Abstract
Background
Protein supplementation improves physiological adaptations to endurance training, but the impact on adaptive changes in the skeletal muscle transcriptome remains elusive. The present analysis was executed to determine the impact of protein supplementation on changes in the skeletal muscle transcriptome following 5-weeks of endurance training.
Results
Skeletal muscle tissue samples from the vastus lateralis were taken before and after 5-weeks of endurance training to assess changes in the skeletal muscle transcriptome. One hundred and 63 genes were differentially expressed after 5-weeks of endurance training in both groups (q-value< 0.05). In addition, the number of genes differentially expressed was higher in the protein group (PRO) (892, q-value< 0.05) when compared with the control group (CON) (440, q-value< 0.05), with no time-by-treatment interaction effect (q-value> 0.05). Endurance training primarily affected expression levels of genes related to extracellular matrix and these changes tended to be greater in PRO than in CON.
Conclusions
Protein supplementation subtly impacts endurance training-induced changes in the skeletal muscle transcriptome. In addition, our transcriptomic analysis revealed that the extracellular matrix may be an important factor for skeletal muscle adaptation in response to endurance training. This trial was registered at clinicaltrials.gov as NCT03462381, March 12, 2018.
Trial registration
This trial was registered at clinicaltrials.gov as NCT03462381.
Similar content being viewed by others
Background
Skeletal muscle is an extraordinary malleable tissue which is demonstrated by its rapid remodeling and adaptation to exercise training [1, 2]. Repetitive bouts of endurance exercise, e.g. endurance training, lead to various metabolic and morphological adaptations in skeletal muscle [3, 4]. At the myocellular level, long term skeletal muscle adaptation is supposed to be the result of repeated modifications in transcriptional and translational responses of each exercise bout thereby increasing the synthesis of specific proteins required for remodeling [5,6,7,8]. However, training-induced changes in baseline transcriptome have also shown to play an important role [9,10,11]. Skeletal muscle transcriptome analysis provides an unbiased examination of the molecular alterations to exercise training, thereby potentially unravelling novel pathways involved in adaption to endurance training [12,13,14].
Protein feeding following endurance exercise has shown to affect mRNA-specific pathways involved in extracellular matrix, myogenesis, immunogenic response, and energy metabolism [15], suggesting that repeated post-exercise endurance protein feeding may enhance the adaptive response to endurance training. Whether protein supplementation also impacts the changes in the skeletal muscle transcriptome following a period of endurance training remains to be elucidated. We have recently demonstrated that protein supplementation during endurance training enhances physiological adaptations, where the major part of the adaptations was observed during the first 5-weeks of the 10-weeks training intervention [16]. Therefore, we decided to specifically focus the present analysis on the effect of protein supplementation on changes in skeletal muscle transcriptome during 5-weeks of endurance training. To this end, we assessed the impact of protein supplementation during 5-weeks of endurance training on changes in the skeletal muscle transcriptome. We hypothesize that protein supplementation elicits greater changes in the skeletal muscle transcriptome when compared to carbohydrate supplementation.
Results
Baseline characteristics
In total, four subjects dropped out during the conduction of the study for various reasons. Analysis was executed on the 40 subjects who completed the 5-weeks training program (CON: n = 21 vs PRO: n = 19). Baseline characteristics were not different between groups and can be found in Table 1.
Endurance training program and effect
For more detailed information regarding the endurance training program and supplementation strategy the reader is referred to our recently published paper [16]. Briefly, the monitored training sessions were performed between 0900 and 2100. Exercise training adherence, intensity and supplementation adherence were not different between groups, a . Five weeks of endurance training significantly increased maximal aerobic capacity and skeletal muscle oxidative capacity. Protein supplementation caused a greater gain in maximal aerobic capacity and stimulated lean mass accretion but did not further increase skeletal muscle oxidative capacity and endurance performance (Table 1). A full discussion of the physiological effects of endurance training with or without protein supplementation can be found elsewhere [16].
