Abstract
Glioma is the most prevalent type of primary brain tumor, and glioblastoma multiforme (GBM) is the highest and most deadly type of primary central nervous system (CNS) tumor, affecting a significant number of patients each year, with a median overall survival of approximately 14.6 months after diagnosis. Despite intensive treatment, nearly all GBM patients experience recurrence, with a 5-year survival rate of about 5%. The protective BBB and high tumor heterogeneity prevent the effective delivery of drugs, resulting in the treatment failure of various drugs. The emergence of nanometer-scale diagnosis and treatment methods has provided new promising approaches to overcome these difficulties. Thus, our review focuses on the development of BBB-crossing nanomedicine-enhanced chemotherapy and combined therapy applications for glioma. Meanwhile, we also reviewed the strategies to overcome the blood–brain barrier. Additionally, we discuss recent achievements in the area of brain tumor treatment with nanomedicine and the rational design approach, which will offer recommendations for anti-GBM nanomedicine development.
Highlights
-
Nanomedicine offers the advantages of good biocompatibility, loading multiple drugs, targeting specific cells or tissues, controlling drug release, and even passing the blood–brain barrier (BBB) in treating brain tumors.
-
With the development of multifunctional BBB-crossing nanomedicine, chemotherapeutic drugs can be effectively delivered to brain tumors.
-
Combination chemotherapy and other nanomedicine-based therapies can achieve significant synergistic effects in tumor treatment.
-
Formulating a unified preparation approach to make the obtained nanodrugs more uniform, stable, and controllable is urgently needed.
Similar content being viewed by others
Introduction
Brain tumors refer to various types of tumors in the central nervous system (CNS), which are primarily divided into primary and secondary types (Tang et al. 2019a; Yin et al. 2022). Brain tumors have become one of the most dangerous cancers to humans due to the characteristics of their location, and the treatment burden is increasing every year (Liu et al. 2023a; Dai et al. 2022). More than 300,000 new brain tumor cases were reported worldwide in 2018, and more than 76,000 new cases were recorded in China, accounting for more than a quarter of all cases (Bray et al. 2018; Guo et al. 2022a). In adults, glioma is the most common type of primary brain tumor. The World Health Organization (WHO) divides glioma into grade I–IV astrocytoma grades based on pathological results: I, II [astrocytoma], III [anaplastic astrocytoma], and IV [glioblastoma or GM]; the higher the tumor grade is, the poorer the prognosis. GBM is the most common and fatal form of primary CNS tumor (3.23 per 100,000 person-years), with a median overall survival (OS) of approximately 14.6 months after diagnosis (Ostrom et al. 2021). Despite intensive treatment, nearly all GBM patients experience recurrence (Ringel et al. 2016), and the 5-year survival rate is approximately 5% (Schwartzbaum et al. 2006; Afshari et al. 2021).
Chemotherapy is one of the primary adjuvant treatments for intracranial tumors. Numerous studies have demonstrated the benefits of postoperative chemotherapy for gliomas, particularly in patients with high-grade gliomas and those with childhood cancers. However, chemotherapy is ineffective due to the high invasiveness of tumor cells, tumor heterogeneity, and the presence of the BBB (Zhang et al. 2022; Wang et al. 2021; Li et al. 2023a). For many years, scientists have attempted to effectively deliver chemotherapy drugs to tumor sites while reducing the accumulation of chemotherapy drugs in normal brain and peripheral tissues (Ding et al. 2020). The potential use of nanomaterials in delivery systems offers a novel approach to treating brain tumors (Xue 2019). Nanomedicine, in contrast to traditional therapies, offers the advantages of superior biocompatibility, loading multiple drugs, targeting specific cells or tissues, controlling drug release, and even passing the BBB (Muhammad et al. 2022; Zhao et al. 2019; Guo et al. 2022b). Furthermore, by optimizing the size, shape, ligand density, lipophilicity, and surface chemical modification of nanoparticles, effective nanoparticle accumulation in the brain can be achieved, and the therapeutic effect can be improved (Liu et al. 2023b). These properties make nanomedicine an attractive treatment tool for brain tumors (Thangudu et al. 2020; Zottel et al. 2019; Zhao et al. 2020; Hsu et al. 2021). Additionally, by tweaking the surface chemistry of nanoparticles to engage specific cell types and perform specific tasks, patient-specific protocols can be tailored, thus choosing different nanocarriers for therapeutic molecules that interact with specific cells.
