Rep. 2020;20:100739.
Clauditz A, Resch A, Wieland K-P, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74:4950–3.
Yehia FAZA, Yousef N, Askoura M. Exploring Staphylococcus aureus virulence factors; special emphasis on Staphyloxanthin. Microbiol Biotechnol Lett. 2021;49:467–77.
Shaw L, Golonka E, Potempa J, Foster SJ. The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology (Reading). 2004;150(Pt 1):217–28.
Murphy J, Ramezanpour M, Stach N, Dubin G, Psaltis AJ, Wormald PJ, et al. Staphylococcus Aureus V8 protease disrupts the integrity of the airway epithelial barrier and impairs IL-6 production in vitro. Laryngoscope. 2018;128.
El-Mowafy SA, Shaaban MI, Abd El Galil KH. Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. J Appl Microbiol. 2014;117:1388–99.
Han B, Zheng X, Baruah K, Bossier P. Sodium Ascorbate as a quorum-sensing inhibitor leads to decreased virulence in Vibrio campbellii. Front Microbiol. 2020;11:1054.
Xu C, Dong N, Chen K, Yang X, Zeng P, Hou C, et al. Bactericidal, anti-biofilm, and anti-virulence activity of vitamin C against carbapenem-resistant hypervirulent Klebsiella pneumoniae. iScience. 2022;25:103894.
Pandit S, Ravikumar V, Abdel-Haleem AM, Derouiche A, Mokkapati VRSS, Sihlbom C, et al. Low concentrations of vitamin C reduce the synthesis of extracellular polymers and destabilize bacterial biofilms. Front Microbiol. 2017:8.
Silva LJ, Silva CR, Sá LG, Barroso FD, Cândido TM, Queiroz HA, et al. Antifungal activity of dexamethasone against fluconazole-resistant Candida albicans and its activity against biofilms. Future Microbiol. 2022;17:607–20.
Vollaro A, Esposito A, Antonaki E, Iula VD, D’Alonzo D, Guaragna A, et al. Steroid derivatives as potential antimicrobial agents against Staphylococcus aureus planktonic cells. Microorganisms. 2020;8:468.
Sakiniene E, Bremell T, Tarkowski A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum. 1996;39:1596–605.
Dobay O, Laub K, Stercz B, Kéri A, Balázs B, Tóthpál A, et al. Bicarbonate inhibits bacterial growth and biofilm formation of prevalent cystic fibrosis pathogens. Front Microbiol. 2018;9:2245.
Holland M, Bjanes E, Nizet V, Dillon N. Bicarbonate modulates delafloxacin activity against MDR Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother. 2022;77:433–42.
El-Ganiny AM, Gad AI, El-Sayed MA, Shaldam MA, Abbas HA. The promising anti-virulence activity of candesartan, domperidone, and miconazole on Staphylococcus aureus. Braz J Microbiol. 2022;53:1–18.
Vijayakumar K, Muhilvannan S, Arun VM. Hesperidin inhibits biofilm formation, virulence and staphyloxanthin synthesis in methicillin resistant Staphylococcus aureus by targeting sarA and crtM: an in vitro and in silico approach. World J Microbiol Biotechnol. 2022;38:44.
Abbas HA, Atallah H, El-Sayed MA, El-Ganiny AM. Diclofenac mitigates virulence of multidrug-resistant Staphylococcus aureus. Arch Microbiol. 2020;202:2751–60.
Abbas HA, Elsherbini AM, Shaldam MA. Glyceryl trinitrate blocks staphyloxanthin and biofilm formation in Staphylococcus aureus. Afr Health Sci. 2019;19:1376–84.
Valliammai A, Selvaraj A, Muthuramalingam P, Priya A, Ramesh M, Pandian SK. Staphyloxanthin inhibitory potential of thymol impairs antioxidant fitness, enhances neutrophil mediated killing and alters membrane fluidity of methicillin resistant Staphylococcus aureus. Biomed Pharmacother. 2021;141:111933.
Saïd-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, et al. Global regulation of Staphylococcus aureus genes by rot. J Bacteriol. 2003;185:610–9.
Pelz A, Wieland K-P, Putzbach K, Hentschel P, Albert K, Götz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005;280:32493–8.
Balamurugan P, Praveen Krishna V, Bharath D, Lavanya R, Vairaprakash P, Adline PS. Staphylococcus aureus quorum regulator SarA targeted compound, 2-[(Methylamino)methyl] phenol inhibits biofilm and down-regulates virulence genes. Front Microbiol. 2017;8:1290.
Jenul C, Horswill AR. Regulation of Staphylococcus aureus virulence. Microbiol Spectr. 2019;7:1.
Otto M. Staphylococcus aureus and Staphylococcus epidermidis peptide pheromones produced by the accessory gene regulator agr system. Peptides (NY). 2001;22:1603–8.
Xiao M, Zhao R, Zhang Q, Fan X, O’Sullivan MVN, Li D-F, et al. Genotypic diversity of Staphylococcus aureus α-Hemolysin gene (hla) and its association with clonal background: implications for vaccine development. PLoS One. 2016;11:e0149112.
Murai M, Moriyama H, Hata E, Takeuchi F, Amemura-Maekawa J. Variation and association of fibronectin-binding protein genes fnbA and fnbB in Staphylococcus aureus Japanese isolates. Microbiol Immunol. 2016;60:312–25.
Bernabè G, Dal Pra M, Ronca V, Pauletto A, Marzaro G, Saluzzo F, et al. A novel Aza-derivative inhibits agr quorum sensing signaling and synergizes methicillin-resistant Staphylococcus aureus to clindamycin. Front Microbiol. 2021:12, 610859.
Khan BA, Yeh AJ, Cheung GYC, Otto M. Investigational therapies targeting quorum-sensing for the treatment of Staphylococcus aureus infections. Expert Opin Investig Drugs. 2015;24:689–704.
Jing S, Kong X, Wang L, Wang H, Feng J, Wei L, et al. Quercetin reduces the virulence of S. aureus by targeting ClpP to protect mice from MRSA-induced lethal pneumonia. Microbiol Spectr. 2022;10:e0234021.
Acknowledgements
NA.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding provided.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.A.A, and N.Y.; methodology, S.M.S. and M.M.S.; software, S.M.S.; validation, H.A.A, S.M.S., N. Y and M.M.S.; formal analysis, H.A.A, S.M.S., N. Y and M.M.S.; investigation, H.A.A, S.M.S., N. Y and M.M.S.; data curation, H.A.A, S.M.S. N. Y and M.M.S.; writing-original draft preparation, S.M.S.; writing review and editing, H.A.A, S.M.S., N. Y and M.M.S.; supervision, H.A.A, N. Y and M.M.S. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All mentioned procedures in the animal study section were in accordance with Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press) and the ethical standards of the Zagazig University Institutional Animal Care and Use Committee (ZU-IACUC) with approval number ZU-IACUC/3/F/141/2022; and all experimental protocols were approved by Zagazig University Institutional Animal Care and Use Committee. The study is reported in accordance with ARRIVE guidelines.
Consent for publication
NA.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Saleh, M.M., Yousef, N., Shafik, S.M. et al. Attenuating the virulence of the resistant superbug Staphylococcus aureus bacteria isolated from neonatal sepsis by ascorbic acid, dexamethasone, and sodium bicarbonate. BMC Microbiol 22, 268 (2022). https://doi.org/10.1186/s12866-022-02684-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02684-x