Abstract
Background
Somatic embryogenesis receptor-like kinases (SERKs) are leucine-rich repeat receptor-like kinases associated with various signaling pathways. These kinases have a relationship with stress signals, and they are also believed to be important for regulating plant growth. However, information about this protein family in apple is limited.
Results
Twelve apple SERK genes distributed across eight chromosomes were identified. These genes clustered into three distinct groups in a phylogenetic analysis. All of the encoded proteins contained typical SERK domains. The chromosomal locations, gene/protein structures, synteny, promoter sequences, protein–protein interactions, and physicochemical characteristics of MdSERK genes were analyzed. Bioinformatics analyses demonstrated that gene duplications have likely contributed to the expansion and evolution of SERK genes in the apple genome. Six homologs of SERK genes were identified between apple and Arabidopsis. Quantitative real-time PCR analyses revealed that the MdSERK genes showed different expression patterns in various tissues. Eight MdSERK genes were responsive to stress signals, such as methyl jasmonate, salicylic acid, abscisic acid, and salt (NaCl). The application of exogenous brassinosteroid and auxin increased the growth and endogenous hormone contents of Malus hupehensis seedlings. The expression levels of seven MdSERK genes were significantly upregulated by brassinosteroid and auxin. In addition, several MdSERK genes showed higher expression levels in standard trees of ‘Nagafu 2’ (CF)/CF than in dwarf trees of CF/‘Malling 9’ (M.9), and in CF than in the spur-type bud mutation “Yanfu 6” (YF).
Conclusion
This study represents the first comprehensive investigation of the apple SERK gene family. These data indicate that apple SERKs may function in adaptation to adverse environmental conditions and may also play roles in controlling apple tree growth.
Similar content being viewed by others
Background
Many cell-surface receptors have been identified in plants in recent years. The somatic embryogenesis receptor-like kinases (SERKs) are examples of cell-surface receptors that participate in plant defense responses and growth. SERKs belong to the leucine-rich repeat II receptor-like kinase (LRRII-RLK) group, and have been highly conserved during evolution [1,2,3,4]. The first SERK gene was identified in Daucus carota, and was shown to play an important role in the formation from of embryos from single somatic cells [5]. SERK genes have recently been identified in Arabidopsis thaliana, tomato, rice and Brassica rapa [6,7,8,9].
The subcellular localization of SERK proteins may provide important clues about their function. As receptors for diverse signals (i.e., brassinosteroid (BR) signals, flagellin signals, male sporogenesis, and Mi-1-mediated resistance to potato aphids), SERK proteins mainly localize in the cell membrane [10]. They contain seven conserved domains: a signal peptide (SP) domain; a leu-zipper (ZIP) domain; five Leu-rich repeat (LRR) domains; a Ser-Pro-rich (SPP) domain; a transmembrane (TM) domain; and a cytoplasmic Ser/Thr kinase domain adjacent to the C-terminal domain (CTD) [11]. These conserved domains have important biological functions. In cells, protein transport is mediated by the SP domain [12]; the ZIP domain is essential for specific binding of SERK proteins [7, 12]; the LRR domain is essential for proteins to combine with plasma membranes [13]; the SPP domain is involved in the interaction between proteins and cell walls [7]; the TM domain separates the intracellular and extracellular regions of SERK proteins [14]; the cytoplasmic Ser/Thr kinase domain is related to the phosphorylation state of SERK proteins [13]; and the CTD controls the modification of mRNA precursors [15].
The plant SERK genes form small families. For example, there are five SERK proteins in A. thaliana and four close homologs in rice. However, they have various functions in processes ranging from embryo formation to fertility, defense responses against pathogens and fungi, abiotic stress resistance, and senescence [5, 7, 16, 17]. AtSERK1 mediates ovule and embryo development and enhances embryogenic competence in culture [18]. AtSERK2 functions as important control point for sporophytic development and affects male gametophyte production [19]. Some SERK genes, including OsSERK1–2 and AtSERK3–4, take part in various stress and cell-death processes. OsSERK1 can be activated by blast fungus, host cell death, defense signaling molecules, and other stress signals [6]. OsSERK2 positively regulates immunity in many signaling pathways [20]. AtSERK3 is involved in the containment of dead cells after microbial infection [21], and AtSERK4 functions redundantly with AtSERK3 to regulate a BR-independent cell-death pathway [22].
AtSERK3–4 and AtSERK5-Ler mediate BR signals, and are essential for normal plant growth and development. Brassinosteroid signaling shares close relationships with the signaling pathways of other hormones, including gibberellin (GA), auxin, cytokinin, and abscisic acid (ABA) [23,24,25,26,27,28]. Plant height and stem growth are mainly regulated by BR, and BR cannot perform this function without AtSERK3–5 mediating the signal transduction pathway. In previous studies, overexpression of AtSERK3/BAK1 or AtSERK4/BKK1 caused stem elongation, while the AtSERK3/BAK1 or AtSERK4/BKK1 null mutation led to a semi-dwarf phenotype and reduced sensitivity to BR [29]; in BR insensitive1–5 (bri1–5) and serk3serk4 mutants, overexpressing AtSERK5-Ler with an intact RD motif restored the normal plant phenotype [28].
Since those studies, the important roles of SERK genes in stress responses and growth have been demonstrated in model plants. A few studies have focused on the roles of BR in apple. For instance, BR probably regulates apple seedling size and root formation [30]. Digital gene expression (DGE) analyses have shown that the dwarf phenotype of autotetraploid apple plants is related to the BR signaling pathway [31]; and BR was shown to stimulate the elongation of apple branches in vivo and to take part in mediating apple tree architecture [32, 33]. However, there is little information about SERK genes, which encode important BR signal receptors, in apple, and there have been no thorough and systematic studies on apple SERK genes.
Apple is an important perennial woody fruit crop in temperate regions. High stress resistance and vigorous vegetative growth of apple nursery trees are important attributes to apple producers and breeders. Abiotic stress can impact fruit yield and quality, and inappropriate vegetative growth suppresses early flowering and reduces yields in apple. Most widely grown apple cultivars are sensitive to adverse conditions and exhibit undesirable growth under such conditions, which is problematic for the apple industry. These two important problems are mediated by complex biological processes, including stress responses and hormone regulation. Therefore, the functional identification of apple SERK genes is very important.
To identify available information about the possible roles of SERK genes apple, we conducted a genome-wide search for apple SERK genes. The chromosomal location, gene/protein structure, evolutionary relationships, synteny, promoter sequences, protein–protein interactions, physicochemical characteristics, and expression profiles of these genes were also analyzed. We determined the expression patterns of MdSERK genes in response to stress signals, BR and auxin treatments, and in different graft combinations and apple varieties. The results of this study not only provide insights into SERKs but also form the basis for further studies on their potential functions in apple.
Results
Genome-wide identification, distribution, and multiple sequence alignment of apple SERK genes
In total, 12 candidate apple SERK genes were identified. According to their chromosomal locations, they were named MdSERK1 to MdSERK12. Their deduced polypeptides ranged in length from 554 (MdSERK4) to 1274 (MdSERK11) amino acids (aa), and their predicted molecular weights ranged from 61.48 (MdSERK4) to 143.65 (MdSERK11) kDa (Table 1). The NCBI apple expressed sequence tag (EST) database was screened to evaluate the accuracy of the genomic predictions. All of the putative genes matched to at least one EST (Additional file 1: Table S2). Their protein sequences are shown in Additional file 1: Table S3. Protein sequences were analyzed with ExPASy to predict protein characteristics. Most of the MdSERK proteins were found to be stable with a low instability index (< 40), except for MdSERK1, MdSERK4, and MdSERK5. All MdSERK proteins were hydrophilic according to their grand average of hydropathicity (GRAVY) values. The predominant aa residues were Leu, Ser, and Gly. We also detected Ala and Val in the MdSERK proteins. The aliphatic index values ranged from 81.61 (MdSERK6) to 96.90 (MdSERK9). The predicted isoelectric point (pI) ranged from 5.45 (MdSERK1) to 8.69 (MdSERK11). Seven of the 12 MdSERK proteins comprised acidic amino acids and the others comprised basic amino acids. All MdSERK proteins were predicted to be localized in the cell membrane (Additional file 1: Table S4). The 12 genes were distributed across eight of the 17 Malus × domestica chromosomes (Fig. 1). The number of MdSERK genes ranged from one to three genes per chromosome. One MdSERK gene was detected on chromosomes 2, 3, 9, 11, and 17, and two MdSERK genes were detected on chromosomes 13 and 16. Chromosome 15 contained three MdSERK genes, the maximum number among all chromosomes (Fig. 1).
