Abstract
Background
Arthropods are the largest group in the animal kingdom and are morphologically characterized by heterorhythmic segments. Brachyuran decapod crustaceans undergo brachyurization metamorphosis in the early developmental process, characterized by a reduced abdomen that is folded beneath the cephalothorax and inserted between the pereiopods or in a special cavity. As the main cause of major alterations in the evolution of animal body plans, Hox genes encode transcription factors and are involved in bilaterian anterior-posterior axis patterning.
Results
We found eight Hox genes (labial, proboscipedia, Deformed, zerknüllt, Sex combs reduced, Antennapedia, Ultrabithorax, fushi tarazu, abdominal-A and Abdominal-B) in Eriocheir sinensis. The phylogenetic topology of 13 arthropod Hox genes was closely related to traditional taxonomic groupings. Genome collinearity analysis was performed using genomic data and chromosomal location data of E. sinensis and Portunus trituratus. We found that their chromosomes were highly collinear, and there was a corresponding collinear relationship between the three Hox genes (lab, ftz and Abd-B). The mRNA expression levels of Scr and Antp fluctuated significantly in different developmental stages of E. sinensis, especially in the brachyurization stages. Evolutionary analysis indicated the presence of positively selected sites in Ubx.
Conclusions
In this study, we used genome-wide analysis to identify and analyze all members of the Hox genes in E. sinensis. Our data will contribute to a better understanding of Hox genes in E. sinensis and provide useful molecular evolutionary information for further investigation on their roles in the brachyurization of crabs.
Highlights
Eight Hox genes (lab, Dfd, Scr, ftz, Antp, Ubx, abd-A, and Abd-B) were identified in Eriocheir sinensis.
There was a corresponding collinear relationship between the three Hox genes (lab, ftz and Abd-B) in E. sinensis and Portunus trituratus.
Positively selected sites in Ubx (1 M 0.952*, 3 S 0.998**, 4 Y 0.998**, P = 0.0000) were identified in E. sinensis.
The full-length cDNA of Scr and Antp in E. sinensis were cloned and characterized. Hox genes (Scr and Antp) were differentially expressed during the larval development of E. sinensis.
Similar content being viewed by others
Introduction
In the lifecycle of an organism, the formation of the body and its organs during ontogeny or regeneration is known as morphogenesis. Homeobox genes are essential for controlling embryonic patterning and regulating these anatomical changes. These genes are mainly characterized by an approximately 180 bp (bp) homeodomain conserved region [1]. Homeobox genes were first identified in Drosophila melanogaster [2], followed by sequential discovery in all multicellular organisms, from parasites to vertebrates, plants and fungi, and they are highly evolutionarily conserved [3, 4].
Traditionally, homeobox genes are divided into two subfamilies. One contains genes that are arranged in clusters on chromosomes and expressed along the main anterior-posterior (A-P) axis of the animal body, which are called Hox genes or type-I homeobox genes. The other are non-A-P homeobox genes, which are not arranged in clusters but rather are scattered across different chromosomes, and they are named type-II homeobox genes based on sequence similarity [5]. In particular, Hox genes are specifically related to the regulation of body plan development during the embryonic stage of the life cycle [6]. Hox genes are mostly involved in the morphogenesis and differentiation of tissues or organs such as limbs [7], brain [8], muscle [9], blood [10], and bone [11]. Each Hox gene contains a conserved homeobox domain (homeodomain, HD) involved in the regulation of downstream genes [12]. The homeobox domain is a 60-amino-acid protein sequence with an alpha-helical secondary structure [13]. The activity of these regulatory proteins encoded by homeobox genes is most pronounced at the early embryonic stage, when the embryo is forming a body axis [14]. The expression patterns of Hox genes show a linear pattern: genes at the 3’-end are expressed first and regulate the anterior development of the embryo, while genes at the 5’-end are expressed later and regulate the posterior development of the embryo [15]. This phenomenon is called spatial collinearity and temporal collinearity.
