Abstract
Background
Quorum sensing is a mechanism of cell to cell communication that requires the production and detection of signaling molecules called autoinducers. Although mesophilic bacteria is known to utilize this for synchronization of physiological processes such as bioluminescence, virulence, biofilm formation, motility and cell competency through signaling molecules (acyl homoserine lactones, AI-1; oligopeptides, peptide based system and furanosyl borate diester, AI-2), the phenomenon of quorum sensing in thermophiles is largely unknown.
Results
In this study, proteomes of 106 thermophilic eubacteria and 21 thermophilic archaea have been investigated for the above three major quorum sensing systems to find the existence of quorum sensing in these thermophiles as there are evidences for the formation of biofilms in hot environments. Our investigation demonstrated that AI-1 system is absent in thermophiles. Further, complete peptide based two component systems for quorum sensing was also not found in any thermophile however the traces for the presence of response regulators for peptide based system were found in some of them. BLASTp search using LuxS (AI-2 synthase) protein sequence of Escherichia coli str. K-12 substr. MG1655 and autoinducer-2 receptors (LuxP of Vibrio harveyi, LsrB of E. coli str. K-12 substr. MG1655 and RbsB of Aggregatibacter actinomycetemcomitans) as queries revealed that 17 thermophilic bacteria from phyla Deinococcus- Thermus and Firmicutes possess complete AI-2 system (LuxS and LsrB and/or RbsB). Out of 106 thermophilic eubacteria 18 from phyla Deinococcus- Thermus, Proteobacteria and Firmicutes have only LuxS that might function as AI-2 synthesizing protein whereas, 16 are having only LsrB and/or RbsB which may function as AI-2 receptor in biofilms.
Conclusions
We anticipate that thermophilic bacteria may use elements of LsrB and RbsB operon for AI-2 signal transduction and they may use quorum sensing for purposes like biofilm formation. Nevertheless, thermophiles in which no known quorum sensing system was found may use some unknown mechanisms as the mode of communication. Further information regarding quorum sensing will be explored to develop strategies to disrupt the biofilms of thermophiles.
Similar content being viewed by others
Background
Quorum sensing is a cell density dependent mode of communication used by bacteria to sense their neighbors and allows them to synchronize their behavior. In mesophilic bacteria, quorum sensing is known to regulate various collaborative processes viz. virulence, bioluminescence, competence, biofilm formation, swarming, sporulation, motility, regulation of stress related genes, optimal growth under iron starvation and antibiotic resistance. These processes are controlled by the production and detection of signaling molecules termed as autoinducers [1,2,3,4,5]. Despite such vast information regarding quorum sensing in mesophiles, the information in thermophiles is largely unknown, although the processes like formation of biofilms by thermophilic bacteria and archaea are well recognized [6,7,8].
Although the three major quorum sensing systems (Autoinducer-1, Peptide based and Autoinducer-2) have been investigated in thermophilic bacteria but still there is limited information on the mechanism of quorum sensing in thermophilic environment [9]. Autoinducer-1 (acyl homoserine lactone, AHLs) is known to be heat labile, so this kind of quorum sensing was thought unlikely at high temperature [10]. Peptide based quorum sensing has been observed in hyperthermophilic syntrophic cultures like the association between Thermotoga maritima, which produces H2 as autoinhibitory product and hydrogenotrophic methanogen Methanococcus jannaschii [7]. Here, the inhibition of growth of T. maritima due to H2 is relieved and cell density is enhanced by transfer of H2 to M. jannaschii. Autoinducer-2 (AI-2) is known to be a universal system of bacterial communication. Nichols et al. [11] demonstrated that two hyperthermophiles, a eubacterium T. maritima, and an archaean Pyrococcus furiosus, produced a response to Vibrio harveyi AI-2 assay despite lacking the autoinducer-2 synthase LuxS (also known as S-ribosyl homocysteine lyase or S-ribosylhomocysteinase). They further hypothesized that temperature dependent rearrangement of phosphorylated ribose results in formation of AI-2 in these hyperthermophiles. Perez-Rodriguez et al. [12] demonstrated that the luxS is expressed and quorum sensing is induced in Caminibacter mediatlanticus, an epsilon proteobacterium from deep-sea hydrothermal vents. They further represented the evolutionary linkage of luxS genes among the thermophiles and human pathogens of epsilon proteobacteria. Rao et al., [13] demonstrated the presence of LuxS homologues in certain thermophilic members of Deinococcus-Thermus and Firmicutes. However, to date, no report exists on the exploration of complete autoinducer-2 pathway comprising autoinducer-2, methylthioadenosine/S-adenosylhomocysteine nucleosidase (also known as MTA/SAH nucleosidase or Pfs) and autoinducer-2 receptor in thermophilic bacteria.
LuxS is known to be involved in quorum sensing as well as in activated methyl cycle (AMC). Quorum sensing pathway involves two steps, the conversion of toxic S-adenosyl-homocysteine to S-ribosyl-homocysteine catalyzed by Pfs and then conversion of S-ribosyl-homocysteine to homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD), catalyzed by LuxS. This DPD is further converted to AI-2 (a furanosyl borate diester) (Fig. 1).