Details of domain structures were obtained from multiple sequence alignments (Fig. 2). The MdSERK genes encoded proteins with the expected SERK domains, including (from the N to C termini) the SP domain, the ZIP domain, five LRR domains, the SPP domain, the TM domain, the cytoplasmic Ser/Thr kinase domain, and the CTD (Fig. 2). In the encoded SERKs, the N-terminal SP aa residues were variable except for Leu, Asp, and Glu residues. With only minor sequence variations, the Leu residues of the Leu zipper (highlighted with a yellow box) were essentially conserved. The Leu residues of three of the five Leu-rich repeats (all except for LRR4 and LRR5) were also conserved (highlighted with a yellow box). The SPP and TM domains were not highly conserved. In contrast, the Ser/Thr kinase domain was essentially conserved at Ser and Val (green box) and it also shared a conserved RD motif (red box). The C-terminal domain comprised about 20 aa and had conserved Gly, Leu, Glu, and Trp residues (red box) (Fig. 2). Ten consensus motifs were identified using the MEME motif search tool (Additional file 2: Figure S1; Additional file 1: Table S5). Based on location information, the kinase domain consisted of motifs 1–5 and 8–10; the Leu zipper contained motifs 6 and 7; and the LRR domain included motif 6. The predicted protein structures analysis showed that all MdSERK proteins consisted of α helices, β turns, extended strands, and random coils (Additional file 3: Figure S2, Additional file 1: Table S6). Among these structures, α helices were the most abundant and largest, while β turns were the least abundant and smallest. Random coils were larger than extended strands both in terms of amino acid length and proportion. The transmembrane helices of all MdSERK proteins were also analyzed. At least one transmembrane segment was detected in all MdSERK proteins, except for MdSERK5 (Additional file 4: Figure S3). There was one transmembrane segment in MdSERK2–3, MdSERK6–8, and MdSERK10–12. There were two transmembrane segments in MdSERK1 and MdSERK9, and three in MdSERK4.
Alignment of multiple apple SERK sequences. Conserved sequence characteristics of MdSERKs are indicated with colored underlines (black, signal peptide; gray, leucine zipper; deep red, red, orange, yellow, and green, leucine-rich repeat 1–5, respectively; dark green, serine-proline-rich domain; indigo, transmembrane domain; purple, kinase domain; brown, C-terminal domain). Conserved amino acid residues and RD motif are indicated with colored rectangles
Phylogenetic and gene structure analysis of apple SERK gene family
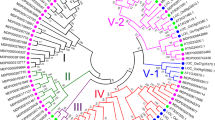
The amino acid sequences of SERKs from various plant species were compared to evaluate the evolutionary relationships among these kinases. Then, an unrooted phylogenetic tree was constructed to identify putative orthologs. In the tree, the tested species clustered into three distinct groups (i.e., A, B, and C) (Fig. 3).
As shown in Fig. 3, in Group A, MdSERK1, and MdSERK8–9 clustered with SISERK3A/B, NbSERK3A/B, MtSERK2–6, AtSERK3–4, AtSERK5-Ler, and others. In Group B, MdSERK4 was closely related to AtSERK1–2, GmSERK1, MtSERK1, and SISERK1, all of which were from different plant species. The remaining MdSERK genes clustered into Group C, which contained genes encoding OsSERK1–2, TaSERKlike3, and PoapSERK2 proteins. There were four sister pairs in apple: MdSERK8/ MdSERK9, MdSERK6/MdSERK11, MdSERK5/MdSERK10, and MdSERK3/MdSERK12.
An unrooted phylogenetic tree was constructed using the protein sequences encoded by the apple SERK genes (Additional file 5: Figure S4). Three gene categories were identified, similar to the phylogenetic groups discussed above. As expected, most SERK genes within the same group had very similar intron/exon distribution patterns in terms of exon length and intron number. Group A had seven members; MdSERK2–3, MdSERK5, MdSERK10 and MdSERK11. These genes were similar in exon length and intron length, and MdSERK6 shared similar intron phases with MdSERK11. Among Group B genes, MdSERK8 and MdSERK9 shared identical intron phases and exon numbers. Group C consisted of only one MdSERK gene with nine exons and eight introns.
Synteny analysis, promoter analysis, and interaction network of apple SERK genes
Because gene duplication events have occurred during the evolution of plant genomes, we searched for MdSERK duplicates. Segmental and tandem duplications were defined based on published criteria [34, 35]. One tandem duplication was identified: MdSERK8 and MdSERK9 on chromosome 15 (Fig. 4). The following five segmental duplications were also detected: MdSERK1 (chromosome 2) and MdSERK8/9 (chromosome 15); MdSERK3 (chromosome 9) and MdSERK12 (chromosome 17); MdSERK5 (chromosome 13) and MdSERK10 (chromosome 16); and MdSERK6 (chromosome 13) and MdSERK11 (chromosome 16) (Fig. 4; Additional file 1: Table S7). A syntenic map between AtSERK and MdSERK was generated (Fig. 5). In total, six SERK orthologs in Arabidopsis and apple were identified. MdSERK1–AtSERK4/5, MdSERK3–AtSERK1/2, and MdSERK8/9–AtSERK3 were located in duplicated genomic regions between the apple and Arabidopsis genomes (Additional file 1: Table S8).
The promoter regions (about 1.5 kb) of MdSERK genes (Additional file 6: Figure S5) were found to contain important cis-acting elements associated with stress and hormone-related responses. At least one stress-specific activation element was present in the promoter of all MdSERK genes. There were one to three cis-acting salicylic acid (SA)-responsive (TCA) elements in all MdSERK genes, except for MdSERK1, MdSERK3–4 and MdSERK7; one to two CGTCA motifs in all MdSERK genes; and one to three ABA-responsive elements (ABREs) in MdSERK1–10. These included the BR-response element G-box or non-E-box in all studied genes except for MdSERK2 and MdSERK8 [36]. There was more than one auxin-responsive element (TGA) in all MdSERK genes except for MdSERK4, MdSERK7, and MdSERK8. All genes contained more than one GA-response element (GARE) motif (i.e., one GARE-motif and a P-box in MdSERK2, two P-boxes in MdSERK3, and two GARE-motifs in MdSERK5) [37] in all other MdSERK genes).
An interaction network for the MdSERK proteins was constructed using A. thaliana orthologs (Additional file 7: Figure S6). Five of the 10 MdSERK genes were orthologs of two out of five AtSERK genes. The MdSERK partners mainly included BAK1/SERK3 and SERK4, which are involved in BR and flagellin signal transduction and in the regulation of stem elongation, leaf development, xylem differentiation, and cell-death responses. MdSERK1, MdSERK4, and MdSERK8–9, which were found to be highly homologous to AtSERK3/AtBAK1, formed relatively strong interactions with BRI1, BKI1, BSK1, BES1, and SERK4. MdSERK2–3 and MdSERK12, MdSERK5 and MdSERK10, and MdSERK6 and MdSERK11 were homologous to A. thaliana NSP-interacting kinases 1–3 (NIK1–3) which are involved in the defense response to geminivirus infection, and exhibited strong interactions with SUPPRESSOR OF ACAULIS 52 (SAC52), which is involved in translational regulation and plant height control.
Apple SERK expression profiles in different tissues
The tissue-specific expression patterns of MdSERK genes were analyzed in various tissues of M. hupehensis seedlings by quantitative real-time polymerase chain reaction (qRT-PCR) (Fig. 6). MdSERK1 and MdSERK7 were highly expressed in shoot tips. MdSERK2/5 and MdSERK6/11 showed the highest expression levels in the phloem. The expression levels of MdSERK4 and MdSERK12 were higher in the xylem and phloem of the stem than in other tissues. MdSERK3 was strongly expressed in the root, and the highest expression levels of MdSERK8–10 were in the leaf.
Expression profiles of apple SERK genes in different tissues. Quantitative reverse transcription polymerase chain reaction analysis of SERK gene expression levels in roots, xylem and phloem of stems, leaves, and shoot tips. Bars show mean ± standard error (n = 3). Overall least significant difference (p < 0.05) was calculated and used to separate means
To elucidate the potential roles and functions of the MdSERK genes in apple, expression profile data for different tissues (seedlings, roots, stems, flowers, fruit, and leaves) with two biological replicates were downloaded from the ArrayExpress database (E-GEOD-42873) (Additional file 8: Figure S7). Almost all MdSERK genes (except for MdSERK8–9 and MdSERK11) were expressed at higher levels in the flower of M74, fruit of M20_100 daf (100 days after flowering) and M.20_harvest, leaf of M49, and fruit of M74_harvest than in other tissues. MdSERK8–9 and MdSERK11 showed low expression levels in almost all tissues, especially in seedlings of Golden delicious (GD), compared with the other MdSERK genes.