Arthropods have maximum species richness in the animal world, and they are also the most morphologically diverse group, accounting for approximately 80% of all animal species [16]. To date, ten ancestral homologs have been confirmed in arthropods: labial (lab), proboscipedia (pb), Deformed (Dfd), zerknüllt (zen), Sex combs reduced (Scr), Antennapedia (Antp), Ultrabithorax (Ubx), fushi tarazu (ftz), abdominal-A (abd-A) and Abdominal-B (Abd-B) [17]. Drosophila melanogaster has eight Hox genes and was found to be divided into two homologous heterogeneous gene clusters on chromosome 3: the antennal foot complex Antp-C (antennapedia complex) gene cluster and the double-thorax complex BX-C (bithorax complex) gene cluster [18]. Antp-C is mainly involved in the developmental regulation of the head and chest, and BX-C is mainly involved in the regulation of posterior thoracic and abdominal development [19].
In the early developmental process, brachyuran decapod crustaceans undergo brachyurization metamorphosis, which is characterized by a reduced abdomen that is folded beneath the cephalothorax and inserted between the pereiopods or in a special cavity [20, 21]. The Chinese mitten crab (Eriocheir sinensis) is a limnic and intertidal crab mainly distributed in eastern and northern China. The juvenile development period of E. sinensis normally consists of five zoeae stages and a megalopa stage, and it subsequently enters the first juvenile crab stage and develops into an adult crab after multiple molts [22]. The most conspicuous feature of morphological change from megalopa to adult crab is brachyurization, including abdominal degeneration. A previous study showed that Hox genes are involved in embryonic development and appendage development in crustaceans [23], but the molecular regulatory mechanisms of brachyurization are poorly studied [24].
In this study, we used genome-wide analysis to identify all members of the Hox gene family between E. sinensis and 12 other arthropod species. These sequences were aligned and used to construct a phylogenetic tree. Moreover, the full-length cDNA of the Scr and Antp genes were cloned and characterized from the Chinese mitten crab (E. sinensis) using expressed sequence tag (EST) and rapid amplification of cDNA end (RACE) techniques. The mRNA expression profiles of Scr and Antp transcripts in various samples (different developmental stages of juvenile crabs) were measured by fluorescent real-time quantitative PCR (RT‒qPCR). The selective pressure of Scr and Antp was determined to reveal the relationship between brachyurization and adaptive evolution of Hox genes in Brachyura crabs. Our data will contribute to a better understanding of Hox genes in E. sinensis and provide useful molecular evolutionary information for further investigation on their roles in brachyurization of crabs.
Result
Identification and analyses of Hox genes in the Chinese mitten crab
Eight Hox genes (lab, Dfd, Scr, ftz, Antp, Ubx, abd-A and Abd-B) were identified through BLAST analysis of the E. sinensis genome database. A sequence alignment of the eight Hox proteins in E. sinensis with those of other species, such as D. melanogaster and L. vannamei, revealed the presence of a residue sequence of YPWM and a conserved domain of homeodomain except Abd-B (Supplementary file S1). We analyzed the physical properties and amino acid composition of the Hox proteins in Chinese mitten crab and found that it was rich in Ala, Gly, Pro, Gln, Ser and Thr (Supplementary file S2 and Supplementary file S3).
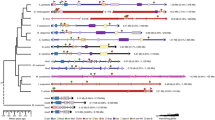
The amino acid sequences of eight Hox proteins from E. sinensis were clustered. The results showed that the eight Hox genes were classified into two major clades. Scr, Dfd, ftz, Ubx and abd-A clustered together, and the other group included Antp, Abd-B and lab. The genomes of E. sinensis and P. trituratus were analyzed for collinearity, and there was a high degree of collinearity among multiple chromosomes (Fig. 1). This reflects the sister group relationship of E. sinensis and P. trituratus. Among them, the Hox genes (lab, ftz and Abd-B) present on chromosome 21 in E. sinensis are colinear with those in the P. trituratus genome, retaining more ancestral traits.