This study is aimed at assessing which type(s) of quorum sensing system(s) is prevalent in thermophiles. For this, we have investigated all the three major quorum sensing systems (Autoinducer-1, Peptide based system and Autoinducer-2) in 106 thermophilic eubacteria and 21 thermophilic archaea. Through extensive database analysis of their proteomes, it was found that no autoinducer-1 like synthase or receptor is present in thermophiles. However traces of the presence of peptide based system have been found in certain thermophilic eubacteria. This study also investigates AI-2 synthase (LuxS), Pfs as well as receptors for autoinducer-2 (LuxP, LsrB and RbsB) by analyzing proteomes of thermophilic bacteria and thermophilic archaea in NCBI. These finding provide new insights into the mode of communication utilized by thermophilies (planktonic/biofilms) at high temperature.
Results
Prevalence of quorum sensing systems in thermophiles
The proteomes of 106 thermophilic eubacteria and 21 thermophilic archaea were searched for the presence of autoinducer-1 system through BLASTp using LuxI and LuxR protein sequences of V. fischeri ES114 as query. Not more than 30% identity with 40% query cover was found in any of the thermophiles. None of the thermophiles was found to have autoinducer-1 system as it is already known that AHLs are degraded at high temperature [10].
Peptide based quorum sensing was searched through Quorumpeps software which comprises all the known quorum sensing signaling peptides [14]. T. maritima which is already reported [7] to exhibit quorum sensing through peptide signaling was the only thermophile found in this study. Next BLASTp search was performed for finding the peptide based signaling in other thermophiles by using protein sequences of five peptide based quorum sensing systems (Additional file 1) as query but very low (less than 35%) identity was found in any of the thermophilic eubacteria (Additional file 2, Additional files 3, 4 and 5). Among the five peptide based sequences used for BLASTp, none of the histidine kinase receptors showed identity with thermophiles while few thermophilic eubacteria had query cover more than 90% and identity more than 28% with response regulator AgrA and ComA (Additional file 2), and MultAlin showed very little conservation (Additional files 3, 4 and 5). None of the thermophilic archaea showed identity with any of the protein sequences (Additional file 1).
Prevalence of enzymes involved in synthesis of DPD
Pfs and LuxS
A total of 106 thermophilic eubacteria were used for the analysis. As shown in Table 2, 40 were having LuxS protein showing identity (36 to 66%) with LuxS of E. coli str. K-12 substr. MG1655. Nitratiruptor sp. SB155–2 was found to have maximum identity (66%) while Anoxybacillus spp. had minimum (36–39%). Further, to complete the analysis for the presence of quorum sensing in thermophiles, search for the presence of LuxS in archaea was performed, but it was not found in any archean (Table 1).
The LuxS sequences of thermophlic bacteria were aligned with those of mesophiles to find the conservation of residues. The “invariant sequence motif”, HXXEH was found in all these bacteria besides amino acids viz. Ser-9, Phe-10, Asp-13, His-14, Ala-19, Pro-20, Val-22, Arg-23, Asp-40, Arg-43, Pro-46, Asn-47, Ala-64, Arg-68, residues 80–95, Gly-97, Pro-125, Cys-132, Gly-133, His-138, Ala-143 and Leu-151 conserved in LuxS protein sequence of thermophilic as well as mesophilic bacteria (Additional file 6).
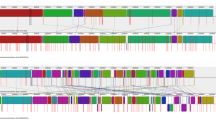
Phylogenetic analysis of LuxS from mesophilic and thermophilic bacteria (Fig. 2) showed nine distinct clades formed. First clade comprising thermophilic Gram positive sporulating bacteria indicated that they shared a common Bacillus spp. (B. cereus/ B. anthracis) as ancestor. Second clade was formed by distinct thermophilic bacteria from different genera showing the evolutionary linkage between T. thermocopriae and C. perfringens as well as between M. hydrothermalis and O. profundus. T. thermocopriae, previously known as Clostridium thermocopriae and C. perfringens are Gram-positive anaerobic bacteria most commonly found in decaying vegetables. On the other hand M. hydrothermalis and O. profundus are deep-sea hydrothermal vent thermophiles. This information indicates that the bacteria prevalent in similar environments share relatedness among their LuxS sequences. In the third clade, S. marcescens and E. coli shared a common ancestor V. harveyi nested within the thermophilic lineages. They all belong to the phylum Proteobacteria. This suggests the thermophilic origin of LuxS among Proteobacteria. Fourth clade was formed by Thermus spp. with T. oshimai being the ancestor as all other LuxS of Thermus spp. are nested within it. Fifth clade showed evolutionary linkage of P. luminescens and S. enterica sharing a common thermophilic ancestor M. silvanus. This again suggested the thermophilic lineage of LuxS. Sixth clade comprised of mesophiles far distinct in evolutionary linkage. Seventh included Meiothermus spp., sharing T. calditterrae as ancestor. Eighth and ninth comprised of Gram positive thermophilic bacteria and Thermoanaerobacter spp. respectively. In ninth clade all Thermoanaerobacteria spp. were sharing a common ancestor (M. chilarophilus). LuxS was highly conserved among mesophiles and thermophiles while the conservation on the basis of 16S rRNA was not observed (Fig. 3).
The first enzyme that leads to detoxification of S-adenosylhomocysteine is MTA/SAH nucleosidase (Pfs), presence of which together with LuxS confirms the synthesis of autoinducer-2 precursor, DPD. Therefore, we searched for the presence of Pfs in thermophilic bacteria by using MTA/SAH nucleosidase sequence of E. coli str. K-12 substr as query (Table 2). Certain thermophilic bacteria which did not possess LuxS were having Pfs e.g. Fervidobacterium islandicum, Fervidobacterium nodosum, Thermosipho petrophila, Thermosipho melanesiensis and Thermotoga sp.. Maximum identity was observed with Anoxybacillus spp. and Geobacillus spp. (53–56%) while the identity was least with Meiothermus ruber (29%) and Thermotoga spp. (30–32%) which indicates that Pfs of Anoxybacillus spp. and Geobacillus spp. is more identical to that of mesophiles. Multiple sequence analysis showed no conservation among Pfs of all bacteria (Additional files 7 and 8).