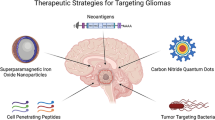
Our review focuses on the development of BBB-crossing nanomedicine enhanced chemotherapy and combined therapy applications for glioma. Additionally, we discuss recent achievements in the area of brain tumor treatment with nanomedicine and the rational design approach, which will offer recommendations for anti-GBM nanomedicine development (Scheme 1).
Current treatment status of glioma
In the past 30 years, much research has been conducted on the treatment of glioma, from radiological and surgical techniques to molecular therapy, although surgical excision is still the preferred treatment for brain tumor patients (Fig. 1) (Ma et al. 2021). However, in most brain tumors, particularly GBM, which grows deeper and causes a vicious infiltration of brain tissue, it is almost impossible to completely resect and easily relapse after treatment (Fig. 2a, b) (Lara-Velazquez et al. 2020; Bucci et al. 2004). After gross total resection (GTR) of the tumor in general, adjuvant radiotherapy [2 Gray (Gy)/day, 5 days/week, 6 weeks plus concurrent daily temozolomide (TMZ, 75 mg/m2)], and six cycles of adjuvant TMZ (150–200 mg/m2, for the first 5 days/28 days per cycle) were administered (Stupp et al. 2005). Unfortunately, conventional radiotherapy and chemotherapy are also ineffective because of the unique microenvironment of GBM. For example, radiation therapy uses X-rays to kill cancer cells by damaging their DNA. Although high-energy electron beams can reach deep into the brain to destroy invasive cancer cells, the hypoxic tumor microenvironment (TME) and inherent radiation resistance impair efficacy (Xie et al. 2019; Cheng et al. 2019). Furthermore, radiation therapy can cause major side effects, such as leukocytic encephalopathy, nerve damage, hair loss, vomiting, infertility, and skin rashes, harming the patient’s physical and mental wellbeing. The use of chemotherapy drugs causes the death of brain tumor cells, and TMZ has shown significant potential in treating glioblastomas and other difficult-to-treat cancers; thus, the Food and Drug Administration (FDA) has recommended that oral TMZ be employed as the standard chemotherapy regimen for GBM and anaplastic astrocytomas (Younis et al. 2019; Sidaway 2017; S.A. Grossman et al. 2010). However, therapeutic drugs cannot be effectively delivered to tumors (98% of small molecules and 100% of biological macromolecules) due to the high complexity of the human brain, high aggressiveness of tumor cells, tumor heterogeneity, and the existence of the blood–brain barrier (BBB) (Fig. 2c) (Pardridge 2015). Insufficient drug accumulation and even acquired tumor resistance are caused by these limitations, resulting in a considerable reduction in the effectiveness of treatment.

Adapted from ref. (Ma et al. 2021) with permission from BMJ copyright 2021
Recent advances in the management of glioma.
Current chemotherapeutic drugs for glioma
Generally, chemotherapy is employed as an adjuvant treatment for glioma after GTR of the tumor. Ideally, chemotherapy should have a specific distribution of drug concentrations at the tumor site, prolonged duration of action, the most effective killing of cancer cells, and minimized side effects on normal tissues. However, numerous factors affect chemotherapy, such as the existence of a blood–brain barrier, tumor heterogeneity, and drug resistance.
FDA-approved anti-glioma agents in chemotherapy
Nitrosoureas [lomustine, semustine, fotemustine, carmustine, and nimostine] can penetrate the blood–brain barrier and be initially employed in chemotherapy for gliomas (Vredenburgh et al. 2007). Occasionally, combination regimens of PCV [procarbazine + lomustine (CCNU) + vincristine] or cytotoxic chemotherapy drugs such as teniposide, etoposide, ifosfamide, cisplatin, or carboplatin have been employed; however, these chemotherapy regimens have limited efficacy and relatively high toxicity (Parasramka et al. 2017).
The clinical application of TMZ is a major advancement in glioma chemotherapy (Strobel et al. 2019). TMZ is a novel alkylating agent with strong permeability to the CNS and is approximately 100% bioavailable upon oral administration, with few side effects (Table 1) (Stupp et al. 2005). TMZ has fewer side effects and excellent tolerance and is easier to administer than traditional cytotoxic chemotherapy drugs. RT concomitant with TMZ for GBM treatment was first described in 2005. In that study, the median survival rate of GBM patients who received TMZ adjuvant chemotherapy was 26.5%, which was higher than that of GBM patients who received RT alone (10.4%) (Stupp et al. 2005).