Phylogenetic analysis of the Hox gene family
We found a total of 112 Hox genes in 13 selected species and mapped their transcription orientation from genomic data with annotation files (Fig. 2). Aligning Hox protein sequences, a total of 112 Hox protein sequences from 13 selected species were used to build a phylogenetic tree using the maximum likelihood approach (Fig. 3, Supplementary file S5). E. sinensis and P. trituberculatus, belonging to Eubrachyura, are sister groups to shrimps and grouped with hermit crabs into one class. Each Hox gene of Eriocheir sinensis was first clustered with the Hox gene of crabs and shrimps. This clustering phenomenon shows that crustaceans are relatively closely related to insects and slightly distantly related to Chelicerata species. The results support the traditional taxonomy.
Adaptive evolution analyses of Hox genes
Different ω ratio models were used to determine if Hox genes in the arthropod species have experienced positive selection. In the site model, the positively selected site with a posterior probability greater than 0.95 was identified only in Ubx. In the branching model, E. sinensis was set as the foreground branch, and the other branches were set as background branches. Out of the seven Hox genes (lab, Scr, Antp, Ubx, ftz, abd-A and Abd-B), the results showed that there was a significant difference in the evolution rate of Ubx and abd-A between E. sinensis and other animals, so Ubx and abd-A were considered rapidly evolving genes. The results of branch-site model A revealed evidence of positive selection on Ubx (1 M 0.952*, 3 S 0.998**, 4 Y 0.998**) among the arthropod lineages (Tables 1, 2 and 3). Setting Brachyura as the foreground branch for selection pressure analysis, we found that in the branch model, the evolution rate of Ubx was different in Brachyura from the background branch. In the branch site model, Antp (57 K 1.000**) had a positive selection site, and its mutation site was the same as the positive selection site of the Chinese mitten crab. This indicates that the Antp gene is possibly involved in the brachyurization development of crabs (Supplementary file S6 and Supplementary file S7).
Full-length cDNA structure of Scr and Antp and mRNA expression of the Antp and Scr genes in E. sinensis during different developmental periods
The full-length cDNA sequences of Scr (1770 bp, KP822930) and Antp (2413 bp, KP822927) were obtained by DNAStar Lasergene 7.1 after the sequence splicing of the Scr and Antp genes. Through analysis, it was found that the full-length cDNA sequence of Scr has a 5’-end untranslated region (UTR) of 424 bp and a 3’-end untranslated region of 350 bp, and its open reading frame (ORF) contains 996 bp, encoding 332 amino acids. The full-length cDNA sequence of Antp has a 5’-end untranslated region (UTR) of 324 bp and a 3’-end untranslated region of 1123 bp, and its ORF contains 966 bp, encoding 322 amino acids. Their open reading frames both contain a conserved domain (Homeobox) (Supplementary files S4).
The mRNA transcripts of Scr and Antp were detected in all tested developmental stages of the Chinese mitten crab. The mRNA expression of Scr in E. sinensis was relatively higher at stages O and Z1 to Z4 and lower at stages Z5, M and J1 to J3 (Fig. 4A). The mRNA expression level of Antp in E. sinensis was the highest at stage O, and the expression of Antp gradually decreased with the development and growth of crabs (Fig. 4B).
Discussion
In this study, we identified a single Hox gene cluster including seven typical homeotic genes and one additional homeotic gene from the genome of E. sinensis. However, orthologs of pb and Hox3 were not found in E. sinensis based on comparison with the sequences of arthropods, including D. melanogaster and L. vannamei. However, because 10 Hox genes were extracted from the transcriptome of the Chinese mitten crab in a previous study [25], the pb and Hox3 genes were not found in this study, perhaps due to incomplete genomic assembly for E. sinensis.