Effects of exogenous hormone and salt treatments on gene expression profiles
To investigate the roles of MdSERK genes in stress responses, MdSERK gene expression patterns in young leaves were separately analyzed after methyl jasmonate (MeJA), SA, ABA, and salt treatments (Fig. 7). MdSERK2/5, MdSERK3, MdSERK6/11, and MdSERK12 were induced by MeJA. The expression level of MdSERK2/5 increased by 0.2- to 5-fold from 3 to 12 h in the MeJA-treated plants. MdSERK3 expression increased by 1.3-, 1.5-, 4-, 0.3-, and 5-fold at 0, 1, 3, 6, and 12 h, respectively, after MeJA treatment. MdSERK6/11 expression increased by about 2-, 0.5-, and 2.5-fold at 0, 6, and 12 h after MeJA treatment. The expression level of MdSERK12 was increased by about 3-, 3.2-, and 6-fold at 1, 3, and 6 h after MeJA treatment. MdSERK2/5 and MdSERK12 expression levels were also increased by about 0.5- to 15-fold at most time points after SA treatment. MdSERK2/5 and MdSERK12 showed higher expression levels in all treatment groups than in control groups from 1 to 12 h. The transcript levels of MdSERK2/5 were higher in the ABA treatment group than in the control group throughout the whole experiment. The relative expression level of MdSERK3 was significantly increased at 1, 6, and 12 h after ABA treatment, and those of MdSERK6/11 were upregulated by about 2-, 13-, and 9-fold at 3, 6, and 12 h after ABA treatment. The NaCl treatment increased the transcript levels of MdSERK4, MdSERK6/11, and MdSERK10 in leaves. MdSERK4 was induced by NaCl at all time points, except for 0 d. The transcript levels of MdSERK6/11 in leaves were about 10-, 8-, 6.5-, and 11.5-fold higher in the NaCl-treated group than in the control group. After the NaCl treatment, MdSERK10 was upregulated by about 7 to 12 times. The transcript levels of the other MdSERK genes were neither increased nor decreased in response to stress treatments (data not shown).
Transcript levels of MdSERKs after hormone and salt treatments. Quantitative reverse transcription polymerase chain reaction analysis of selected apple SERK genes in response to methyl jasmonate (MeJA), salicylic acid (SA), abscisic acid (ABA), and NaCl treatments at different time points (0, 1, 3, 6, and 12 h). EF1-α was used as an internal control. qRT-PCR data are shown relative to 0 h. Bars show mean ± standard error (n = 3). *Significant difference at 0.05 level, **significant difference at 0.01 level
Effects of exogenous BR and auxin treatments on apple growth, endogenous hormone contents, and gene expression profiles in M. hupehensis seedlings
Endogenous BR and auxin were simultaneously analyzed after exogenous hormone applications (Fig. 8). The BR content in shoot tips was high from 2 to 4 weeks after BR treatment (Fig. 8a), and the level of auxin (indole-3-acetic acid, IAA) in the auxin-treated group increased to about 4, 12.3, and 12.4 μg·g− 1 FW at 0, 2, and 3 weeks, respectively, after the auxin treatment (Fig. 8b). The plant height and stem diameter of M. hupehensis seedlings were measured after treatments with BR and auxin. The plant height had increased significantly by 2, 3, and 4 weeks after hormone application (Fig. 9a and Fig. 9b). The plants in the BR treatment groups were about 4 cm taller than those in the control group at 4 weeks after treatment (Fig. 9a). The height increased by about 9 cm at 4 weeks after the auxin treatment (Fig. 9b). Compared with control plants, those treated with BR had a significantly greater stem diameter from 1 to 4 weeks after the treatment (Fig. 9c). Like the BR treatment, the auxin treatment increased the lateral growth of stems. Compared with control plants, the auxin-treated plants showed 0.2-, 0.21-, 0.22-, and 0.28-mm thicker stems at 1, 2, 3, and 4 weeks, respectively, after the auxin treatment (Fig. 9d).
Endogenous hormone contents in shoot tips of control, brassinosteroid (BR)-treated, and auxin-treated plants. a Contents of BR in control and BR-treated groups. b Content of auxin in control and auxin-treated groups. Bars show mean ± standard error (n = 3). *Significant difference at 0.05 level, **significant difference at 0.01 level
Height of Malus hupehensis seedlings after brassinosteroid (BR) and auxin treatments. Plant height was measured at 0, 1, 2, 3 and 4 weeks after BR (a) and auxin (b) treatments. Bars show mean ± standard error (n = 3). Stem diameter was measured at 0, 1, 2, 3 and 4 weeks after BR (c) and auxin (d) treatments. *Significant difference at 0.05 level, **Significant difference at 0.01 level
The MdSERK expression levels were evaluated in the shoot tips of M. hupehensis after the hormone treatments (Fig. 10). The transcript levels of several MdSERK genes were significantly upregulated at most time points after the BR treatment. For instance, the transcript level of MdSERK1 was increased by about 1.2-, 2.1-, and 3-fold at 0, 2, and 4 weeks after the BR treatment. The transcript levels of MdSERK2/5 and MdSERK3 were increased by about 0.3- to 3-fold at 0, 1, 2, and 4 weeks after the BR treatment. The transcript level of MdSERK4 was increased by 0.7- to 3-fold at 0, 2, 3, and 4 weeks after the BR treatment. MdSERK8 was upregulated in BR-treated shoot tips compared with the control at all time points, except at 2 weeks. The MdSERK9 expression levels were increased by approximately 2- to 15-fold, compared with that in the control, at most time points after the exogenous BR treatment.
Expression of SERK genes in response to brassinosteroid (BR) and auxin treatments. Quantitative reverse transcription polymerase chain reaction analysis of apple SERK genes in response to BR and auxin treatments at different time points (0, 1, 2, 3, and 4 weeks). EF1-α was used as an internal control. qRT-PCR data are shown relative to 0 weeks. Bars show mean ± standard error (n = 3). *Significant difference at 0.05 level, **significant difference at 0.01 level
MdSERK1, MdSERK8, and MdSERK9 were significantly upregulated after the auxin treatment (Fig. 10). MdSERK1 and MdSERK9 were induced by approximately 0.6- to 15-fold after auxin treatment, and the transcript level of MdSERK8 was increased by about 3- to 8-fold at 1, 3, and 4 weeks after the auxin treatment. The transcript levels of other MdSERK genes either increased or decreased at different time points in response to BR and auxin treatments (data not shown).
Shoot growth and gene expression levels in different grafting combinations and branch-types
The grafting combination of CF/CF (‘Nagafu No. 2’ (CF) on its own rootstock) grew faster than did CF/M.9 (CF on the dwarf rootstock M.9) after bud break, and the primary shoot length of CF/CF was greater than that of CF/M9 at 130 (by about 13 cm) and 155 (by about 42 cm) days after bud break (DABB) (Additional file 9: Figure S8). To investigate whether MdSERKs are involved in regulating apple tree height, MdSERK expression patterns were analyzed in the shoot tips of the two grafting combinations (Fig. 11a). MdSERK1 showed higher expression levels in CF/CF trees than in CF/M.9 trees at 105, 130, and 155 DABB, but was repressed at 55 DABB in CF/CF trees. MdSERK4 and MdSERK8–9 were expressed at higher levels in CF/CF trees than in CF/M.9 trees from 55 to 155 DABB. MdSERK4 expression was 3- to 75-fold higher in CF/CF trees than in CF/M.9 trees from 55 to 155 DABB. MdSERK8 and MdSERK9 also showed higher expression levels (by about 20- to 145-fold) in CF/CF trees than in CF/M.9 trees throughout the trial period. The expression patterns of the remaining MdSERK genes were irregular after bud break (data not shown).
Comparison of SERK expression between grafting combinations (CF/CF vs. CF/M9) and cultivars (YF vs. CF). Quantitative reverse transcription polymerase chain reaction analysis of apple SERK genes in (a) CF/CF and CF/M9 after bud break (55, 80, 105, 130, and 155 days), and in (b) YF and CF after bud break (65, 85, 105, 125, and 145 days). EF1-α was used as an internal control. qRT-PCR data are shown relative to 55 days. Bars are mean ± standard error (n = 3). *Significant difference at 0.05 level, **significant difference at 0.01 level
A previous study showed that shoot length and internode length are shorter in YF (the spur-type bud mutation of “Yanfu 6”) than in CF, and that YF stops growing earlier than CF [38]. We also investigated MdSERK expression patterns in YF and CF shoot tips during the shoot growth period (Fig. 11b). Seven MdSERK genes showed high expression levels in CF. MdSERK2/5 and MdSERK3 were induced at 65, 85, 105, and 145 DABB in the shoot tips of CF. In CF, the expression levels of MdSERK6/11 and MdSERK9 were high from 85 to 145 DABB. MdSERK9 expression was upregulated by about 2-, 1.5-, and 5-fold at 85, 125, and 145 DABB in standard-type CF. The expression of other MdSERK genes showed no particular pattern in YF and CF, like in CF/CF and CF/M.9.