TMZ is available in oral and injectable forms and works by inducing genomic DNA alkylation at the N7 and O6 positions of guanine and the N3 position of adenine, resulting in nucleotide mismatch and death of tumor cells (Strobel et al. 2019). However, TMZ sensitivity is significantly affected by the level of O6-methylguanine-DNA methyltransferase, which can remove TMZ-induced alkylation from nucleotides and has been employed as a predictor of responsiveness to alkylating drugs (Tomar et al. 2021; Qiu et al. 2014). Furthermore, the mechanisms of TMZ resistance in glioma also involve DNA damage repair (DNA mismatch repair, base resection repair), abnormal cell signaling pathways (p53-mediated signaling pathway, reactive oxygen species-mediated signaling pathway, RTK-related signaling pathway, transforming growth factor β-mediated signaling pathway, Wnt/β-catenin signaling pathway), glioma stem cells, TME (hypoxic microenvironment, exosome), epidermal growth factor receptor, etc. (Messaoudi et al. 2015; Beckta et al. 2019; Wade et al. 2013; Wee and Wang 2017; Jiang et al. 2019; Jia et al. 2018).
The first antiangiogenic therapy approved by the FDA for use in patients with cancer is BV. This recombinant humanized monoclonal antibody prevents angiogenesis by inhibiting VEGF-A, which can hinder the growth of new blood vessels in tumor tissues (Kreisl et al. 2009; Chinot et al. 2014; Gilbert et al. 2014). However, Ameratunga concluded that BV therapy does not significantly enhance the OS of patients with primary glioblastoma (Ameratunga et al. 2018). Thus, it is primarily recommended for recurrent glioblastomas, particularly when it is difficult to determine whether postoperative neuroimaging changes and radiochemical therapies are direct radiation responses or tumor recurrence.
Potential natural anti-glioma agents
Several FDA-approved drugs and their mechanisms of action have been identified, but researchers are currently focusing on some natural drugs with potential anti-glioma properties. For example, in 1998, Sanchez et al. published an article on anti-glioma. Additionally, Guzman et al. reported the antitumor potential of cannabinoids in animal models in 2003 (Guzman 2003). Thus, the therapeutic potential of cannabinoids has attracted the attention of numerous researchers in the field. Neurostatin, an O-acetylated derivative of ganglioside GD1b, has powerful antitumor properties both in vitro and in vivo and shows natural antitumor properties against astrocytoma division and astroblasts (Pais et al. 2013). Beatriz Valle-Argos and coworkers discovered that the compound antitumoral (neurostatin) inhibits the proliferation of U373MG and C6 glioma cells by activating cell cycle inhibitors, including p27 and p21 (Valle-Argos et al. 2010). Perillyl alcohol is a monoterpene that can be found in the essential oil of plants such as citrus fruits and lavender, and the results of a phase I/II study showed that it may be effective against gliomas. Perillyl alcohol is known to stop cancer by stopping Ras protein from working, but it also works on NF-κB, TGF, and different cell cycle proteins (Chen et al. 2018). Kuo Yong and coworkers characterized the anti-glioma activities of 12 compounds isolated from the marine-associated fungus Penicillium sp. ZZ1750. Among them, penipyridinone B, questiomycin A, and xanthocillin X showed potent anti-glioma activity (Yong et al. 2022).
Obstacles of chemotherapeutic agents for glioma treatment
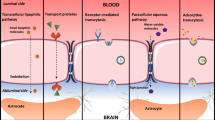
Numerous physiological barriers prevent most chemotherapies from having a practical therapeutic effect on GBM, including the BBB, blood–brain–tumor barrier, GBM cancer stem cell resistance, and highly heterogeneous GBM (Tellingen et al. 2015; Tam et al. 2020). As previously stated, a significant obstacle to drug delivery to the tumor is the BBB; essentially, the BBB is a dynamic interface between the blood and the brain tissue that selectively inhibits the passage of most substances, thus protecting the CNS from potentially neurotoxic substances (Fig. 3a) (Zou et al. 2021). This not only helps keep the CNS in a steady physiologic state but also prevents therapeutic drugs from entering the brain tissue. In patients with GBM, the BBB is damaged by infiltrating tumor cells. However, BBB disruption does not constitute a complete loss of its biological mechanism, and the mix of heterogeneous destruction with intact areas is sufficient to prevent drug access to tumor cells (Ganipineni et al. 2018). This phenomenon is called the "blood–brain–tumor barrier," and it can block chemotherapy agents from reaching tumor lesions and offer a barrier to the treatment of CNS tumors (Fig. 3b) (Ganipineni et al. 2018; Saraiva et al. 2016).