Previous studies have found that the repertoire of Hox genes differs among different species. D. melanogaster, L. vannamei, Daphnia pulex and Daphnia magna are represented by complete sequences for all ten Hox genes [26, 27]. However, only nine Hox genes from Paracyclopina nana were identified, all except Hox3 [28]. A Hox3 gene ortholog was also not present in the Hox gene cluster of Macrobrachium olfersii [29]. Moreover, pb, Hox3 and ftz were missing in two species of copepods, Tigriopus japonicus and T. kingsejongensis [30]. Because Hox gene clusters are expressed along the main anterior-posterior (A-P) axis, dynamic mutation in the Hox gene causes the ectopic development of a given organ. Normal development of D. melanogaster results in a pair of balancing rods in the posterior thorax, but a mutation of Ubx will cause flies to grow a pair of wings in the posterior thorax [31]. The data of Hox gene sequences in arthropods, especially crustaceans, will contribute to explaining the common morphological changes and evolutionary process in crustaceans during metamorphosis.
Compared with higher vertebrates, studies on Hox genes in arthropods lag far behind. Hox genes have been reported to play important roles in cell division, paired appendage development, axial morphogenesis regulation and skeleton maintenance [32, 33]. The basal expression levels of Hox genes demonstrated different patterns of expression over different developmental stages. We selected Scr and Antp for further investigation because we only cloned these two Hox genes (failed to acquire the full-length cDNA of other Hox genes in E. sinensis) in the early stage of our experiment (before the genome of E. sinensis was determined) and examined the mRNA expression patterns of these two genes. In this experiment, the mRNA expression patterns of the Scr and Antp genes were detected at all 10 stages of juvenile development in E. sinensis, suggesting that Scr and Antp may play a regulatory role in the juvenile development of E. sinensis. Previous studies indicated that Scr controls the development of the insect prothorax [34], and Antp is involved in regulating morphological changes in the abdominal appendages in crustaceans [35, 36]. The main morphological changes in brachyurization development mainly occurred in stages Z5, M and J1 of E. sinensis (Fig. 4C), and Scr and Antp mRNA were expressed at the lowest levels in stages Z5 ~ J3, indicating the possibility that these Hox genes are partially involved in brachyurization and further developmental regulation. The thoracic function of E. sinensis is increased with shortened abdomen, whereas the abdominal function is weakened, certain abdominal segments heal and fold under the cephalothorax, and the motor function is lost. The mRNA expression of Scr and Antp changed dramatically during their respective larval developmental phases, mirroring changes in the morphology of E. sinensis larvae. During the egg stage, when the Chinese mitten crab individuals were fully formed, the relative expression of the Scr and Antp gene mRNAs peaked. In contrast, during the Z5 stage, their expression reached its lowest point, which caused abdominal degeneration in E. sinensis. This significant change (p < 0.01) implied that these two genes might be involved in regulating the short tailing process of E. sinensis. Young et al. indicated that Hox genes are highly expressed in the vertebrate posterior embryonic region and play a role in posterior tissue generation [37]. This finding fits with the so-called dose effect that quantitative variation in the levels of Hox gene products can affect segment morphology in subtle ways [38].
The rate of gene evolution depends on the substitution rate of the nucleotides in the nucleic acid molecule over a certain period of time, and the selection pressure reacts to the rate of gene evolution. Based on selective pressure analyses of Hox genes, E. sinensis was set as the foreground branching clade in the branching model and branch site model. The results revealed that six Hox genes had no positive selection signal, but Antp and Ubx did, indicating that the evolutionary rates of Antp and Ubx were perhaps different among different species. A previous study reported that in the early course of gene evolution, the evolutionary rate of the Hox gene in actinopterygian and sarcopterygian fishes is faster than that in terrestrial vertebrates [39]. The different rates of Hox gene evolution imply that Hox genes may regulate animal morphology to adapt to the environment. Overall, there have been a relatively detailed investigations of the evolution and expression pattern of Hox genes in Brachyura crabs, but further work is needed to focus on the molecular evolutionary mechanism and functions in developmental regulation of brachyurization.