Discussion
SERKs have been shown to play important roles in the response to stress signals and in regulating plant growth, but they have only been systematically identified in model plants so far [1,2,3,4, 39]. The functional identification of all members in this family in apple, a woody fruit tree, had not yet been reported. Therefore, in this study, we conducted the first genome-wide identification and functional prediction of SERKs in apple. These results provide insights for further functional identification of SERK genes.
Genome-wide identification and characteristics analysis of SERK genes in apple
Twelve apple SERK proteins were identified (Fig. 1; Table 1). All MdSERK proteins contained conserved SERK domains: the SP domain, the ZIP domain, five LRR domains, an SPP domain, a TM domain, a cytoplasmic Ser/Thr kinase domain, and the CTD (Fig. 2; Additional file 2: Figure S1). Structural analyses of the MdSERK proteins revealed conservation in the proportion of α helices, β sheets, extended strands, and random coils (Additional file 3: Figure S2; Additional file 1: Table S6). Protein structure determines its function, and these observations suggested that the MdSERK proteins have similar secondary structures and functions. Several characteristics, including protein length, molecular weight, instability index, GRAVY value, major aa content, and aliphatic index were compared (Table 1 and Additional file 1: Table S4). Most of the MdSERK proteins showed similar length and molecular weight to those of AtSERKs, indicating that MdSERKs are high molecular weight proteins [39]. Almost all of MdSERK proteins were predicted to be stable according to their low instability index (< 40). All MdSERK proteins had very similar GRAVY values and high aliphatic indexes, which may be associated with the contents of major hydrophilic and aliphatic amino acids (i.e., Leu, Ser, Gly, Ala, and Val). Most MdSERK proteins contained at least one transmembrane segment (Additional file 4: Figure S3), and all of them were predicted to locate in the cell membrane (Additional file 1: Table S4), consistent with their predicted functions as cell membrane receptors [29].
Evolutionary and syntenic relationships of SERK genes
In the unrooted phylogenetic tree, the putative apple SERK proteins were grouped into different classes (Fig. 3). The 12 apple SERK genes were distributed into three groups, which may have different roles. Group A contained eight apple SERKs (MdSERK2–3, MdSERK5–7, and MdSERK10–12). The proteins encoded by these genes may play roles in plant immunity, stress resistance, and organ development, based on their similarities to OsSERK1–2, TaSERKlike3, and PoapSERKlike2 [6, 20, 40]. MdSERK1 and MdSERK8–9 were found to have close relationship with AtSERK3–4, AtSERK5-Ler, MtSERK1–6, GmSERK2, SISERK3A/B, and NbSERK3A/B in Group B. The proteins encoded by these genes are likely to be involved in BR-mediated biological processes, including stem growth, leaf development, root formation, and cell division, as well as in biotic and abiotic stress responses [4, 29]. MdSERK4 shared high homology with AtSERK1–2 and MtSERK2, which play roles in embryo development and disease resistance [6, 18]. There were several sister pairs in the combined tree, including four apple/apple pairs. The coding sequences and gene structures of the apple sister pairs were highly conserved (Fig. 3; Additional file 2: Figure S1; Additional file 5: Figure S4). All the MdSERKs were similar in their conserved domains, including the SP domain, the ZIP domain, five LRR domains, the SPP domain, the TM domain, the cytoplasmic Ser/Thr kinase domain, and the CTD. They all contained an intact RD motif, which is essential for SERK protein function [28]. These findings suggested that the functions of these apple SERK genes may be various and redundant.
Our results showed that there are more SERK genes in apple than in A. thaliana [39]. Gene duplication events may have played a significant role in the expansion of the apple SERK gene family. Tandem duplications and segmental duplications are the major evolutionary patterns [40]. Some gene duplication events for SPL, CYS, and bZIP genes have been characterized [41,42,43]. One tandem and five segmental duplications were found among the MdSERK genes (Fig. 4). Apple has undergone a genome-wide duplication that led to the formation of the present 17 chromosomes from an initial nine ancestral chromosomes. This event may have played an important role in the expansion of MdSERK genes [44], and may explain why there are more MdSERK genes than AtSERK genes.
Gene function in less-studied plants can be better understood through genomic comparison with a well-identified species, such as Arabidopsis. In our study, six SERK orthologs were identified from apple and Arabidopsis (Fig. 5; Additional file 1: Table S8). Both AtSERK4 and AtSERK5-Ler are involved in the response to cell death and stem elongation [4, 22, 29]. MdSERK1 showed strong similarity to these genes, suggesting that it may have similar roles. MdSERK3 was identified as a homolog of AtSERK1 and AtSERK2, which play important roles in reproductive growth [18, 19]. AtSERK3 (also known as AtBAK1) is involved in BR signal transduction, plant growth, and stress responses [22, 32]. MdSERK8 and MdSERK9 were identified as homologs of AtSERK3, indicating they may have diverse functions. The functions of these MdSERK genes should be confirmed experimentally in further studies.
Expression and potential functional analysis of MdSERK genes
The 12 analyzed MdSERK genes were expressed differently among the roots, xylem, and phloem of stems, and the leaves and shoot tips of M. hupehensis seedlings (Fig. 6), consistent with SERK expression patterns in other species [8, 41, 42]. Almost all of the analyzed MdSERK genes showed higher expression levels in the xylem, phloem, or shoot tips than in other organs. Therefore, these SERKs may play critical roles in stem development. We also analyzed the expression of MdSERK genes in different tissues based on ArrayExpress data (Additional file 8: Figure S7). The highest expression levels of MdSERK genes were in the flowers, fruit, and leaves, indicating that they may play important roles in flower, fruit, and leaf development. The various expression patterns might be related to gene chromosomal locations, characteristics, and structures. The experimental and digital investigation data indicated that MdSERK genes are involved in many aspects of apple development. These findings should be confirmed by further functional analyses.
Several studies have reported that SERK genes are involved in defense signals and are master regulators of abiotic and biotic stresses in many plant species [6, 20, 43, 44]. OsSERK1 is associated with host cell death, blast fungus, and defense signaling molecules [6]. The regulation of the rice immune receptors XA21 and XA3 by OsSERK2 confers immunity to bacterial leaf blight [20]. Salinity and powdery mildew induce SERKs in barley [45]. AtSERK3/AtBAK1 interact with flagellin-sensitive 2 (FLS2) and elongation factor Tu receptor, and are involved in flagellin sensing and binding of a bacterial elicitor, respectively [46, 47]. Plant cell death was shown to be controlled by AtSERK3/AtBAK1 and AtSERK4/AtBKK1 [21]. Previous studies have confirmed that SA, MeJA, and ABA have important roles in plant resistance [48,49,50]. Plants can upregulate many resistance genes, such as those encoding WRKY, YTH domain-containing RNA-binding proteins (YTPs), and cystatins (CYS), to adapt to salt stress [48, 50]. However, little is known about the potential roles of SERK genes in regulating stress responses in apple, or whether apple SERK genes are associated with MeJA, SA, ABA, and salt signals. Therefore, we monitored the expression patterns of apple SERK genes under various stress conditions. Some MdSERK genes were induced by several signals, including MeJA, SA, ABA, and salt stress (Fig. 7). For example, MeJA induced the expression of MdSERK2/5, MdSERK3, MdSERK6/11, and MdSERK12; SA treatment increased the expression levels of MdSERK2/5 and MdSERK12; ABA treatment increased the transcript levels of MdSERK2/5, MdSERK3, and MdSERK6/11; and salt treatment upregulated MdSERK4, MdSERK6/11, and MdSERK10. Stress-specific elements (MBSs, TCA elements, CGTCA motifs, and ABREs) may partly explain the responses of some MdSERKs to MeJA, SA, and ABA signals (Additional file 6: Figure S5). These results suggested that these MdSERK genes may play important roles in plant responses to various stresses (SA, MeJA, ABA, and salt) through complex regulatory mechanisms.