Strategies to overcome the blood–brain barrier
Since the development of molecular biology, people have gained a better understanding of the BBB. Studies have shown that certain biological, chemical, and physical stimuli can alter BBB permeability (Chen and Liu 2012).
Biological stimulation
Biological stimulation mainly involves inflammatory modulators and vasoactive mediators, such as bradykinin, histamine, and vascular endothelial growth factor, which can act on relevant receptors and enhance BBB permeability (Thangudu et al. 2020; Zhao et al. 2020; Liao et al. 2019). Vazana et al. discovered that excessive glutamate release enhances vascular permeability in the cerebral cortex of mice by activating NMDA receptors using in vivo microscopy. High-intensity magnetic stimulation may improve BBB permeability and facilitate drug delivery during neuron activation. Furthermore, in a three-dimensional external blood–tumor barrier (BTB) model, Wang et al. demonstrated that histamine could enhance BTB permeability by decreasing the expression levels of proteins related to tight junctions, such as ZO-1, occludin, and claudin-5, and the levels of H2 receptors are directly correlated with BTB permeability. However, the molecular mechanisms of viruses are biomaterials that can stimulate the blood–brain barrier to open. By upregulating the expression of chemokines, it disrupts the structure of the blood–brain barrier and degrades the protein at the brain–blood junction, allowing substances to penetrate the CNS (Kuang et al. 2009).
Chemical stimulation
Some chemical stimuli can also modulate tight junctions to improve BBB permeability, resulting in favorable conditions for drug delivery to the brain. High concentrations of arabinose and mannitol solutions can increase BBB permeability by constricting endothelial cells, allowing molecules to pass through the BBB to reach the brain (Doolittle et al. 2000). Some pharmaceutical excipients can reversibly open the BBB. For instance, in a rat model, oleic acid, a protein kinase C activator, demonstrated reversible BBB opening to Evans blue albumin and γ-aminoisobutyric acid when administered by arterial infusion (Sztriha and Betz 1991). Sodium dodecyl sulfate (SDS) is a commonly used medicinal excipient that has been demonstrated to elicit reversible and dose-dependent changes in BBB permeability in a rat model (Saija et al. 1997).
Physical stimulation
In comparison to the abovementioned two approaches, it is safer to use physical stimulation approaches, such as focused ultrasound (FUS), microwaves, lasers, and magnetic fields. Mechanical or thermal stimulation increases the space between the capillary endothelial cells of the brain (Chen et al. 2017). Destruction of the BBB can increase the accumulation of therapeutic drugs in the brain; however, a damaged BBB can also allow toxins to penetrate the brain and cause neurological dysfunction. Thus, the ideal destruction approach for the BBB should be controllable, temporary, reversible, specific, and selective. By combining ultrasound with microbubbles (MBs), ultrasound targeted microbubble destruction can open the BBB noninvasively and reversibly (Hynynen et al. 2001). Upon exposure to sonication, MBs begin to oscillate at a frequency similar to US. During stable cavitation, mechanical stress will be generated, resulting in additional disruption of the tight junctions and increased permeability of the BBB (Lee et al. 2017). It should be noted, however, that inertial cavitation might lead to MB collapse with microjetting, fragmentation and shock-wave generation, which may cause harm to the vascular endothelial cells (Novell et al. 2020). Thus, it is generally considered that stable cavitation (applying a few hundred kPa) is the best way to open the BBB (Meng et al. 2019). In addition, FUS-induced medication delivery is now being investigated in GBM (Alli et al. 2018), Parkinson's disease (PD) (Karakatsani et al. 2019), and Alzheimer's disease (AD) (Weber-Adrian et al. 2019).
Nanocarrier-based chemotherapy therapy for glioma
Preclinical and clinical studies have demonstrated that many factors can affect the efficacy of combination therapy, resulting in disappointing clinical outcomes in brain tumor patients. The BBB appears to be the primary source of dissonance in GBM, and traditional chemotherapeutics are limited by their poor targeting of the tumor, short circulation times in vivo, inability to penetrate the tissue, and low therapeutic index. As nanotechnology progresses, researchers are developing various types of nanomedicine for delivering anti-brain tumor drugs, including polymer nanoparticles, micelles, liposomes, and inorganic nanoparticles based on the characteristics of brain tumor tissue and TME (Afshari et al. 2022). This approach offers high drug stability, sustained release potential, and low drug toxicity and is also known as the nanoparticle drug delivery system (NDDS).