Phylogenetic analysis to find the evolutionary relationship of MTA/SAH nucleosidase in thermophiles and E. coli str. K-12 substr. showed that among the LuxS positive species only Gram positive thermophiles, and Thermotoga spp. showed evolutionary relationship while all others were far distant in evolution (Fig. 4).
Prevalence of SAH hydrolase
SAH hydrolase (S-adenosyl-L-homocysteine hydrolase) is involved in one step pathway for the conversion of SAH to homocysteine and adenosine without producing autoinducer-2 (Fig. 1). SAH hydrolase was found to be highly conserved among bacteria and was present in a large number of thermophiles (Tables1 and 2). Among archaea, SAH hydrolase was found in Thermococcus barophilus, T. chitonophagus, T. gammatolerans, T. kodakarensis, T. litoralis, Pyrolobus fumarii, P. abyssi, P. furiosus, Pyrococcus horikoshii, Picrophilus torridus, Metallosphaera sedula and Aeropyrum pernix with identity between 40 to 43% by using SAH hydrolase of Ralstonia solanacearum as query (Table 1). However none of the known quorum sensing system was found in thermophilic archaea. M. silvanus and M. chliarophilus had autoinducer-2 system as well as SAH hydrolase. All other thermophilic bacteria had only SAH hydrolase and no LuxS or autoinducer-2 system in them which shows that they have role in detoxification of SAH only (Table 2). MultAlin showed the highly conserved regions in SAH hydrolases (Additional files 9 and 10) of mesophilic and thermophilic bacteria.
Phylogenetic analysis showed four different clades (Fig. 5). LuxS negative Firmicutes formed a separate clade that indicates they all utilize one step pathway for the detoxification of SAH and their SAH hydrolases are evolutionarily identical. Thermococcus spp. and Pyrococcus spp. are forming a clade together that shows the high similarity and evolutionary linkage among their SAH hydrolases. Moreover microorganisms growing in similar environments may have acquired SAH hydrolase by horizontal gene transfer. A clade was formed by Thermotoga spp. sharing common ancestor (C. platensis). Last clade indicates events of horizontal gene transfer from M. hydrothermalis and M. silvalnus to Proteobacteria and Thermodesulfobacter and from Proteobacteria to Aquificae. T. thiophilus, T. atlanticus and T. indicus are sulfur reducing whereas S. azorense and S. subterraneum are sulfur oxidizing bacteria. They may have evolved together in evolution and may have acquired SAH hydrolase by horizontal gene transfer.
Prevalence of AI-2 receptor proteins
LuxP and LsrB
LuxP was not found in any of the thermophilic bacteria through BLASTp using LuxP of Vibrio harveyi as query sequence. LsrB type autoinducer-2 ABC transporter substrate-binding proteins were present in O. profundus, M. silvanus, A. geothermalis, T. napthophila, M. thermoacetica and C. subterraneus and were absent in all other thermophilc bacteria. In LsrB positive strains the identity was 25–62% with query sequence. Maximum identity was with O. profundus (62%). LsrB type protein was present in few such thermophiles which did not possess LuxS e.g. T. naphthophila, Moorella sp. and C. subterraneus which indicates that these thermophiles can respond to autoinducer-2 but do not produce it or they are involved in pentose sugar transport. M. silvanus and A. geothermalis were the two thermophiles which possessed LuxS, Pfs and LsrB, hence the complete autoinducer-2 producing cascade. Further Oceanithermus profundus possessed LuxS and LsrB but no Pfs. All the LsrB sequences from mesophilic and thermophilic bacteria were aligned to find the conservation (Additional file 11). Few residues were conserved such as Lys-39, Gly-52, Gly-61, Pro-75, Gln-84, Ala-115, Gly-119 and Gly-262. Our results showed that the LsrB of E. coli str. K-12 substr. MG1655 had very less identity with thermophilic bacterial autoinducer-2 ABC substrate binding protein although the query cover was more than 90%. Phylogenetic analysis was performed to find the evolutionary relationship among autoinducer-2 binding LsrB protein in bacteria but no relationship was found except for Firmicutes A. geothermalis, G. thermocatenulatus that shared a common ancestor M. glycerini (Fig. 6).
Rbs B
In vitro competition assays demonstrated that LsrB and RbsB both are capable of binding AI-2 in A. actinomycetemcomitans [35]. RbsB homologs were searched in thermophilic bacteria by BLASTp using RbsB of A. actinomycetemcomitans as query sequence. High conservation was observed by MultAlin alignment (Additional file 12), maximum identity being with Thermoanaerobacter spp. (61–62%) and minimum with Meiothermus spp. (31–33%). This suggests that RbsB is highly conserved in bacteria, whether thermophiles or mesophiles. Phylogenetic analysis was done further to observe the evolutionary relationship (Fig. 7). Thermophilic spore formers are forming clade together. Kosmotoga spp. showed common ancestor Petrotoga mobilis while T. thermocopriae and C. subterraneus are sharing T. thermosaccharolyticum as ancestor. All of them belong to phylum Firmicutes which may indicate that Firmicutes have acquired RbsB by horizontal gene transfer, all other thermophiles were far apart in the evolution of LsrB.