Muscle transcriptome
Endurance training differentially expressed gene in the muscle transcriptome in both the CON and the PRO group. The activity of more genes was altered by endurance training in the PRO group than in the CON group (893 vs. 441, respectively, F-test q-value < 0.05). Table 2 shows the top 20 significant genes based on level of significance for both CON group and PRO group. Among the top 20 significant genes for the CON group are genes related to extracellular matrix organization including collagen type IV alpha chain (COL4A2), collagen type IV alpha 1 chain (COL4A1), laminin subunit alpha 4 (LAMA4), laminin subunit beta 1 (LAMB1) and alpha-2-macroglobulin (A2M). Top 20 significant genes for the PRO group were comparable with those of the CON group and relate to extracellular matrix organization including collagen type III alpha 1 chain (COL3A1), secreted protein acidic and cysteine rich (SPARC), collagen type IV alpha 2 chain (COL4A2), collagen type IV alpha 1 chain (COL4A1), laminin subunit alpha 4 (LAMA4), peroxidasin (PXDN), laminin subunit beta 1 (LAMB1), alpha-2-macroglbulin (A2M) and nidogen 1 (NID1).
Effect of protein supplementation
Figure 1 (Venn diagram) shows the number of genes regulated as a result of endurance training for each the CON group and the PRO group and the groups combined. After 5-weeks of endurance training, gene expression count was greater in the PRO group compared with CON. In addition, the top 20 and overall gene transcript change in muscle transcriptome was consistently greater in the PRO group when compared to the CON group (Fig. 2). Figure 3 shows a heatmap of the genes that were differentially expressed by endurance training in both the CON group and PRO group (40 genes, F-test q-value< 0.0001). The changes in gene expression following 5-weeks of endurance training did not markedly differ between the CON group and PRO group (time-by treatment interaction, F-test q-value> 0.05). No major differences can be observed with regard to training response between the PRO and CON group. Gene-set-enrichment analysis showed a similar result, as gene sets that were significant for the CON group were generally also significant for the PRO group.
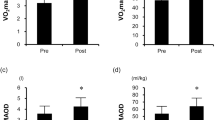
Scatterplots with line of identity to visualize the magnitude of change in muscle transcriptome per group. Figs. A & B are based on the total number of genes changed per group (184 for CON (a) and 384 for PRO (b), F-test q-value< 0.05). Figs. C & D are based on the top 20 significant genes changes in the CON (c) and PRO (d) group
Biological processes
Based on all significant genes altered (F-test q-value< 0.05) in each group, gene ontology biological processes revealed extracellular matrix organization as the process with the highest change in gene expression profile in both the CON group and the PRO group (Table 3). In the CON group 25 genes were linked to extracellular matrix organization whereas 55 genes in the PRO group. Accordingly, gene set enrichment analysis (Table 4) showed time-by treatment interaction for extracellular matrix organization processes such as extracellular matrix receptor interaction (q-value< 0.001), extracellular matrix glycoproteins (q-value = 0.006) and collagen formation (q-value = 0.041). Gene set enrichment also showed significant increases in energy metabolism and oxidative phosphorylation with no clear differences between the CON group and the PRO group (q-value> 0.05).
Discussion
We have recently demonstrated that protein supplementation enhances physiological adaptations to endurance training. The greater physiological adaptations elicited by protein supplementation were mainly observed during the first 5 weeks of training of a 10 week endurance training intervention [16]. Likewise, changes in the skeletal muscle transcriptome were primarily observed during the first 5 weeks of training with no further changes from week 5 to 10 weeks of training. Therefore, to gain further insight regarding the effects of protein supplementation during endurance training on changes in the skeletal muscle transcriptome, the present analysis focused on changes in skeletal muscle transcriptome during 5 weeks of endurance training.
Five weeks of endurance training increased maximal aerobic capacity. Adding protein supplementation elicited a greater increase in maximal aerobic capacity and stimulated lean mass gain. For a more detailed discussion on the changes in physiological outcome measures the reader is referred to our recently published paper [16]. At the skeletal muscle transcriptional level, endurance training caused relatively small (FC < 2) but consistent and statistically robust changes in the skeletal muscle transcriptome. Furthermore, changes in the skeletal muscle transcriptome tended to be greater in the PRO group as compared to the CON group. However, the differences in changes in the skeletal muscle transcriptome between the two groups are far less clear. This lack of clear differences in skeletal muscle gene expression transcripts between the PRO and CON group is likely due to timing of muscle tissue sampling, low sample size and high inter-individual variation.
In this study we demonstrated that the physiological adaptive response to endurance training was accompanied by significant changes in the skeletal muscle transcriptome. Gene set enrichment analysis showed that endurance training caused significant changes in gene expression transcripts involved in extracellular matrix, which is in line with previous reports that have investigated changes in skeletal muscle transcriptome following prolonged endurance training [13, 14]. Several upregulated genes among the top 20 genes are involved in extracellular matrix organization, including COL4A2, COL4A1, LAMA41, LAMB1 and A2M. The results of gene-ontology biological processes and gene set enrichment analysis are consistent with the top 20 genes, showing increased extracellular matrix remodeling. The observed changes in gene expressions transcripts related to extracellular matrix remodeling tended to be more pronounced in the PRO group than the CON group. The latter suggests that the greater changes in skeletal muscle transcriptome, in particular the extracellular matrix, may reflect the greater physiological adaptations observed in the PRO group (e.g. greater gain in VO2max and stimulation of lean mass accretion).