Based on their different modes of action, NDDSs can be divided into active targeting NDDSs, passive targeting NDDSs, and environmentally responsive NDDSs (Fig. 4). Nanocarriers containing chemotherapeutic drugs can be transported primarily by carrier-mediated transport and receptor-mediated transport, as well as by adsorption-mediated transport and cell-mediated transport to cross the BBB and deliver therapeutic drugs directly to the site of the lesion to achieve effective treatment. Furthermore, exosome therapy has received extensive attention as a method for delivering drugs to the CNS. As an intercellular communication system, exosomes are natural nanoparticles secreted by human cells. They can promote the transfer of molecules between cells and have demonstrated good tolerance in human clinical trials. Wang (Pais et al. 2013) and coworkers constructed neutrophil exosome-based drug delivery systems in response to an inflammatory microenvironment in gliomas. It enhances the efficiency of drug delivery across the BBB and tumor targeting and provides a method for noninvasive drug delivery targeting in the CNS. TMZ is the first-line chemotherapy medication of choice for glioma patients. However, drug resistance limits the efficacy of TMZ and its clinical application. Thus, to reduce the resistance of glioma to TMZ, Hui Shi employed the copolymer angiopep-2 (A2)-modified polymeric micelle (A2PEC) as the nanocarrier, embedding TMZ in the core by intermolecular hydrophobic interactions, and a small interfering RNA (SiPLK1) was electrostatically complexed with the cationic layer (called TMZ-A2PEC/siPLK1). Their results revealed that TMZ-loaded nanocomplexes were delivered across the BBB and to glioma cells with remarkable efficiency. The TMZ-loaded nanocomplex can improve TMZ sensitivity and significantly inhibit glioma growth (Fig. 5a) (Shi et al. 2020). Among the different types of nanoparticles, liposomes have a unique history of successful clinical translation as drug and gene delivery vectors. Recently, it has become widely accepted that nanoparticles can recruit biomolecules from biological fluids, such as blood, and form a complex layer around the nanoparticles called the 'biomolecular corona (BC)'. Antonietta Arcella and colleagues synthesized a set of TMZ-encapsulating nanomedicines made of four cationic liposomes. Their research determined that the form of liposome-BC showing high levels of targeting fingerprints could enhance the ability to cross the BBB and anticancer efficacy (Fig. 5b) (Arcella et al. 2018). These results indicate that therapeutic molecules can be delivered through the blood–brain barrier by adjusting the surface chemical and biological properties of nanoparticles, and by selecting different nanocarriers for specific cell types.

Adapted from Ref. (Shi et al. 2020) with permission from Dove Medical Press copyright 2020; adapted from ref. (Arcella et al. 2018) with permission from the American Chemical Society copyright 2018
Nanocarrier strategy enhances chemotherapy in glioma. a The TMZ-A2PEC/siPLK1 drug formation and delivery system (Shi et al. 2020). b Design liposomes employing plasma proteins most likely to bind blood–brain barrier receptors to exploit the biomolecular corona (BC) in targeted brain drug delivery (Arcella et al. 2018).
Combined therapy for glioma
Although researchers have investigated various approaches to improve the efficiency of chemotherapy, their defects make eliminating glioma difficult. Combination chemotherapy and other therapies can achieve significant synergistic effects in tumor treatment (chemodynamic therapy (CDT), sonodynamic therapy (SDT), immunotherapy, and photodynamic therapy (PDT)), achieving a synergistic anticancer effect that reduces toxicity and visibly enhances the elimination of glioma (Table 2).