Conclusion
Here, we explored eight Hox genes from the chromosomal level genome of the Chinese mitten crab E. sinensis. A total of 112 putative Hox genes were identified in 13 arthropod species. According to the collinear analysis, there are a large number of collinear fragments between E. sinensis and P. trituratus, which implies that they accumulate less variation with shorter differentiation times and retain more ancestral traits. Evolutionary analyses indicated the presence of positively selected sites in Ubx. In the branch model, the Ubx and abd-A genes in E. sinensis were identified as rapidly evolving genes. The full-length cDNA sequences of Scr and Antp were cloned, and the mRNA expression patterns of the two Hox genes in E. sinensis were determined at different developmental stages. The expression levels of Scr and Antp in E. sinensis were the highest at stage O and fluctuated significantly in different developmental periods. Our data will contribute to a better understanding of the Hox gene family in E. sinensis and provide useful molecular evolutionary information for further investigation into their roles in the brachyurization of crabs.
Materials and methods
Sample collection
Tissue samples of E. sinensis were collected from the healthy Chinese mitten crabs that were bred in an aquatic nursery of Rudong County, Nantong City, Jiangsu Province, China. The samples were collected at the fertilized egg stage (stage O), all zoea stages (stage Z1 ~ Z5), megalopa stage (stage M) and juvenile crab stages (stage J1 ~ J3, with approximately 30 mg for each sample). These fresh tissues were stabilized immediately in RNAlater RNA Stabilization Reagent (QIAGEN, Germany), kept overnight at + 4 °C, and finally stored at − 20 °C before RNA isolation.
Preparation of total RNA
Following the manufacturer’s instructions, we used an RNeasy Mini Kit (Qiagen, Germany) to extract total RNA from different samples from the Chinese mitten crab. The RNA concentration and purity were assessed spectrophotometrically by measuring the absorbance of the solution at 260 and 280 nm in a biophotometer (Eppendorf, Germany). The RNA fragmentation state was evaluated by 1% agarose denatured gel electrophoresis.
Phylogenetic analysis of Hox genes
For comparative genomics analyses, we used all the annotated genes in the Chinese mitten crab and thirteen other arthropod species. Genome assemblies of 13 species were obtained from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) and GigaDB database (http://gigadb.org/) (Table 4). The Hox amino acid sequences of Litopenaeus vannamei and Drosophila melanogaster were used as queries. BLASTP in BLAST v2.2.23 was used for similarity searches in the genomes of the selected species. An e-value of 10− 5 was used as an initial cutoff to identify significant matches. Hits < 100 codons and overlapping sequences were excluded.
The ProtParam software tool at the ExPASy portal (https://web.expasy.org/protparam/) was used to calculate the amino acid percentages of Hox gene-encoded proteins. CLUSTALW 2.0.10 [40] was used to compare the homology of Hox protein sequences in E. sinensis, D. melanogaster and L. vannamei. The gene structure of the Hox genes and the distribution of motifs and domains at each sequence were visualized using TBtools version 1.074 [41]. Mapping of gene family members in chromosome locations was performed using the R 4.0.5 package (“ggplot2” and “gggenes”) [42]. Amino acid sequences were aligned with MAFFT, and phylogenetic analyses were performed with IQtree on the Phylosuit platform. The phylogenetic analysis of Hox protein sequences used the JTT model for maximum likelihood analysis with 1000 bootstrap replicates. Using genomic data and chromosomal location data of E. sinensis and Portunus trituratus, cross-species genome collinearity analysis was performed using MCScanX software and then visualized in Circos 0.67.