The extracellular matrix is composed of collagen, glycoproteins and proteoglycans [17]. Moreover, extracellular matrix remodeling is a primary adaptation to endurance training [4]. The extracellular matrix is important for muscle cell development, structure maintenance, force transmission, and tissue remodeling through the modulation of growth factors and extracellular molecule interactions [18]. Extracellular matrix degradation is an important morphological adaptation by allowing growth of new capillaries from existing ones in response to endurance training [19,20,21,22,23,24]. Whether the exercise-induced growth of capillaries was further stimulated by protein supplementation and contributed to the larger increase in maximal aerobic capacity cannot be concluded from these data.
Our observation that protein supplementation may increases extracellular matrix remodeling to endurance training is new and further elaborates on previous work, which demonstrates that addition of protein to post-exercise carbohydrate-lipid nutrition differentially alters the transcriptome involved in tissue structure and remodeling through regulation of extracellular matrix [15]. General skeletal muscle adaptations to exercise training include regulation of angiogenesis, mitochondrial biogenesis, myogenesis and alterations in structural support such as the extracellular matrix [25, 26]. There is surprisingly little known about the role of the extracellular matrix in response to endurance training. Our data show that the gene expression transcriptional response to endurance training in skeletal muscle is related to extracellular matrix components and that protein supplementation tended to enlarge this adaptive response. In this study, it could be that the extent in which the extracellular matrix remodeled reflects the degree of muscle growth. Lean mass substantially increased in the protein group and this was accompanied by stronger regulations in gene expression transcripts related to extracellular matrix remodeling. Previous research postulated that remodeling of the extracellular matrix is required for exercise-training induced muscle growth [27].
In contrast to the observed effect of protein supplementation on physiological adaptation, we were unable to find a clear additional effect of protein supplementation on the skeletal muscle transcriptome besides the extracellular matrix. It is possible that the effects of protein supplementation already started to manifest during the early hours of recovery from exercise, when mRNA abundance generally peaks [8, 28]. Although the precise mechanisms by which protein supplementation elicited a greater increase in maximal aerobic capacity to endurance training cannot be derived from this analysis, it is likely that protein supplementation enhanced the gene/protein expression changes after each exercise session thereby improving skeletal muscle tissue adaptation, resulting in cumulatively meaningful changes in recovery and phenotypic adaptation over a prolonged period of time.
Conclusion
Thus far, much attention has been given to the acute molecular responses to a single bout of exercise, and the current theory suggests that acute signals predict/drive phenotypic adaptation over time. For example, the AMP-activated protein kinase and peroxisome proliferator-activated receptor-y coactivator-1ɑ, have been proposed as primary regulators of muscle tissue adaptation in response to endurance training [29,30,31,32]. Whether these genes are truly critical for metabolic and performance adaptations to endurance training has yet to be determined. Indeed, training-induced changes in baseline transcriptome have also shown to play an important role [9,10,11]. Our transcriptomic analysis revealed that the extracellular matrix may be an important factor for skeletal muscle adaptation in response to endurance training. Thus, we argue that mRNA expression changes in human skeletal muscle during later stages of recovery from a single bout of endurance exercise reflect more prolonged molecular responses to short-term energy and ionic homeostasis challenges rather than chronic steady-state adaptation to endurance training [7]. Protein supplementation subtly impacts endurance training-induced changes in the skeletal muscle transcriptome. In addition, our transcriptomic analysis revealed that the extracellular matrix may be an important factor for skeletal muscle adaptation in response to endurance training.
Methods
Subjects
The investigation was approved by the Medical Ethical Committee of Wageningen University, in accordance with the Declaration of Helsinki. This trial was registered at clinicaltrials.gov as NCT03462381 and adheres to CONSORT guidelines for clinical trials. A detailed description of subject participation, experimental design, endurance training program, supplemental strategy, whole-body physiological outcome measures can be found in our previous publication [16]. A schematic overview of the study protocol can be found in Fig. 4.