Nanomedicine chemotherapy combined with gene therapy
Gene therapy has attracted considerable media attention as a new approach to treating brain tumors. It is based on introducing exogenous genes into target cells using vectors to achieve the desired therapeutic effect (Arnone et al. 2021). However, achieving effective and safe gene delivery and expression for gene therapy development remains challenging. When performing gene therapy for gliomas, it is crucial to choose the right carrier. Although viral vectors are the most effective approach for gene delivery, their use is limited due to their low safety, limited DNA loading capacity, complex assembly, and high cost. Nonviral vectors are relatively safe, but their transfection efficiency is very low (Caffery et al. 2019). Thus, the direction and focus of future research will be on how to enhance the carrier’s delivery capability and security. Nanomaterials offer numerous advantages, including low immunogenicity, high volume, high transfection efficiency, ease of production, and low cost, making them excellent candidates for gene delivery vectors. Wang et al. employed polyethyleneimine (PEI) and biodegradable poly[(poly(2-diisopropylamino/2-mercaptoethylamine) ethyl aspartate], (PAsp (DIP/MEA)) to construct a diblock copolymer [PEI-PAsp(DIP/MEA)]. Then, nanovesicles were assembled for the delivery of the cytosine deaminase (CD) gene and 5-fluorocytosine (pCMVCD/5-FC) into glioma cells (Fig. 6a) (Wang et al. 2014). This nanovesicle is a multifunctional nanovesicle carrier that is pH and reduction sensitive, and the nanovesicles can be highly effective in cotransferring genes and drugs when the particle size and surface charge are controlled appropriately. By effective transfection and expression of the CD gene, cytosine deaminase transforms the nontoxic prodrug 5-FC into its toxic active metabolite 5-fluorouracil, which can destroy RNA and DNA synthesis, thus inhibiting tumor cell growth. Zhang and coworkers successfully developed gene therapy-based iron oxide nanoparticles (FA/Pt-siGPX4@IONPs) with folate (FA) encapsulated on their surface, and the drugs were found to show selective glioblastoma cell targeting effects both in vitro and in vivo. A substantial increase in iron levels (Fe2+ and Fe3+) is observed after IONPs are injected intracellularly, causing Fenton reactions between Fe2+, Fe3+, and intracellular H2O2, creating reactive oxygen species (ROS) that trigger ferroptosis. Pt is responsible for damaging DNA, resulting in apoptosis, while the coreleased si-GPX4 inhibited GPX4 expression and improved the efficiency of ferroptosis (Fig. 6b) (Zhang et al. 2020a). The effectiveness of this approach opens the way for designing more effective and personalized GBM combination therapy.
Nanomedicine chemotherapy combined with immunotherapy
Immunotherapy, a type of treatment that involves stimulating the host's immune system to kill cancer cells while inhibiting their escape from the immune system, has shown significant success in treating various solid cancers. Clinical applications of immunotherapy offer significant benefits to cancer patients. Thus, immunotherapy has been extensively investigated for treating glioblastoma. However, preclinical investigations using immune checkpoint inhibitors (Zeng et al. 2013), dendritic cell vaccines (Mitchell et al. 2015), and chimeric antigen receptor T cells have shown significant immune responses in animal models (Weiss et al. 2018). Several phase III clinical trials failed to achieve corresponding OS benefits.(Reardon et al. 2020; Wu et al. 2021) These results may be related to the intrinsic characteristics of GBM cells (loss of neoantigens targeting therapeutic T cells) (Jackson et al. 2014) and extrinsic mechanisms (systemic immunological suppression) compromising the effects of immunotherapy (Wu et al. 2021). Additionally, current methods for delivering neoantigens, such as injecting neoantigen peptides with adjuvants (such as oil emulsions), result in precipitation, accumulation, and inflammation at the injection site, reducing lymphatic drainage; due to reduced immune tolerance (Janjua et al. 2021). Thus, new approaches to improve antigen-presenting cell delivery to lymphoid tissues are required to achieve effective antitumor immunity. Scheetz et al. developed synthetic high-density lipoprotein (SHDL)-based protein immunotherapy vectors that loaded cytosine guanine (CpG) and tumor-specific neoantigens that targeted GBM and caused immune-mediated tumor regression in mice (Fig. 7a) (Scheetz et al. 2020). Their findings demonstrated that the combination of neoantigen peptide sHDL/CpG and anti-PD-L1 monoclonal antibody induced a specific T cell response, resulting in the regression of in situ GL261 gliomas in 33% of mice and achieving long-term survival and immune memory. Ruan and coworkers proposed a combination therapy employing gold nanoparticles (AuNPs) that included chemotherapy, autophagy inhibition, and PD-L1 immunosuppressive agents (Fig. 7b) (Ruan et al. 2019). Specifically, doxorubicin (DOX) and the autophagy inhibitor hydroxychloroquine (HCQ) were coloaded onto pod protease-responsive aggregable gold nanoparticles (AuNPs-D&H-A-A&C). However, D&H-A-A&C can improve DOX and HCQ accumulation at the glioma tumor site after administration and can inhibit DOX-induced autophagy through HCQ and destroy the protective autophagy flow of cells, reducing glioma cells resistance to DOX. However, intraperitoneal injection of PD-L1 monoclonal antibody can block the immunosuppressive pathway, enhance the immunogenicity of anti-GBM, and limit the secretion of immunosuppressive factors and the differentiation of immunosuppressive cell. According to the study’s in vivo results, the treatment regimen dramatically increased the therapeutic efficacy of GBM in mice, enhanced immunity and memory, and reduced the likelihood of GBM recurrence. In summary, the combination of chemotherapy and immunotherapy uses the development of nanotechnology incorporating chemotherapy drugs with or without anti-PD-L1 immune checkpoint blockade. Compared to traditional immunotherapies, it realizes the precise release of antibodies while reducing the dosage of chemotherapy drugs, facilitating the improvement of the immune system and alleviating systemic side effects.