STRING analysis of LuxS
Deinococcus-Thermus, Firmicutes and Proteobacteria were the only phyla producing LuxS. Therefore, we have selected one member from each of these phyla for STRING analysis to find the proteins linked to LuxS as well as to find the possible role of LuxS in thermphilic bacteria. It is known that if Pfs and LuxS both are present than the pathway will lead to the production of DPD, a precursor of autoinducer-2. Sequence of LuxS protein of M. ruber, member of Deinococcus-Thermus phylum; G. thermoglucosidasius, member of Firmicutes phylum and C. mediatlanticus, member of Proteobacteria phylum were used for STRING analysis (Additional files 13, 14 and 15). Methionine synthase, cysteine synthase and MTA nucleosidase were linked to LuxS in all these thermophilic bacteria while cobalamin-independent methionine synthase, metE (5-methyltetrahydropteroyltri-L-glutamate) and homoserine dehydrogenase were linked to LuxS in M. ruber and C. mediatlanticus only. O-acetyl homoserine sulfhydrylase and cys/met metabolism pyridoxial phosphate dependent protein were present in M. ruber and G. thermoglucosidasius only. Presence of both LuxS and S-adenosylhomocysteine nucleosidase in all of them indicates that they are using two step pathways for the detoxification of SAH (Fig. 1). Presence of LuxS may further leads to the production of AI-2 in these thermophilic bacteria.
Discussion
The way thermophilic bacteria adapt themselves in hot environment has become the topic of interest since 1960’s after the discovery of T. aquaticus. Presence of evidences for quorum sensing in mesophiles demands the investigation of quorum sensing mechanism(s) utilized by thermophiles. Thermophilic bacteria viz. T. thermophilus and thermophilic archaea viz. P. furiosus have been shown to form biofilms [45]. These thermophiles with other thermophilic biofilm formers are included in this study to suggest that these may interact with each other by quorum sensing. Previous work on the presence of peptide based quorum sensing in T. maritima [7], temperature dependent formation of AI-2 in T. maritima and archaean P. furiosus [11] and the expression of LuxS in C. mediatlanticus led us to search for quorum sensing systems present in thermophilic community.
In this study, the most prevalent quorum sensing systems were investigated. AI-1 system was not found in thermophiles which is in accordance with the results of Schopf et al. [10] that suggests the heat labile nature of AI-1 system. However, few thermophilic eubacteria showed traces for the presence of peptide based quorum sensing system (Table 2) but the identity with their mesophilic counterparts was very low. Only few members of phyla Firmicutes, Deinococcus-Thermus, Chloroflexi and Thermodesulfobacteria showed some identity with the response regulator AgrA and ComA but none of them showed any good identity with receptor histidine kinases which may suggest the presence of incomplete two component system in thermophilic eubacteria or they do not posseses any peptide based system or there may be some unknown proteins involved in two component peptide based system for thermophiles.
Search for MTA/SAH nucleosidase (Pfs), autoinducer-2 synthase (LuxS) and SAH hydrolase in the proteomes of thermophilic bacteria showed that the universal autoinducer-2 type of quorum sensing is the mode of communication. Pfs and SAH hydrolase are important enzymes like LuxS as they play role in the detoxification of SAH [20,21,22]. The Pfs mutant of Neisseria meningitidis completely stopped the release of AI-2 due to the accumulation of inhibitors such as MTA and toxic SAH [23]. These results were further supported by the previous work in which Pfs and LuxS inhibitors showed reduction in biofilm formation and did not produce autoinducer-2 [24,25,26]. Absence of LuxS and presence of Pfs and SAH hydrolase in Fervidobacterium islandicum, F. nodosum, Thermosipho melanesiensis, Thermotoga sp. and an archean Aeropyrum pernix in this work predicts that Pfs and SAH hydrolase play role in the detoxification of SAH and they do not produce AI-2. Therefore, in these bacteria due to the lack of LuxS no autoinducer-2 is synthesized.
Phylogenetic analysis of LuxS in thermophiles and mesophiles did not correlate with their 16S rRNA phylogeny, that indicates LuxS is more conserved in bacteria of different phyla and environment and mesophilic bacteria may have acquired LuxS from thermophiles (Fig. 2). Further LuxS from themophilic bacteria of same phylum were evolutionarily closer than from other phyla. This finding was supported by the Perez-Rodriguez et al. [12] who showed the LuxS gene flow from thermophiles to mesophilic epsilonproteobacteria. This work showed the events of horizontal luxS gene transfer, as certain thermophilic bacteria inhabiting similar environment were having LuxS that was evolutionarily more identical. Anoxybacillus spp. and Geobacilllus spp prevalent in dairy industry, B. anthracis and B. cereus bearing close phenotypic and genotypic resemblance, T. thermocopriae and C. perfringens found in decaying vegetables, M. hydrothermalis and O. profundus, inhabitants of deep sea hydrothermal vent, S. marcescens and E.coli, pathogenic bacteria, S. enterica (human pathogen) and P. rhabdus (insect pathogen) were having more identical LuxS. Further, V. harveyi, S. marcescens and E.coli were nested within thermophilic lineage as Nitratiruptor sp. SB155–2 is known to be phylogenetically related to epsilon-proteobacterial pathogens [27]. Thus this evolutionarily identical LuxS among proteobacteria may be the result of this relation.