Previous studies have concluded that the SERKs-mediated BR signaling is important for regulating plant growth. Previous studies have shown that serk mutants display abnormal growth, including dwarfism, delayed growth, stamen abortion, and a narrow-leaf phenotype [24,25,26]. Auxin was the first plant hormone shown to play important roles in many aspects of plant growth, including cell differentiation [51], plant structure [52], and apical meristem dominance [53]. Early research showed that auxin is involved in BR synthesis and signal transduction in model plants [54], and participates in the regulation of BR-responsive gene expression [55,56,57,58]. However, the relationship between SERK genes and BR or auxin signaling pathways in apple has not been explored. In this study, MdSERK1–5 and MdSERK8–9 in shoot tips were induced by BR, and the expression levels of MdSERK1 and MdSERK8–9 were increased by an auxin treatment (Fig. 10). The application of exogenous BR and auxin in separate experiments increased endogenous BR and IAA contents (Fig. 8), and induced horizontal and vertical growth of the stem (Fig. 9). These results indicate that there may be a complicated mechanism by which auxin regulates SERK genes in apple, and that these MdSERK genes might have a positive role in regulating apple tree growth by mediating BR and auxin signals.
Previous studies have shown that dwarf rootstocks can inhibit apple tree growth [52, 59, 60]. SERK3–5 encode important regulators of plant growth [24,25,26]. However, it was unknown whether MdSERK genes participate in controlling apple tree growth on dwarf rootstocks. In this study, the transcript levels of MdSERK1, MdSERK4, and MdSERK8–9 (homologs of the important growth regulators AtSERK4/5 and AtSERK3) (Fig. 3) varied considerably among trees with different vigor (Fig. 11a and Additional file 7: Figure S6). These observations were consistent with their expression patterns in response to exogenous auxin treatment (Fig. 10). Compared with CF/CF, the dwarf phenotype of CF/M.9 apple trees may be caused by a lowered auxin content, according to previous studies [52, 60,61,62]. There was at least one TGA element (auxin-responsive element) in the promoters of MdSERK1 and MdSERK9 (Additional file 6: Figure S5). These results indicated that MdSERK1 and MdSERK8–9 may be involved in the regulation of the dwarf phenotype of CF/M.9 trees through auxin signaling, and that MdSERK4 may also play an important role in apple tree growth. However, few studies have focused on the roles of SERK proteins in the dwarfing mechanism of apple rootstocks. Our study has highlighted potential roles of MdSERK genes in the dwarfism of apple trees. Further research should explore the potential relationships between MdSERK proteins and dwarfism.
The MdSERK genes exhibited different expression patterns between CF and YF apple cultivars, which differ in their shoot elongation [38]. MdSERK2–3, MdSERK5–6, MdSERK8–9, and MdSERK11 showed higher expression levels in CF than in YF during the shoot growth period (Fig. 11b), suggesting that they may be responsible for the growth difference between YF and CF. This expression pattern might be related to GA and auxin levels, as these hormones control the growth of YF trees [38]. At least one GARE or auxin-response motif was identified in the promoters of MdSERK2–3, MdSERK5–6, MdSERK8–9, and MdSERK11 (Additional file 6: Figure S5), and close relationships between BR and GA or auxin have been identified in other plants [30, 36, 63]. The synteny analysis showed that MdSERK8/9 and AtSERK3 (an important regulator of shoot growth) may be heterogenous homologs (Fig. 5). These results indicate that MdSERK genes may be involved in the response to GA and auxin signals to regulate the growth of YF trees. This hypothesis needs to be confirmed experimentally in further studies.
Conclusion
This study represents the first genome-wide analysis of the apple SERK family. Detailed bioinformatics analyses including gene chromosomal location, multiple sequence alignment, phylogenetic, synteny, chemical characterization, gene structure, promoter sequence, and protein–protein interaction analyses were conducted. The expression patterns of MdSERKs in different tissues, in response to various stresses and hormone treatments, and in different grafting combinations and apple cultivars were analyzed. Eight MdSERK genes (MdSERK2–6 and MdSERK10–12), especially MdSERK2 and MdSERK5, were predicted to play important roles in stress responses. Like SERK3–5 in Arabidopsis, most MdSERK genes (MdSERK1–6, MdSERK8–9, and MdSERK11), especially MdSERK8 and MdSERK9, appeared to be involved in regulating plant size. The data generated in this study provide information about the activities of apple SERK genes and help to support hypotheses on the involvement of SERK genes in stress responses and growth regulation. Future research should focus on validating the functions of MdSERK genes in various stress responses, and their roles in controlling apple tree size.
Methods
Chromosomal location, multiple sequence alignment, protein structure, chemical characterization, subcellular localization, phylogenetic, and intron/exon structure analyses of apple SERK genes
Known AtSERK (AtSERK1–4 and AtSERK5-Ler) protein sequences [7] were used as queries to search the Apple Genome Database (GDR) (https://www.rosaceae.org/). Pfam (http://pfam.xfam.org/) and SMART (http://smart.embl-heidelberg.de/) were used to confirm the presence of conserved pfam codes and SERK domains. Apple sequences lacking the characteristic domains present in AtSERK protein sequences were eliminated from further analyses.
Details of the chromosomal locations of MdSERK genes were obtained through BLASTN searches of the GDR database (GDR; http://www.rosaceae.org/). The MdSERK genes were mapped to chromosomes using MapDraw [64]. A BLASTN search of apple expressed sequence tag (EST) datasets (https://www.rosaceae.org/tools/ncbi_blast) from the Apple Genome Database was conducted to find the corresponding record for each putative SERK gene.
Multiple aa sequences were aligned using DNAMAN (version 6.0). The MEME program (http://meme-suite.org/) was used to identify conserved motifs, with the following parameters: number of repetitions-any; maximum number of motifs-10; optimum motif width-6 to 200 amino acid residues. Transmembrane helices were analyzed using the TMHMM server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) and the PHYRE server v. 2.0 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index). MdSERK protein three-dimensional structures were predicted using NPS (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html). The ExPASy program (http://web.expasy.org/protparam/) was used to predict MdSERK protein characteristics. The subcellular localization of MdSERKs was predicted using Plant-mPLoc (http:// www.csbio.sjtu.edu.cn/bioinf/plant-multi/). Phylogenetic trees were prepared with MEGA7 using the maximum likelihood method, complete deletion, and bootstrap tests with 1000 replications. The intron/exon structures of apple SERK genes were analyzed using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/).
Synteny analysis, identification of cis-acting elements in promoters of apple SERK genes, and SERK protein interaction networks
Tandem and segmental duplications of apple genes can produce homologous genes. The SERK genes were considered as duplicates if the aligned gene sequences were at least 70% identical, and the length of the matching sequences corresponded to at least 70% of the longer gene [65, 66]. Duplicated genes separated by five or fewer genes within a 100-kb region on the same chromosome were considered as tandem duplicates [34]. Coparalogs located on duplicated blocks of different chromosomes were considered to be segmental duplications, as proposed by other researchers [38]. Syntenic blocks in apple as well as those between the apple and A. thaliana genomes were downloaded from the plant Genome Duplication Database (http://chibba.agtec.uga.edu/duplication) [39, 67] to identify segmentally duplicated MdSERK genes, and apple and A. thaliana SERK homologs. Circos (version 0.63) (http://circos.ca/) was used to visualize the chromosomal locations of SERK genes.
The upstream regions (− 1.5 kb upstream of the transcription start site) of apple SERK genes were used as queries to search the MEME (http://meme-suite.org/) and PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) databases to identify putative cis-elements.
Arabidopsis is the most popular model plant species and the functions of AtSERK genes have been well characterized. The role of similar genes in other species can be predicted based on their homologs in Arabidopsis [50]. Therefore, to further analyze the roles of apple SERK genes and the relationship between them and other genes, the interolog proteins from Arabidopsis were used to construct an interaction network using STRING 10 (http://string-db.org/) (option value > 0.800).
Plant materials and treatments
The following six plant materials were used for four experimental treatments in this study: ‘NagafuNo. 2’ (CF)/ Malling 26 (M.26), M. hupehensis seedlings, CF/Malling 9 (M.9)-CF/CF, and CF/M.26-‘Yanfu 6’ (YF)/M.26. These materials were subjected to stress treatments, BR and auxin treatments, and used for grafting combination and branch-type comparisons, as described below.
Stress treatment For the stress experiments, scion buds of CF were grafted onto 1-year-old M.26 rootstock in 2014. After grafting, trees were grown in pots containing culture medium (mixture of garden soil and river sand at a 1: 1 ratio) and were drip-irrigated in a net house. In 2016, young leaves (third to fifth fully expanded leaves beneath the shoot apex) of CF/M.26 were treated with 50 μM MeJA, 100 μM SA, or 300 μM ABA at 55 DABB. The MeJA, SA, and ABA were purchased from Sigma (Deisenhofen, Germany) and were dissolved in ethanol. Leaves sprayed with sterile distilled water served as controls. Young leaves were harvested from both the control and treated lines at 0, 1, 3, 6, and 12 h after treatments [68]. The salt treatment was performed by irrigating plants with water containing 200 mM NaCl (dissolved in sterile distilled water). Trees irrigated with the same volume of sterile distilled water served as controls. Young leaves from salt-treated and control trees were collected at 0, 2, 4, 6, and 8 d after treatment [69].