Analyses of evolutionary pressure
We used comparisons of nonsynonymous/synonymous substitution ratios (dN/dS) to quantify natural selection. Different types of selection were represented by different values: ω < 1 means purifying selection, ω = 1 indicates neutral selection, and ω > 1 means positive selection. Due to the incomplete sequence of Dfd, we only performed selective pressure analysis on the other seven Hox genes (lab, Scr, Antp, Ubx, ftz, abd-A and Abd-B) in E. sinensis. Analyses of selective pressure were conducted using the Codeml program implemented in the Phylogenetic Analysis by Maximum Likelihood (PAML4.9) package. Different Hox genes in crustacean species have experienced positive selection. The selected site model (Site model), branch model (Branch model), and branch site model (Branch-site model) were used to test for the presence of positive branches on the divergent clades of the crustaceans for Hox.
Full-length cDNA amplification and the mRNA expression patterns of Antp and Scr during different periods
Gene-specific primers for Scr and Antp gene amplification were designed based on the high-throughput sequencing of the Chinese mitten crab transcriptome sequence obtained earlier in our laboratory (data unpublished). The primers are shown in Table 5. The cDNA was synthesized using a 3’ Full RACE Core Set with PrimeScript™ RTase (Qiagen, Germany). PCR amplification was followed by using TaKaRa LA Taq with GC Buffer (TaKaRa, Dalian, China). The β-actin gene (GenBank accession no. HM053699.1) of E. sinensis, as an effective internal control [26, 43], was selected to calibrate the cDNA template. Gene-specific primers for RT‒qPCR were designed according to the sequences of the two genes (Scr and Antp), and these primers are shown in Table 5. Real-time quantitative PCR (RT‒qPCR) was conducted using the SYBR® Premix Ex-Taq™ II kit (TaKaRa, Dalian, China). The RT‒qPCR cycling conditions were as follows: initial denaturation at 95 °C/30 sec; 40 cycles of 95 °C/5 sec and 60 °C/30 sec; 95 °C/15 sec; 60 °C/1 min, 95 °C/15 sec. The RT‒qPCR assay was carried out in triplicate on 96-well plates. Employing the formula RATE = 2−ΔΔCt, the comparative Ct method was used to analyze the relative expression levels of Scr and Antp. Statistical analysis of the Scr and Antp gene expression data was performed using STATISTICA 10.0. One-way analysis of variance (ANOVA) with Tukey’s post hoc tests were performed to assess statistical significance, in which statistical significance was accepted at p ≤ 0.05 and marked with asterisks or different letters.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Kaufman TC, Lewis R, Wakimoto B. Cytogenetic analysis of chromosome 3 in Drosophila melanogaster: the homoeotic gene complex in polytene chromosome interval 84a-B[J]. Genetics. 1980;94(1):115–33.
Gehring WJ, Affolter M, Bürglin T. Homeodomain Proteins[J]. Annual Rev Biochem Annual Reviews. 1994;63(1):487–526.
Krumlauf R. Hox genes, clusters and collinearity[J]. Int J Dev Biol. 2018;62(11–12):659–63.
Holland PWH. Evolution of homeobox genes[J]. Wires Dev Biol. 2013;2(1):31–45.
Mark M, Rijli FM, Chambon P. Homeobox genes in embryogenesis and pathogenesis[J]. Pediatr Res. 1997;42(4):421–9.
Grenier JK, Garber TL, Warren R, et al. Evolution of the entire arthropod hox gene set predated the origin and radiation of the onychophoran/arthropod clade[J]. Curr Biol. 1997;7(8):547–53.
Izpisúa-Belmonte J-C, Duboule D. Homeobox genes and pattern formation in the vertebrate limb[J]. Dev Biol. 1992;152(1):26–36.
Vollmer J-Y, Clerc RG. Homeobox genes in the developing mouse Brain[J]. J Neurochem. 1998;71(1):1–19.
Tsumagari K, Baribault C, Terragni J et al. DNA methylation and differentiation: Hox genes in muscle cells[J]. Epigenetics & Chromatin, BioMed Central, 2013, 6(1): 25.