Further, this study showed that some thermophilic bacteria viz. M. hydrothermalis, M. taiwanesis, M. cerbereus, M. rufus, O. profundus and T. aquaticus have LuxS but no Pfs. However, except M. hydrothermalis all of them possess SAH hydrolase. This indicates that here the detoxification of S-adenosylhomocysteine (SAH) occurs through SAH hydrolase since Pfs is absent in them. However, except O. profundus all of them lack the known receptors for AI-2 which predicts that they may not be involved in AI-2 type quorum sensing and their LuxS carries out the metabolic process such as the production of homocysteine for converting it back to methionine in AMC. However, the possibility of the presence of some unknown AI-2 receptors in them cannot be ruled out.
Multiple sequence alignment of LuxS of thermophilic and mesophilic bacteria showed the presence of invariant HXEEH motif which is in accordance with the work done by Keersmaecker et al. [28]. Further, our analysis showed that all thermophilic bacteria possess amino acids in the similar positions as in persistant hub which is the characterstic of extremophilic LuxS protein as described by Bhattacharyya and Vishveshwara [29]. M. silvanus and M. chilarophilus both showed autoinducer-2 synthesizing complete pathway as well as SAH hydrolases. Presence of both pathways in these two thermophiles allows them to recycle methionine more economically which is further supported by the work of Schell et al. [30] that showed the presence of both of these pathways in Bifidobacterium longum. Except M. silvanus and M. chilarophilus, all other thermophiles lacking complete autoinducer-2 system have SAH hydrolases which indicates that they may have role in detoxification of SAH to homocysteine and adenosine without synthesizing AI-2. Our results are further supported by Winzer et al. [31] who showed the presence of only SAH hydrolase and no autoinducer-2 synthesizing machinery in Leptospira interrogans. Nevertheless, the possibility of unknown mechanisms to synthesize AI-2 exists.
LuxP was not found in any of the thermophilic bacteria, corroborating the previous work showing the LuxP type receptors are only limited to Vibrionales and Oceanospirillales [32, 33].
Another well known receptor for autoinducer-2 was found to be LsrB. There are evidences for the presence of LsrB in Enterobacteriaceae and Rhizobiaceae and Bacillaceae families [34]. Autoinducer-2 ABC transporter which shares identity with LsrB ABC transporter was found in some thermophilic bacteria in this study. M. silvanus, O. profundus, A. geothermalis, C. subterraneus, M. thermoacetica and T. naphthophila were the only thermophiles having autoinducer-2 ABC transporter identical to LsrB. Our results showed that M. silvanus, A. geothermalis and O. profundus possess both Pfs and LuxS as well as LsrB, complete autoinducer-2 quorum sensing circuit which can produce as well as detect autoinducer-2. However, all of these thermophiles posseses RbsB as well. It indicates that they may utilize two receptors for autoinducer-2 detection like in Aggegatibacter actinomycetemcomitans [35] where LsrB protein competes with the LuxP of Vibrio harveyi during autoinducer-2 bioassay and inhibits the interaction of AI-2 with LuxP. They further showed that LsrB competes with LuxP for the AI-2 produced by V. harveyi while RbsB competes with LuxP for the AI-2 produced by A. actinomycetemcomitans. It may be concluded from our work that C. subterraneus, M. thermoacetica and T. naphthophila possess autoinducer-2 ABC transporter protein which is identical to LsrB ABC transporter but they all lacked LuxS protein which indicates that they might respond to autoinducer-2 produced by other bacteria in biofilms without producing it themselves. This is in accordance with the results of Rezzonico and Duffy [32] which showed the presence of orphan lsr operon in R. sphaeroides and S.meliloti that lack luxS. It may be possible for bacteria to detect the AI-2 signal even if it does not produce it. Pseudomonas aeruginosa does not contain luxS but in the presence of enzymatically produced AI-2 or in co-culture with Streptococcus mitis, some of its virulence gene promoters were upregulated [36, 37]. Similar findings were observed in some mixed biofilm formation [38].
In previous reports, RbsB has been speculated as another receptor for autoinducer-2 in Borellia burgdorferi and Actinobacillus actinomycetemcomitans [35, 39]. Our study found a large number of thermophilic bacteria having D-ribose binding protein identical to RbsB of A. actinomycetemcomitans (Additional file 12). Ribose ABC-transporter is distributed ubiquitously among thermophilic bacteria regardless of the prevalence of Pfs and LuxS and these results are in accordance with the previous work [32]. It may thus work as the autoinducer-2 receptor in thermophilic bacteria lacking LuxP and LsrB.
Furthermore, there are certain mesophilic bacteria (Actinobacillus pleuropneumoniae, Borrelia burgdorferi, Helicobacter pylori, Mycobacterium avium and Streptococcus spp.) which are known to form biofilms and other quorum sensing phenotypes and produce AI-2 but lack the known LuxP, LsrB and RbsB like receptors [40,41,42,43]. Similarly in many thermophiles no known receptor for AI-2 has been found, so unknown alternative receptors might exist for them. Presence of LuxS and autoinducer-2 receptor in thermophiles may explain their role in quorum sensing. Presence of autoinducer-2 receptor but lack of autoinducer-2 synthase in certain thermophiles may explain their interactions with neighbours in biofilms regardless of producing autoinducer-2 synthase but responding to autoinducer-2. Peptide based or other unknown quorum sensing systems may be the mode of communication used by some thermophilic archaea as no autoinducer-1 or autoinducer-2 system is present in them.