Hormone treatment On March 1 in 2015 and 2016, two batches of M. hupehensis seeds were respectively planted in an experimental orchard at the Northwest Agriculture and Forestry University in Yangling (108°04′E, 34°16′N), China. When the two batches of seedlings reached the 8–10 leaf stage, they were transplanted into ½-strength Hoagland’s nutrient solution, and grown under greenhouse conditions (24 h cycle of 12-h light (800 μmol m− 2 s− 1) at 25 ± 1 °C, followed by 12-h dark at 15 ± 1 °C) on April 1 in 2015 and 2016. Equal-sized M. hupehensis seedlings, which were apomictic and very uniform in their genotype and phenotype [30, 70, 71], were used to assess the effects of BR (in 2015) and auxin (in 2016) treatments, and were evaluated at 2 months after the treatments. Using a hydroponic system, BR and auxin experiments were performed in greenhouse from June 1 to June 28 in 2015 and 2016, respectively. For the BR treatment, equal-sized seedlings (about 9.5 cm height) were treated with 0.5 mg/L 2,4-epibrassinolide (a BR) in aerated ½-strength Hoagland’s nutrient solution from June 1 to June 28, 2015. The optimum concentration of BR was determined based on previous studies [30, 72]. The BR was purchased from Sigma and was dissolved in ethanol. From June 1 to June 28 of 2016, the second batch of M. hupehensis seedlings (about 9 cm height) was cultured in aerated ½-strength Hoagland’s nutrient solution containing 0.1 mg/L 1-naphthylacetic acid (NAA, an auxin analogue, Sigma), which had been dissolved in ethanol. The optimal concentration of NAA was determined based on previous studies [73, 74]. Seedlings cultured in aerated ½-strength Hoagland’s nutrient solution and treated with the same volume of distilled water served as controls in 2015 and 2016. During the experimental process in both years, plants were grown in ½-strength Hoagland’s nutrient solution in black open-topped plastic containers (50 cm × 35 cm × 15 cm) and the solution was replaced every week. The seedlings were positioned at the top of the containers using a polystyrene foam board with holes. The seedlings were held firmly in the holes by sponges. There were 30 seedlings per container, and they were aerated by an air pump. Roots (consisting of the new root and root tips), xylem and phloem of stems (near the apices of newly growing shoots and 3–4 mm in diameter), leaves (third to fifth fully expanded young leaves beneath the shoot apices), and shoot tips (shoot apices) were collected at 0, 1, 2, 3, and 4 weeks after the hormone treatments. Roots were cleaned with distilled water and then excess liquid was removed with paper towels.
Comparison of grafting combinations We compared CF on its own roots with CF on the dwarf rootstock M.9 (CF/CF vs. CF/M.9). The scion buds from CF were grafted onto 1-year old CF (vigorous rootstock) and M.9 (dwarf rootstock) in 2012 and then planted in a net house in an experimental plot at the Yangling National Apple Improvement Center, Yangling, China (34.31°N, 108.04°E). The growth conditions of the trees were the same as those described in the ‘Stress treatment’ section. In 2013, the shoot tips (shoot apices) of CF/CF and CF/M.9 were collected for analysis at 55, 80, 105, 130, and 155 DABB.
Branch-type comparisons In 2009, standard-type CF and the short-branched spur-type mutant YF were each grafted onto Malling 26 (M.26) using standard horticultural practices. The grafted plants were grown in an experimental plot at the Yangling National Apple Improvement Center, Yangling, China (34.31°N, 108.04°E). In 2013, branch tips (branch apices) were sampled for RT-qPCR analysis of MdSERK gene expression at 65, 85, 105, 125, and 145 DABB.
To minimize sampling error, all samples were sampled using three biological replicates with six trees per replicate. All samples were collected in the same manner, and were cut into pieces and immediately placed in an ultra-low temperature freezer.
Measurements of tree growth and endogenous hormones in M. hupehensis seedlings
Hormone treatments The plant height of M. hupehensis seedlings was measured at 0, 1, 2, 3, and 4 weeks after BR or auxin treatment in 2015 and 2016. Using a steel tape measure, the plant height was measured from the basal stem to the primary shoot apex. Stem diameter was the average value of the stem diameter measured in two directions with a Vernier caliper. Using ELISA, the endogenous BR and IAA contents in shoot tips were measure after the BR treatment (2015) and the auxin treatment (2016) as described elsewhere [75, 76].
Comparison of grafting combinations The primary shoot length (from the graft union to the primary shoot apex) of CF/CF and CF/M.9 was measured at 55, 80, 105, 130, and 155 DABB with a steel tape measure.
Branch-type comparison The growth characteristics of YF and CF were investigated and have been published elsewhere [38].
The above data were measured with three biological replicates with six trees per replicate to minimize experimental error.
Apple SERK gene expression analysis
Total RNA was extracted using the CTAB method [77]. RNase-free DNase I (Invitrogen, Shanghai, China) was used to remove any residual genomic DNA. First-strand cDNA was synthesized from 2 μg total RNA using the SYBR Prime Script RT-PCR Kit II (TaKaRa, Dalian, China). The cDNA samples were diluted to about 150 ng/μL, and 1 μL (in a final volume of 10 μL) was used for qRT-PCR assays, which were conducted using three technical replicates and three biological replicates. Primers specific for MdSERK genes were designed using Primer 6 and Primer 3 (Additional file 1: Table S1). The specificity of primers was checked in GDR (https://www.rosaceae.org/). The coding sequences of MdSERK2 and MdSERK5, and of MdSERK6 and MdSERK11 were extremely similar, so it was impossible to separate them by PCR using different pairs of primers. Therefore, only two pairs of primers were designed to amplify these genes. The qRT-PCR analyses were conducted using the SYBR Green qPCR kit (TaKaRa) and the Bio-Rad CFX 134 Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA. The PCR amplification conditions were as follows: 95 °C for 5 min; 40 cycles of 94 °C for 20 s, 59 °C for 20 s, and 72 °C for 10 s. The apple EF1-α gene [27, 68] served as the internal standard. Relative gene expression levels were calculated using the 2−ΔΔCt method [78, 79].
The young leaves of CF/M.26 were used to extract RNA, which was used for functional analyses of MdSERKs in response to stresses. The shoot tips of M. hupehensis seedlings, CF/CF, CF/M.9, and CF-YF were used to analyze the roles of MdSERKs in apple tree growth. The shoot tips are essential for plant architecture [27, 38]. We extracted RNA from the roots, xylem, and phloem of stems, leaves, and shoot tips of M. hupehensis seedlings in the control group to investigate the tissue expression patterns of MdSERK genes. Tissue expression patterns among different apple varieties and tissues were also investigated using data downloaded from the online ArrayExpress database (https://www.ebi.ac.uk/arrayexpress/, 240E-GEOD-42873).
Statistical analysis
Standard values and standard error of experimental data were calculated using Microsoft Excel 2010. Analyses of the significance of differences were conducted using the Data Processing System (DPS, v7.05; Zhejiang University, Hangzhou, China).
Abbreviations
- 100daa:
-
100 days after flower
- ABA:
-
abscisic acid
- BR:
-
brassinosteroid
- bri1–5:
-
BR insensitive1–5
- CF:
-
Nagafu 2
- CTD:
-
C-terminal domain
- CYS:
-
cystatins
- DABB:
-
days after bud break
- DGE:
-
digital gene expression
- DPS:
-
Data Processing System
- EST:
-
expressed sequence tag
- GA:
-
gibberellin
- GDR:
-
Apple Genome Database
- IAA:
-
Indole-3-acetic acid
- LRRII-RLK:
-
leucine-rich repeat II receptor-like kinase
- LRRs:
-
leu-rich repeat
- M.9:
-
Malling 9
- Mb:
-
megabases
- MeJA:
-
methyl jasmonate
- NAA:
-
1-naphthylacetic acid
- NIK1–3:
-
NSP-interacting kinase1–3
- qRT-PCR:
-
quantitative reverse transcription polymerase chain reaction
- SA:
-
salicylic acid
- SAC52:
-
SUPPRESSOR OF ACAULIS 52
- SERKs:
-
Somatic embryogenesis receptor-like kinases
- SP:
-
signal peptide
- SPP:
-
serine-proline-rich
- TM:
-
transmembrane
- YF:
-
Yanfu 6
- YTPs:
-
YTH domain-containing RNA-binding proteins
- ZIP:
-
leu-zipper
References
Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tor M, de Vries S, Zipfel C. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–55.
Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S. The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell. 2005;17:3337–49.
Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol. 2008;148:611–9.
Wu XM, Kou SJ, Liu YL, Fang YN, Xu Q, Guo WW. Genome-wide analysis of small RNAs in nonembryogenic and embryogenic tissues of citrus: microRNA- and siRNA-mediated transcript cleavage involved in somatic embryogenesis. Plant Biotechnol J. 2015;13:383–94.
Schmidt E, Guzzo F, Toonen M, SC DV. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–62.
Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 2005;222:107–17.
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt E, Boutilier K, Grossniklaus U, de Vries SC. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001;127:803–16.
Mantelin S, Peng H, Li B, Atamian HS, Takken FLW, Kaloshian I. The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J. 2011;67:459–71.
Zhang S, Li C, Li Q, Wang QN, Huang SH, Zhang YF, Wang XF. Functional divergence of BAK1 genes from Brassica rapa in regulating plant architecture. Genet Mol Res. 2015;14:14587–96.
KH Nam, J Li. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–12.
Baudino S, Hansen S, Brettschneider R, Hecht VF, Dresselhaus T, Lorz H, Dumas C, Rogowsky PM. Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta. 2001;213:1–10.
Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–64.
Shah H, Gadella T, van Erp H, Hecht V, de Vries SC. Subcellular localization and oligomerization of the Arabidopsis thaliana somatic embryogenesis receptor kinase 1 protein. J Mol Biol. 2001;309:641–55.
Shah K, Russinova E, Gadella T, Willemse J, de Vries SC. The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes Dev. 2002;16:1707–20.
Garg M, Ramdas N, Vijayalakshmi M, Shivashankar GV, Sarin A. The C-terminal domain (CTD) in linker histones antagonizes anti-apoptotic proteins to modulate apoptotic outcomes at the mitochondrion. Cell Death Dis. 2014;5:e1058.
Nolan KE, Kurdyukov S, Rose RJ. Characterisation of the legume SERK-NIK gene superfamily including splice variants: implications for development and defense. BMC Plant Biol. 2011;11:44.
Song D, Li G, Song F, Zheng Z. Molecular characterization and expression analysis of OsBISERK1, a gene encoding a leucine-rich repeat receptor-like kinase, during disease resistance responses in rice. Mol Biol Rep. 2008;35:275–83.
Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–38.
Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell. 2005;17:3350–61.
Chen X, Zuo S, Schwessinger B, Chern M, Canlas PE, Ruan D, Zhou X, Wang J, Daudi A, Petzold CJ, et al. An XA21-associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors. Mol Plant. 2014;7:874–92.
Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Abu Qamar S, Mengiste T, Betsuyaku S, Parker JE, Muessig C, et al. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol. 2007;17:1116–22.
He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. BAK1 and BKK1 regulate Brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol. 2007;17:1109–15.
Jin Y, Tang R, Wang H, Jiang C, Bao Y, Yang Y, Liang M, Kong F, Li B, Zhang H. Overexpression of Populus trichocarpa CYP85A3 promotes growth and biomass production in transgenic trees. Plant Biotechnol J. 2017;15:1309–21.
Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–8.
Haubrick LL, Assmann SM. Brassinosteroids and plant function: some clues, more puzzles. Plant Cell Environ. 2006;29:446–57.
Kir G, Ye H, Nelissen H, Neelakandan AK, Kusnandar AS, Luo A, Inze D, Sylvester AW, Yin Y, Becraft PW. RNA interference knockdown of BRASSINOSTEROID INSENSITIVE1 in maize reveals novel functions for brassinosteroid signaling in controlling plant architecture. Plant Physiol. 2015;169:826.
Zheng L, Ma J, Song C, An N, Zhang D, Zhao C, Qi S, Han M. Genome-wide identification and expression profiling analysis of brassinolide signal transduction genes regulating apple tree architecture. Acta Physiol Plant. 2017;39:177-96.
Wu W, Wu Y, Gao Y, Li M, Yin H, Lv M, Zhao J, Li J, He K. Somatic embryogenesis receptor-like kinase 5 in the ecotype Landsberg erecta of Arabidopsis is a functional RD LRR-RLK in regulating brassinosteroid signaling and cell death control. Front Plant Sci. 2015;6:852.
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–22.
Mao J, Zhang D, Li K, Liu Z, Liu X, Song C, Li G, Zhao C, Ma J, Han M. Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Plant Growth Regul. 2017;82:391–401.
Ma Y, Xue H, Zhang L, Zhang F, Ou C, Wang F, Zhang Z. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica). Sci Rep. 2016;6:26719.
Pereira-Netto AB, Roessner U, Fujioka S, Bacic A, Asami T, Yoshida S, Clouse SD. Shooting control by brassinosteroids: metabolomic analysis and effect of brassinazole on Malus prunifolia, the Marubakaido apple rootstock. Tree Physiol. 2009;29:607–20.
Zheng X, Zhao Y, Shan D, Shi K, Wang L, Li Q, Wang N, Zhou J, Yao J, Xue Y, et al. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 2017;217:1086–98.
Wang L, Guo K, Li Y, Tu Y, Hu H, Wang B, Cui X, Peng L. Expression profiling and integrative analysis of the CESA/CSL superfamily in rice. BMC Plant Biol. 2010;10:282-198.
Wei F, Coe E, Nelson W, Bharti AK, Engler F, Butler E, Kim H, Goicoechea JL, Chen M, Lee S, et al. Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genet. 2007;3:1254–63.
Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B. Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell. 2015;27:2261–72.
Gubler F, Jacobsen JV. Gibberellin-responsive elements in the promoter of a barley high-pI alpha-amylase gene. Plant Cell. 1992;4:1435–41.
Song C, Zhang D, Zheng L, Zhang J, Zhang B, Luo W, Li Y, Li G, Ma J, Han M. miRNA and degradome sequencing reveal miRNA and their target genes that may mediate shoot growth in spur type mutant "Yanfu 6". Front Plant Sci. 2017;8:441.
Aan Den Toorn M, Albrecht C, de Vries S. On the origin of SERKs: bioinformatics analysis of the somatic embryogenesis receptor kinases. Mol Plant. 2015;8:762–82.
Singh A, Breja P, Khurana JP, Khurana P. Wheat brassinosteroid-Insensitive1 (TaBRI1) interacts with members of TaSERK gene gamily and cause early flowering and seed yield enhancement in Arabidopsis. PLoS One. 2016;11:e0153273.
Ahmadi B, Masoomi-Aladizgeh F, Shariatpanahi ME, Azadi P, Keshavarz-Alizadeh M. Molecular characterization and expression analysis of SERK1 and SERK2 in Brassica napus L.: implication for microspore embryogenesis and plant regeneration. Plant Cell Rep. 2016;35:185–93.
Singla B, Khurana JP, Khurana P. Structural characterization and expression analysis of the SERK/SERL gene family in rice (Oryza sativa). Int J Plant Genomics. 2009;539:402-10.
Sweetlove LJ, Nielsen J, Fernie AR. Engineering central metabolism - a grand challenge for plant biologists. Plant J. 2017;90:749–63.
Zhang D, Deng X, Fu F, Lin H. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta. 2015;241:875–85.
Li Y, Liu C, Guo G, He T, Chen Z, Gao R, Xu H, Faheem M, Lu R, Huang J. Expression analysis of three SERK-like genes in barley under abiotic and biotic stresses. J Plant Interact. 2017;12:279–85.
Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nuernberger T, Jones JDG, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497.
Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A. 2007;104:12217–22.
Wang N, Guo T, Sun X, Jia X, Wang P, Shao Y, Liang B, Gong X, Ma F. Functions of two Malus hupehensis (Pamp.) Rehd. YTPs (MhYTP1 and MhYTP2) in biotic- and abiotic-stress responses. Plant Sci. 2017;261:18–27.
de Jong AJ, Cordewener J, Schiava FL, Terzi M, Vande-kerckhove J, Kammen AV, de Vries SC. A carrot somatic embryo mutant is rescued by chitinase. Plant Cell. 1992;4(4):425−33.
Wei W, Hu Y, Han Y, Zhang K, Zhao F, Feng J. The WRKY transcription factors in the diploid woodland strawberry Fragaria vesca: identification and expression analysis under biotic and abiotic stresses. Plant Physiol Biochem. 2016;105:129–44.
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108:18512–7.