Abuhantash M, Collins EM, Thompson A. Role of the HOXA cluster in HSC emergence and blood cancer[J]. Biochem Soc Trans. 2021;49(4):1817–27.
Song JY, Pineault KM, Dones JM, et al. Hox genes maintain critical roles in the adult skeleton[J]. Volume 117. National Academy of Sciences; 2020. pp. 7296–304. 13.
Treisman J, Harris E, Wilson D, et al. The homeodomain: a new face for the helix-turn-helix?[J]. BioEssays. 1992;14(3):145–50.
Bürglin TR, Affolter M. Homeodomain proteins: an update[J]. Volume 125. Springer Berlin Heidelberg: Chromosoma; 2016. pp. 497–521. 3.
McGinnis W, Krumlauf R. Homeobox genes and axial patterning[J]. Cell. 1992;68(2):283–302.
Dressler GR, Gruss P. Anterior boundaries of hox gene expression in mesoderm-derived structures correlate with the linear gene order along the chromosome[J]. Differentiation. 1989;41(3):193–201.
Pascual-Anaya J, D’Aniello S, Kuratani S, et al. Evolution of hox gene clusters in deuterostomes[J]. BMC Dev Biol. 2013;13:26.
Akam M. Hox genes in arthropod development and evolution[J]. Biol Bull. 1998;195(3):373–4.
Lohman FP, Medema JK, Gibbs S, et al. Expression of the SPRR cornification genes is differentially affected by carcinogenic transformation[J]. Exp Cell Res. 1997;231(1):141–8.
Lewis RA, Wakimoto BT, Denell RE, et al. Genetic analysis of the Antennapedia gene complex (Ant-C) and adjacent chromosomal regions of Drosophila melanogaster. II. Polytene chromosome segments 84A-84B1,2[J]. Genetics. 1980;95(2):383–97.
Števčić Z, Systematic, Zoology. [Oxford University Press, Society of Systematic Biologists, Taylor & Francis, Ltd.], 1971, 20(3): 331–40.
Guinot D, Bouchard J-M. Evolution of the abdominal holding systems of brachyuran crabs (Crustacea, Decapoda, Brachyura)[J]. Zoosystema. 1998;20:613–94.
Song C, Cui Z, Hui M, et al. Comparative transcriptomic analysis provides insights into the molecular basis of brachyurization and adaptation to benthic lifestyle in Eriocheir sinensis[J]. Gene. 2015;558(1):88–98.
Li P, Zha J, Sun H, et al. Identification of differentially expressed genes during the brachyurization of the chinese Mitten crab Eriocheir japonica sinensis[J]. Biochem Genet. 2011;49(9):645–55.
Averof M, Akam M. Hox genes and the diversification of insect and crustacean body plans[J]. Nature. 1995;376(6539):420–3.
Li P. Cloning and analysis on morphology and sex regulation related hox gene family and fruitless gene in Eriocheir sinensis[J]. Qingdao: Institute of Oceanology, Chinese Academy of Sciences; 2017.
Sun X. The primary structure and expression study of hox genes in pacific white shrimp, Litopeneaus vannamei[D]. Qingdao: Institute of Oceanology,Chinese Academy of Science; 2015.
Colbourne J, Pfrender ME, Gilbert D, et al. The ecoresponsive genome of Daphnia pulex[J]. Volume 331. Science (New York, N.Y.),; 2011. pp. 555–61. 6017.
Kim H-S, Kim B-M, Lee B-Y, et al. Identification of hox genes and rearrangements within the single homeobox (hox) cluster (192.8 kb) of the cyclopoid copepod (Paracyclopina nana)[J]. J Experimental Zool Part B: Mol Dev Evol. 2016;326(2):105–9.
Jaramillo ML, Ammar D, Quispe RL, et al. Identification of hox genes and their expression profiles during embryonic development of the emerging model organism, Macrobrachium olfersii. J Experimental Zool Part B: Mol Dev Evol. 2022;2022(Jan 1):1–9.