Conclusion
Thermophiles are one of the major groups of extremophile. They are known to form biofilms at high temperature. Identifying the phenomenon to form biofilms at high temperature may reveal how these microorganisms adapted themselves at such adverse conditions. As mentioned in previous studies, AI-1 and oligopeptides are damaged by hydrolytic cleavage whereas; AI-2 is quiet stable at different pH range and temperatures [12, 44]. Therefore AI-2 might be the mode of communication used by thermophilic bacteria. Other unknown quorum sensing systems may be prevalent in thermophiles which have to be explored further. To conclude, our study is an attempt to understand the mechanism of quorum sensing used by thermophiles as information regarding quorum sensing has not been explored in most of the thermophiles. Further this information can be utilized for developing strategies to disrupt the biofilms formed by these thermophiles at high temperature.
Methods
Analysis for the presence of quorum sensing systems (AI-1, peptide based, AI-2) in thermophiles
A total of 106 thermophilic bacteria from 10 different phyla and 21 thermophilic archaea from 3 different phyla were analyzed for the presence of quorum sensing proteins through NCBI protein database (http:// www.ncbi.nlm.nih.gov). Homologues of autoinducer synthases (LuxI for AI-1 and LuxS for AI-2), their receptors (LuxR for AI-1 and LuxP, LsrB and RbsB for AI-2), MTA/SAH nucleosidase (Pfs) and SAH hydrolase were searched in thermophiles through BLASTp (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Quorum sensing signaling peptides were explored using Quorumpeps database [14]. Protein sequences of receptor histidine kinases and response regulators involved in peptide based quorum sensing systems viz. AgrC/AgrA, FsrC/FsrA, RapC/RapA, ComP/ComA and ComD/ ComE were used as queries for BLASTp.
Protein sequences of LuxI/LuxR of Vibrio fischeri (accession number YP_206882.1 and AAW87995.1), LuxS of Escherichia coli str. K-12 substr. MG1655 (accession number NP_417172.1), LuxP of V. harveyi (accession number AAA20837.2), LsrB of E. coli str. K-12 substr. MG1655 (accession number AMC98721.1), RbsB of Aggregatibacter actinomycetemcomitans (accession number WP_050846150.1), Pfs protein of E. coli str. K-12 substr. MG1655 (accession number NP_414701.1), S-adenosyl-L-homocysteine hydrolase of Ralstonia solanacearum (accession no. KFZ93620.1), AgrC/AgrA of Staphylococcus aureus (accession number ABX60402.2 and ALY21623.1), FsrC/FsrA of Enterococcus faecalis (accession number AFO44434.1 and AFO44436.1), RapC/RapA and ComP/ComA of Bacillus subtilis (accession number AAT75294.1, KIX82477.1, AKN15283.1 and BAB13494.1) and ComD/ ComE (accession number AAC44896.1 and APJ35576.1) of Streptococcus pneumoniae were used as queries for BLASTp.
Sequences of quorum sensing proteins in thermophiles having query cover more than 85% with identity more than 35% (except for Pfs and LsrB, as during BLASTp analysis some of the protein sequences in thermophiles are showing names similar to Pfs and LsrB but with identity less than 35%) were aligned using MultAlin with the LuxS homologes in mesophilic bacteria such as Serratia marcescens (ALE94556.1), Campylobacter jejuni subsp. jejuni ATCC 33560 (EIB44085.1), Salmonella enterica (AAR88507.1), E. coli str. K-12 substr. MG1655 (NP_417172.1), Bacillus cereus H3081.97 (EDZ54884), Bacillus anthracis str. Ames (AAP28724.1), Photorhabdus luminescens (OCA55726.1), V. harveyi (AAD17292.1). Further MultAlin was done by aligning SAH hydrolases of thermophiles and mesophiles (Pseudomonas syringae pv. actinidiae, accession no. GAO96569.1; Xanthomonas campestris pv. musacearum NCPPB 4384, accession no. KFA32367.1; Nitrosomonas europaea ATCC 19718, accession no. CAD84571.1; Mycobacterium tuberculosis K, accession no. AIB49973.1) having SAH hydrolase to find the conservation in all of them.
Phylogenetic analysis
Phylogenetic trees were constructed for LuxS, Pfs, LsrB, RbsB, SAH hydrolase as well as for 16S rRNA genes of thermophiles and mesophiles by aligning the sequences using MEGA (Molecular Evolutionary Genetics Analysis) 6.0 software and neighbor-joining (NJ) method [15]. Bootstrap test using 1000 replicates was shown next to the branches [16]. The evolutionary distances were computed using the p-distance method [17].
STRING analysis of LuxS
STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) analysis [18, 19] of LuxS of one representative member of each phylum of thermophilic bacteria was performed to find the interacting protein. STRING analysis was used to find the common proteins linked to LuxS in different phylum.
Abbreviations
- AI-1:
-
Autoinducer-1
- AI-2:
-
Autoinducer-2
- AMC:
-
Activated methyl cycle
- MTA:
-
Methylthioadenosine
- SAH hydrolase:
-
S-adenosyl-L-homocysteine hydrolase
- SAH nucleosidase:
-
S-adenosylhomocysteine nucleosidase
- DPD:
-
4,5-dihydroxy-2,3-pentanedione
- NCBI:
-
National Center for Biotechnology Information
- BLAST:
-
Basic Local Alignment Search Tool
- MEGA:
-
Molecular Evolutionary Genetics Analysis
- STRING:
-
Search Tool for the Retrieval of Interacting Genes/Proteins
- AHL:
-
Acyl homoserine lactone
- ABC transporter:
-
ATP-binding cassette transporter
- metE:
-
5-methyltetrahydropteroyltri-L-glutamate
References
Waters CM, Bassler BL. Quorum sensing cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46.
James D, Shao H, Lamont RJ, Demuth DR. The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals. Infect Immun. 2006;74:4021–9.
Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J, Swords WE. Indirect pathogenicity of Haemophilus influenza and Moraxella cataeehalis in polymicrobial otitis media occurs via interspecies quorum sensing. M Bio. 2010;1:102–10.
Furlani RE, Yeagley AA, Melander C. A flexible approach to 1, 4-di-substituted 2-aminoimidazoles that inhibit and disperse biofilms and potentiate the effects of beta-lactams against multi-drug resistant bacteria. Eur J Med Chem. 2013;62:59–70.
Lin L, Li T, Dai S, Yu J, Chen X, Wang L, Wang Y, Hua Y, Tian B. Autoinducer-2 signaling is involved in regulation of stress-related genes of Deinococcus radiodurans. Arch Microbiol. 2016;198:43–51.
Lapaglia C, Hartzell PL. Stress-induced production of biofilm in the hyperthermophile Archaeoglobus fulgidus. Appl Environ Microbiol. 1997;63(8):3158–63.
Johnson M, Montero C, Connors S, Shockley K, Bridger S, Kelly R. Population density-dependent regulation of exopolysaccharide formation in the hyperthermophilic bacterium Thermotoga, maritima. Mol Microbiol. 2005;55:664–74.
Zhang G, Zhang F, Ding G, Li J, Guo X, Zhu J, Zhou L, Cai S, Liu X, Luo Y, Zhang G, Shi W, Dong X. Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J. 2012;6:1–9.
Montgomery K, Charlesworth JC, LeBard R, Visscher PT, Burns BP. Quorum sensing in extreme environments. Life (Basel). 2013;3(1):131–48.
Schopf S, Wanner G, Rachel R, Wirth R. An archaeal bi-species biofilm formed by Pyrococcus furiosus and Methanopyrus kandleri. Arch Microbiol. 2008;190:371–7.
Nichols J, Johnson M, Chou C, Kelly R. Temperature, not LuxS, mediates AI-2 formation in hydrothermal habitats. FEMS Microbiol Ecol. 2009;68:173–81.
Pérez-Rodríguez I, Bolognini M, Ricci J, Bini E, Vetriani C. From deep-sea volcanoes to human pathogens: a conserved quorum-sensing signal in Epsilonproteobacteria. ISME J. 2015;9:1222–34.
Rao RM, Pasha SN, Sowdhamini R. Genome-wide survey and phylogeny of S-Ribosylhomocysteinase (LuxS) enzyme in bacterial genomes. BMC Genomics. 2016;17:742.
Wynendaele E, Bronselaer A, Nielandt J, D’Hondt M, Stalmans S, Bracke N, Verbeke F, Van De Wiele C, De Tré G, De Spiegeleer B. Quorumpeps database: chemical space, microbial origin and functionality of quorum sensing peptides. Nucleic Acids Res. 2013;41(D1):D655–D65.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–25.
Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125(1):1–15.
Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press; 2000.
Snel B, Lehmann G, Bork P, Huynen MA. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000;28(18):3442–4.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–52.
Sun J, Daniel R, Wagner-Dobler I, Zeng AP. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol Biol. 2004;4:36.
Winzer K, Hardie KR, Burgess N, Doherty N, Kirke D, Holden MT, et al. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiol. 2002a;148:909–22.
Markham GD, Pajares MA. Structure-function relationships in methionine adenosyltransferases. Cell Mol Life Sci. 2009;66:636–48.
Heurlier K, Vendeville A, Halliday N, Green A, Winzer K, Tang CM, Hardie KR. Growth deficiencies of Neisseria meningitidis pfs and luxS mutants are not due to inactivation of quorum sensing. J Bacteriol. 2009;191:1293–302.
Singh V, Luo M, Brown RL, Norris GE, Schramm VL. Transition-state structure of Neisseria meningitides 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase. J Am Chem Soc. 2007;129:13831–3.
Gutierrez JA, Crowder T, Rinaldo-Matthis A, Ho MC, Almo SC, Schramm VL. Transition state analogs of 5′-methylthioadenosine nucleosidase disrupt quorum sensing. Nat Chem Biol. 2009;5:251–7.
Malladi VL, Sobczak AJ, Meyer TM, Pei D, Wnuk SF. Inhibition of LuxS by S-ribosylhomocysteine analogues containing a [4-aza] ribose ring. Bioorg Med Chem. 2011;19:5507–19.
Nakagawa S, Takaki Y, Shimamura S, Reysenbach AL, Takai K, Horikoshi K. Deep-sea vent ε-proteobacterial genomes provide insights into emergence of pathogens. PNAS. 2007;104(29):12146–50.
De Keersmaecker SCJ, Sonck K, Vanderleyden J. Let LuxS speak up in AI-2 signaling. Trends Microbiol. 2006;14:114–9.
Bhattacharyya M, Vishveshwara S. Functional correlation of bacterial LuxS with their quaternary associations: interface analysis of the structure networks. BMC Struct Biol. 2009;9:8.
Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. PNAS. 2002;99(22):14422–7.
Winzer K, Hardie KR, Williams P. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. Adv Appl Microbiol. 2003;53:291–396.
Rezzonico F, Duffy B. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiol. 2008;8:154.
Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2012;37(2):156–81.
Pereira CS, de Regt AK, Brito PH, Miller ST, Xavier KB. Identification of functional LsrB-like autoinducer-2 receptors. J Bacteriol. 2009;191:6975–87.
Shao H, James D, Lamont RL, Demuth DR. Differential interaction of Aggregatibacter (Actinobacillus) actinomycetemcomitans LsrB and RbsB proteins with autoinducer 2. J Bacteriol. 2007;189(15):5559–65.