Schematic overview of the study protocol. Forty subjects completed 10 wk. of progressive endurance training while consuming either 25 g carbohydrates or 25 g protein post-exercise and daily before sleep. All measurements were assessed before, midterm (week 6) and after (week 12). Strongest effect of protein supplementation was observed following 5 weeks of endurance training. To gain more insight into mechanisms underlying greater physiological adaptation as a result of protein supplementation we analyzed skeletal muscle transcriptome data from baseline to midterm. Black dots: measurement points, bleu dots: exercise sessions. Grey part: contains physiological and microarray data analyzed for this manuscript
Muscle biopsies, sample preparation and microarray analysis
Baseline (week 0) and post-intervention fasted muscle biopsies were taken as described by Bergstrom (1974) [33], and the procedure used can be found elsewhere [16]. Total RNA was isolated from the skeletal muscle tissue by using Trizol reagent (Invitrogen, Breda, Netherlands). Thereafter, RNA was purified using the Qiagen RNeasy Micro kit (Qiagen, Venlo, Netherlands), and RNA quality was checked using an Agilent 2100 bioanalyzer (Agilent Technologies, Amsterdam, Netherlands). Total RNA (100 ng) was labelled using an Affymetrix WT plus reagent kit (Life Technologies, Bleiswijk, Netherlands) and hybridized to human whole genome Genechip Human Gene 2.1 ST arrays, (Life Technologies, Bleiswijk, Netherlands). Sample labelling, hybridization to chips, and image scanning were performed according manufacturer’s instructions.
Statistics
Statistical analysis of gene expression changes was performed using limma R library [34]. Contrasts were set for endurance training effect in both groups and an interaction term was used to determine the effect of protein supplementation (protein group versus the control group). P-values were calculated using Intensity Based Moderated t-tests (IBMT) [35]. Significant genes were first selected using the False Discovery Rate Adjusted F-statistic p-value < 0.05. Unadjusted p-values below 0.01 for the contrasts were considered statistically significant within the genes that passed the F-test. Gene set enrichment analysis was done using pre-ranked lists ranked by the t-values from the limma contrasts [36, 37]. We used the most recent library of canonical pathways from The Molecular Signatures Database (MsigDb) [36]. An adjusted p-value (q-value) of 0.10 was considered significant for the gene rest enrichment analysis results. Venn diagram and Heatmaps were made using the ComplexHeatmap library [38] and GraphPad Prism 8.01 for Windows (San Diego, CA). EnrichR was used to determine differences in GO biological processes [39, 40]. A detailed description of the statistical analysis used for the physiological data can be found in our previous publication [16].
Availability of data and materials
Microarray data will be publicly available at Gene Expressions Omnibus (GEO) repository and as supporting file: GPL28236.
Abbreviations
- AMP-activated protein kinase:
-
Adenosine monophosphate-activated protein kinase
- A2M:
-
And alpha-2-macroglobulin
- CON:
-
Control group
- COL4A1:
-
Collagen type IV alpha 1 chain
- COL4A2:
-
Collagen type IV alpha chain
- COL3A1:
-
Collagen type III alpha 1 chain
- IBMT:
-
Intensity Based Moderated t-tests
- LAMA4:
-
Laminin subunit alpha 4
- LAMB1:
-
Laminin subunit beta 1
- mRNA:
-
Messenger ribonucleic acid
- NID1:
-
Nidogen 1
- PXDN:
-
Peroxidasin
- PRO:
-
Protein group
- SPARC:
-
Secreted protein acidic and cysteine rich
- MsigDb:
-
The Molecular Signatures Database
References
Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–38.
Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159(4):738–49.
Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, Britton SL, Bouchard C, Koch LG, Timmons JA. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol. 2011;110(1):46–59.
Timmons JA, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J, Sundberg CJ. Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol. 2005;3:19.
Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Phys Endocrinol Metab. 2000;279(4):E806–14.
Hildebrandt AL, Pilegaard H, Neufer PD. Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration. Am J Phys Endocrinol Metab. 2003;285(5):E1021–7.
Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19(11):1498–500.
Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2):162–84.
Hjorth M, Norheim F, Meen AJ, Pourteymour S, Lee S, Holen T, Jensen J, Birkeland KI, Martinov VN, Langleite TM, et al. The effect of acute and long-term physical activity on extracellular matrix and serglycin in human skeletal muscle. Physiol Rep. 2015;3(8).
Johnson ML, Lanza IR, Short DK, Asmann YW, Nair KS. Chronically endurance-trained individuals preserve skeletal muscle mitochondrial gene expression with age but differences within age groups remain. Physiol Rep. 2014;2(12).