Kim D-H, Jeong H, Kim M-S et al. Identification and characterization of homeobox gene clusters in harpacticoid and calanoid copepods[J]. J Experimental Zool Part B: Mol Dev Evol, 2021, 2021 Dec 2(n/a).
Li H. Hua. Hox genes and their evolutionary mechanisms[J]. Chin J Zool. 2011;46(1):136–42.
Mallo M. Reassessing the role of hox genes during vertebrate development and evolution[J]. Trends Genet. 2018;34(3):209–17.
Mallo M, Alonso CR. The regulation of hox gene expression during animal development[J]. Development. 2013;140(19):3951–63.
Elias-Neto M, Belles X. Tergal and pleural structures contribute to the formation of ectopic prothoracic wings in cockroaches[J]. Volume 3. Royal Society Open Science, The Royal Society; 2016. p. 160347. 8.
Khadjeh S, Turetzek N, Pechmann M, et al. Divergent role of the hox gene Antennapedia in spiders is responsible for the convergent evolution of abdominal limb repression[J]. Proc Natl Acad Sci United States Am Natl Acad Sci. 2012;109(13):4921–6.
Shiga Y, Yasumoto R, Yamagata H, et al. Evolving role of Antennapedia protein in arthropod limb patterning[J]. Development. 2002;129(15):3555–61.
Young T, Rowland JE, Van de Ven C, et al. Cdx and hox genes differentially regulate posterior axial growth in mammalian embryos[J]. Dev Cell. 2009;17(4):516–26.
Lewis EB. A gene complex controlling segmentation in Drosophila melanogaster[J]. Nature. 1978;276(5688):565–70.
Málaga-Trillo E, Meyer A. Genome duplications and accelerated evolution of hox genes and cluster architecture in teleost fishes1[J]. Am Zool. 2001;41(3):676–86.
Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX[J]. Current Protocols in Bioinformatics, 2003, 00(1): 2.3.1–2.3.22.
Chen C, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data[J]. Mol Plant. 2020;13(8):1194–202.
R Core Team. R: A language and environment for statistical computing.[J]. R Foundation for Statistical Computing, Vienna, Austria., 2018.
Jin X-K, Li W-W, Cheng L, et al. Two novel short C-type lectin from chinese mitten crab, Eriocheir sinensis, are induced in response to LPS challenged.[J]. Fish Shellfish Immunol. 2012;33(5):1149–58.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 31000954 to PL), Natural Science Foundation of the Jiangsu Higher Education Institutions (19KJA330001) awarded to PL, and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_1614) awarded to ZYC, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 31000954 to PL), Natural Science Foundation of the Jiangsu Higher Education Institutions (19KJA330001) awarded to PL, and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_1614) awarded to ZYC, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
PL and KYZ designed and supervised the experiments. SSC and XFJ performed the experiments. SSC, LJX, ZYC, XFJ and PL analyzed data. SSC, YJ, and PL prepared the manuscript. All authors discussed the results, and implications and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In the present work, the experimental procedures complied with current laws on animal welfare and research in China and the Guide for the Care and Use of Laboratory Animals (8th edition), and were approved by the Institutional Animal Care and Use Committee of Nanjing Normal University and were conducted in accordance with related guidelines [SYXK (Jiangsu) 2020-0047 and IACUC-20220258]. The authors confirmed that animals did not suffer unnecessarily at any stage of experiments in this study.
Consent for publication
Not applicable.
Competing interests
The authors declared that there are no conflicts interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Jiang, X., Xia, L. et al. The identification, adaptive evolutionary analyses and mRNA expression levels of homeobox (hox) genes in the Chinese mitten crab Eriocheir sinensis. BMC Genomics 24, 436 (2023). https://doi.org/10.1186/s12864-023-09489-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09489-w