Li H, Li X, Wang Z, Fu Y, Ai Q, Dong Y, et al. Autoinducer-2 regulates Pseudomonas aeruginosa PAO1 biofilm formation and virulence production in a dose-dependent manner. BMC Microbiol. 2015a;15:192.
Song S, Du L, Yu J, Ai Q, Pan Y, Fu Y, et al. Does Streptococcus mitis, a neonatal oropharyngeal bacterium, influence the pathogenicity of Pseudomonas aeruginosa? Microbes Infect. 2015;17:710–6.
McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J Bacteriol. 2003;185:274–84.
Stevenson B, von Lackum K, Wattier RL, McAlister JD, Miller JC, Babb K. Quorum sensing by the Lyme disease spirochete. Microbes Infect. 2003;5:991–7.
Li L, Xu Z, Zhou Y, Li T, Sun L, Chen H, Zhou R. Analysis on Actinobacillus pleuropneumoniae LuxS regulated genes reveals pleiotropic roles of LuxS/AI-2 on biofilm formation, adhesion ability and iron metabolism. Microb Pathog. 2011;50:293–302.
Babb K, von Lackum K, Wattier RL, Riley SP, Stevenson B. Synthesis of autoinducer 2 by the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 2005;187:3079–87.
Rader BA, Wreden C, Hicks KG, Sweeney EG, Ottemann KM, Guillemin K. Helicobacter pylori perceives the quorum-sensing molecule AI-2 as a chemorepellent via the chemoreceptor TlpB. Microbiol. 2011;157:2445–55.
Geier H, Mostowy S, Cangelosi GA, Behr MA, Ford TE. Autoinducer-2 triggers the oxidative stress response in Mycobacterium avium, leading to biofilm formation. Appl Environ Microbiol. 2008;74:1798–804.
Globisch D, Lowery CA, McCague KC, Janda KD. Uncharacterized 4, 5-Dihydroxy-2, 3-Pentanedione (DPD) molecules revealed through NMR spectroscopy: implications for a greater signaling diversity in bacterial species. Angew Chem Int Ed Engl. 2012;51:4204–8.
Niou YK, Wu WL, Lin LC, Yu MS, Shu HY, Yang HH, et al. Role of galE on biofilm formation by Thermus spp. Biochem Biophys res Commun [internet]. Elsevier Inc. 2009;390:313–8.
Acknowledgments
AK is grateful to University Grants Commission for Maulana Azad National Junior Research Fellowship.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors listed, have made contributions for the fulfillment of this work. AK and PS designed the work, analyzed the data and contributed to writing manuscript. PS and NC finalized the results and discussion and revised the complete data critically. All authors contributed to writing, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Peptide based quorum sensing systems. (PDF 10 kb)
Additional file 2:
Prevalence of peptide based quorum sensing system in thermophilic eubacteria. (PDF 125 kb)
Additional file 3:
Multiple sequence alignment of AgrA protein from Staphylococcus aureus and thermophilic eubacteria by MultAlin. (PDF 40 kb)
Additional file 4
Multiple sequence alignment of FsrA protein from Enterococcus faecalis D32 and thermophilic eubacteria by MultAlin. (PDF 30 kb)
Additional file 5:
Multiple sequence alignment of ComA protein from Bacillus subtilis and thermophilic eubacteria by MultAlin. (PDF 120 kb)
Additional file 6:
Multiple sequence alignment of LuxS protein from mesophilic and thermophilic eubacteria by MultAlin. Invariant residues are highlighted in red colour. The conserved HTLEH motif and other conserved residues are highlighted within boxes. (PDF 111 kb)
Additional file 7:
Multiple sequence alignment of MTA/SAH nucleosidase from mesophilic and thermophilic eubacteria by MultAlin. (PDF 88 kb)
Additional file 8:
Multiple sequence alignment of MTA/SAH nucleosidase from thermophilic eubacteria by MultAlin. There is no conservation among Pfs protein sequences. (PDF 138 kb)
Additional file 9:
Multiple sequence alignment of SAH hydrolase from thermophilic and mesophilic eubacteria by MultAlin. High conservation among SAH hydrolases has been observed. (PDF 274 kb)
Additional file 10:
Multiple sequence alignment of SAH hydrolase from thermophilic eubacteria by MultAlin. High conservation among SAH hydrolases has been observed. (PDF 323 kb)
Additional file 11:
Multiple sequence alignment of LsrB from mesophilic and thermophilic eubacteria by MultAlin. (PDF 105 kb)
Additional file 12:
Multiple sequence alignment of RbsB protein from mesophilic and thermophilic eubacteria by MultAlin. Conserved residues are highlighted in red within boxes. (PDF 142 kb)
Additional file 13:
STRING analysis of LuxS protein of Meiothermus ruber. (PDF 202 kb)
Additional file 14:
STRING analysis of LuxS protein of Geobacillus thermoglucosidasius. (PDF 225 kb)
Additional file 15
STRING analysis of LuxS protein of Caminibacter mediatlanticus. (PDF 90 kb)
Additional file 16:
NCBI accession number of genomes of thermophililc archaea used in the study. (PDF 24 kb)
Additional file 17:
NCBI accession number of genomes of thermophililc bacteria used in the study. (PDF 137 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kaur, A., Capalash, N. & Sharma, P. Quorum sensing in thermophiles: prevalence of autoinducer-2 system. BMC Microbiol 18, 62 (2018). https://doi.org/10.1186/s12866-018-1204-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-018-1204-x