Popov DV, Makhnovskii PA, Shagimardanova EI, Gazizova GR, Lysenko EA, Gusev OA, Vinogradova OL. Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle. Am J Phys Endocrinol Metab. 2019;316(4):E605–e614.
Vissing K, Schjerling P. Simplified data access on human skeletal muscle transcriptome responses to differentiated exercise. Scientific Data. 2014;1:140041.
Nishida Y, Tanaka H, Tobina T, Murakami K, Shono N, Shindo M, Ogawa W, Yoshioka M, St-Amand J. Regulation of muscle genes by moderate exercise. Int J Sports Med. 2010;31(9):656–70.
Radom-Aizik S, Hayek S, Shahar I, Rechavi G, Kaminski N, Ben-Dov I. Effects of aerobic training on gene expression in skeletal muscle of elderly men. Med Sci Sports Exerc. 2005;37(10):1680–96.
Rowlands DS, Thomson JS, Timmons BW, Raymond F, Fuerholz A, Mansourian R, Zwahlen MC, Metairon S, Glover E, Stellingwerff T, et al. Transcriptome and translational signaling following endurance exercise in trained skeletal muscle: impact of dietary protein. Physiol Genomics. 2011;43(17):1004–20.
Knuiman P, van Loon LJC, Wouters J, Hopman M, Mensink M. Protein supplementation elicits greater gains in maximal oxygen uptake capacity and stimulates lean mass accretion during prolonged endurance training: a double-blind randomized controlled trial. Am J Clin Nutr. 2019;110(2):508–18.
Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278(15):12601–4.
Katz BZ, Yamada KM. Integrins in morphogenesis and signaling. Biochimie. 1997;79(8):467–76.
Jensen L, Pilegaard H, Neufer PD, Hellsten Y. Effect of acute exercise and exercise training on VEGF splice variants in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R397–402.
Hoppeler H, Baum O, Lurman G, Mueller M. Molecular mechanisms of muscle plasticity with exercise. Comp Physiol. 2011;1(3):1383–412.
Saltin B, PDJHoPSM G. Skeletal muscle adaptability: significance for metabolism and performance. 1983;10:555–631.
Sundberg CJ. Exercise and training during graded leg ischaemia in healthy man with special reference to effects on skeletal muscle. Acta Physiol Scand Suppl. 1994;615:1–50.
Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–89.
Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, Madri JA, Hudlicka O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279(4):H1540–7.
Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. 2013;591(Pt 9):2319–31.
Yan Z, Okutsu M, Akhtar YN, Lira VA. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol. 2011;110(1):264–74.
Goody MF, Sher RB, Henry CA. Hanging on for the ride: adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease. Dev Biol. 2015;401(1):75–91.
Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–810.
Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287(5):C1311–9.
Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9.
Jorgensen SB, Jensen TE, Richter EA. Role of AMPK in skeletal muscle gene adaptation in relation to exercise. Appl Physiol Nutr Metab. 2007;32(5):904–11.
Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Phys Endocrinol Metab. 2008;294(2):E463–74.
Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–16.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Sartor MA, Tomlinson CR, Wesselkamper SC, Sivaganesan S, Leikauf GD, Medvedovic M. Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC Bioinformatics. 2006;7:538.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50.
Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267.
Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (Oxford, England). 2016;32(18):2847–9.
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–7.
Acknowledgements
We would like to thank Lonneke Albert MSc and Koen Manusama MSc and other students for their help during the execution of the study. We would like to thank Dr. Urszula Kudla and Dr. Astrid Horstman from Friesland Campina for providing the study drinks as a gift.
Funding
This study was part of the EAT2MOVE project and supported by a grant from the Province of Gelderland, proposal PS2014–49. Study drinks were a gift by Friesland Campina. Friesland Campina did not designed the research and has not been involved by the study execution, data collection/analysis and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
PK, MH and MM designed the research; PK conducted the research; PK, RH and MB analyzed the data; PK wrote the paper. PK had primary responsibility for final content. All authors read, provided feedback and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The investigation was approved by the Medical Ethical Committee of Wageningen University, in accordance with the Declaration of Helsinki. This trial was registered at clinicaltrials.gov as NCT03462381 and adheres to CONSORT guidelines for clinical trials. All subjects volunteered in the present study gave full-written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare that are directly relevant to the contents of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Knuiman, P., Hangelbroek, R., Boekschoten, M. et al. Impact of protein supplementation during endurance training on changes in skeletal muscle transcriptome. BMC Genomics 21, 397 (2020). https://doi.org/10.1186/s12864-020-6686-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-020-6686-x