Song C, Zhang D, Zhang J, Zheng L, Zhao C, Ma J, An N, Han M. Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol Plant. 2016;38.
Balzan S, Johal GS, Carraro N. The role of auxin transporters in monocots development. Front Plant Sci. 2014;5:393.
Mouchel CF, Osmont KS, Hardtke CS. BRX mediates feedback between brassinosteroid levels and auxin signalling in root growth. Nature. 2006;443:458–61.
Nolan KE, Irwanto RR, Rose RJ. Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol. 2003;133:218–30.
Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 2005;222(1):107−17.
Friedrichsen DM, Nemhauser J, Muramitsu T, Maloof JN, Alonso J, Ecker JR, Furuya M, Chory J. Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics. 2002;162:1445–56.
Goda H, Sawa S, Asami T, Fujioka S, Shimada Y, Yoshida S. Comprehensive comparison brassinosteroid-regulated of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2004;134:1555–73.
Li HL, Zhang H, Yu C, Ma L, Wang Y, Zhang XZ, Han ZH. Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock. Acta Physiol Plant. 2012;34:235–44.
Feng Y, Zhang X, Wu T, Xu X, Han Z, Wang Y. Methylation effect on IPT5b gene expression determines cytokinin biosynthesis in apple rootstock. Biochem Biophys Res Commun. 2017;482:604–9.
Kamboj JS, Browning G, Quinlan JD, Blake PS, Baker DA. Polar transport of [H-3]-IAA in apical shoot segments of different apple rootstocks. J Hortic Sci. 1997;72:773–80.
Michalczuk L. Indole-3-acetic acid level in wood, bark and cambial sap of apple rootstocks differing in growth vigour. Acta Physiol Plant. 2002;24:131–6.
Vert G, Walcher CL, Chory J, Nemhauser JL. Integration of auxin and brassinosteroid pathways by auxin response factor 2. Proc Natl Acad Sci U S A. 2008;105:9829–34.
Liu R, Meng J. MapDraw: a Microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Yichuan. 2003;25:317–21.
Gu ZL, Cavalcanti A, Chen FC, Bouman P, Li WH. Extent of gene duplication in the genomes of Drosophila, nematode, and yeast. Mol Biol Evol. 2002;19:256–62.
Yang S, Zhang X, Yue J, Tian D, Chen J. Recent duplications dominate NBS-encoding gene expansion in two woody species. Mol Genet Genomics. 2008;280:187–98.
Tang H, Wang X, Bowers JE, Ming R, Alam M, Paterson AH. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res. 2008;18:1944–54.
Li J, Hou H, Li X, Xiang J, Yin X, Gao H, Zheng Y, Bassett CL, Wang X. Genome-wide identification and analysis of the SBP-box family genes in apple (Malus × domestica Borkh.). Plant Physiol Biochem. 2013;70:100–14.
Li X, Guo R, Li J, Singer SD, Zhang Y, Yin X, Zheng Y, Fan C, Wang X. Genome-wide identification and analysis of the aldehyde dehydrogenase (ALDH) gene superfamily in apple (Malus × domestica Borkh.). PLANT PHYSIOL BIOCH. 2013;71:268–82.
Spielman M, Vinkenoog R, Scott RJ. Genetic mechanisms of apomixis. Philos Trans R Soc Lond B Biol. 2003;358:1095–103.
Spillane C, Steimer A, Grossniklaus U. Apomixis in agriculture: the quest for clonal seeds. Sex Plant Reprod. 2001;14:179–87.
Bajguz A, Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem. 2009;47:1–8.
Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–32.
Bonhomme F, Kurz B, Melzer S, Bernier G, Jacqmard A. Cytokinin and gibberellin activate SaMADS a, a gene apparently involved in regulation of the floral transition in Sinapis alba. Plant J. 2000;24:103–11.
Dobrev PI, Kaminek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A. 2002;950:21–9.
Ding J, Mao L, Wang S, Yuan B, Feng Y. Determination of endogenous brassinosteroids in plant tissues using solid-phase extraction with double layered cartridge followed by high-performance liquid chromatography-tandem mass spectrometry. Phytochem Analysis. 2013;24:386–94.
Jaakola L, Pirttila AM, Halonen M, Hohtola A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol. 2001;19:201–3.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods. 2001;25:402–8.
Zhao J, Guo R, Guo C, Hou H, Wang X, Gao H. Evolutionary and expression analyses of the apple basic leucine zipper transcription factor family. Front Plant Sci. 2016;7:376.
Acknowledgments
We thank Jennifer Smith, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by National Science and Technology Supporting Project (2013BAD20B03), the National Apple Industry Technology System of Agriculture Ministry of China (CARS-28), the Innovation Project of Science and Technology of Shaanxi Province (2016TZC-N-11-6), and the Key Research Project of Shaanxi Province (2017ZDXM-NY-019).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and additional files.
Author information
Authors and Affiliations
Author notes
Mingyu Han is deceased. This paper is dedicated to his memory.
- Mingyu Han
Contributions
Liwei Zheng, Juanjuan Ma, Jiangping Mao, Sheng Fan, Dong Zhang, and Caiping Zhao performed the experiments; Liwei Zheng drafted the manuscript and analyzed the data; Mingyu Han revised the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Malus × domestica (‘NagafuNo. 2’ (CF)/Malling 26 (M.26), Malus hupehensis seedlings, CF/ Malling 9 (M.9)-CF/CF, and CF/M.26-‘Yanfu 6’(YF)/M.26) are widely planted in China. They are not listed in Appendices I, II, and III of the Convention on the Trade in Endangered Species of Wild Fauna and Flora (https://cites.org/eng/app/appendices.php). All the materials used in our study were grown in the Apple Demonstration Nursery of Yangling Modern Agriculture Technology Park (Northwest A&F University). Sample collection complied with institutional, national, and international guidelines. This article does not contain any studies with human participants or animals performed by any of the authors. No specific permits were required.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Primers and their sequences used for qRT-PCR analyses. a Internal control. Table S2. Best hits for putative apple SERK proteins in BLAST searches against apple ESTa assemblies. a Downloaded from NCBI database. Table S3. Sequences of MdSERK proteins. Table S4. Amino acid composition, physical and chemical characteristics, and subcellular localization of MdSERK proteins. aGrand average of hydropathicity bThree main amino acids in each protein (A, Ala; S, Ser; G, Gly; L, Leu; V, Val). Table S5. Motif sequences identified using MEME tools. Motif numbers correspond to motifs in Additional file 2: Figure S1. Table S6. Secondary structures of MdSERK proteins. Table S7. Synteny analysis of MdSERK genes. Table S8. Synteny analysis of MdSERK and AtSERK genes. (DOCX 37 kb)
Additional file 2:
Figure S1. Distribution of conserved motifs among apple SERK family members. Ten putative motifs are indicated with numbers in colored boxes. Names of all members and combined E-values are provided on the left; motif sizes are indicated below. Please see Table S5. for details of motifs. (TIF 540 kb)
Additional file 3:
Figure S2. Predicted dimensional structures of MdSERK proteins. (TIF 5808 kb)
Additional file 4:
Figure S3. Transmembrane topology analysis of MdSERK proteins. Transmembrane helices of the MdSERK proteins were predicted with TMHMM server v2.0. Red peaks indicate predicted transmembrane helices. (TIF 163 kb)
Additional file 5:
Figure S4. Intron/exon organization of apple SERK genes. Phylogenetic analysis and intron/exon organization of apple SERK genes. Numbers above or below branches indicate bootstrap values. Differently colored areas correspond to genes in each group. (TIF 417 kb)
Additional file 6:
Figure S5. Promoter sequences of selected apple SERK genes. Promoter (1.5-kb) sequences of 12 MdSERK genes were analyzed with PlantCARE and MEME software. (TIF 6224 kb)
Additional file 7:
Figure S6. Interaction network for proteins encoded by apple SERK genes according to Arabidopsis thaliana orthologs. Line thickness is related to combined score. Homologous genes in apple are indicated in red font in parentheses. (TIF 1893 kb)
Additional file 8:
Figure S7. Heat map showing MdSERK gene transcript levels in different tissues. Relative transcript levels are based on ArrayExpress data. (TIF 5289 kb)
Additional file 9:
Figure S8. Investigation of primary shoot length in different grafting combinations. Primary shoot length of CF after grafting on CF and M.9 rootstocks at 55, 80, 105, 130 and 155 DABB. (TIF 5663 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zheng, L., Ma, J., Mao, J. et al. Genome-wide identification of SERK genes in apple and analyses of their role in stress responses and growth. BMC Genomics 19, 962 (2018). https://doi.org/10.1186/s12864-018-5342-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-018-5342-1