Nanomedicine chemotherapy combined with sonodynamic therapy
Generally, it is accepted that ultrasound (US) is a mechanical vibration wave of objects capable of penetrating bulky tissues, and it is used in various clinical applications (Ning et al. 2022; Guo et al. 2023). Sonodynamic therapy (SDT) is a new cancer treatment approach that employs US to focus sound energy on deep tissue and then activates acoustic sensitizers to transform oxygen-containing media into various ROS [primarily singlet oxygen (1O2)], cavitation bubble effects, and thermal effects to eradicate malignant tumor cells and has been rapidly developed as an alternative treatment for tumors (Qian et al. 2017; Chen et al. 2021; Ji et al. 2021). SDT has unique advantages in the treatment of glioma because of the strong penetration depth of ultrasound.(Wang et al. 2020a, 2020b) For GBM, the BBB’s existence limits suitable chemotherapy agents, and US can open the BBB noninvasively and reversibly increase the uptake of chemotherapeutic agents by cancer cells (Hynynen et al. 2001). DOX is one of the numerous anthracycline antitumor drugs available; however, it is difficult for Dox to pass through the BBB, so it is usually treated by intratumoral injection, resulting in poor drug compliance and a high risk for patients. In Wang et al.'s research, a US-activatable porphyrin-phospholipid liposome (pp-lipo) incorporating DOX was effectively synthesized (Fig. 8a) (Wang et al. 2018). Their research demonstrated that the assistance of low-intensity focused ultrasound enhances the uptake of the nanomedicine DOX-pp-lipo by tumor cells while also enhancing the nuclear uptake and concentration of DOX in nuclear sites and the cytosol. Wu et al. effectively synthesized IR780/PTL nanoparticles (NPs) by self-assembly, conjugating the ROS-responsive thioketal linkers to the paclitaxel (PTX) prodrug (PTX-TL, denoted as PTL) and the sonosensitizer IR780 was loaded into the hydrophobic core, which can be cleaved by ROS and induce paclitaxel release during the SDT process (Fig. 8b) (Wang et al. 2014). ROS can be generated by the IR780/PTL-NPs during US irradiation (1 MHz, 0.2–0.4 W/cm−2, 3 min), thus triggering apoptosis of U87 cells and enhancing the release of PTX directly via ROS-sensitive TL degradation. Additionally, US irradiation was employed to achieve a controlled release of PTX at the tumor sites (1 MHz, 0.4 W/cm−2, 3 min). Furthermore, tumor growth was considerably diminished without any visible side toxicity. Overall, the combination of chemotherapy with SDT has the potential to be a pivotal approach for the successful ablation of GBMs because of the combined effects of chemosensitivity improvement and SDT-induced apoptosis.
Nanomedicine chemotherapy combined with chemodynamic therapy
CDT is an emerging cancer treatment approach that treats cancer using CDT agents (e.g., Fe2+, Mn2+, Cu2+, and Ti3+ ions) to produce hydroxyl radicals (·OH) from hydrogen peroxide (H2O2) in a manner similar to Fenton or Fenton-like reactions, with excellent therapeutic effects and high biosafety (Tang et al. 2019b). CDT causes oxidative stress in tumor cells that destroys proteins, lipids, and other biomolecular substances, eventually killing them (Zhang et al. 2016a). However, transforming CDT into clinical therapy remains a challenge. First, the high PH of tumor lesions hinders Fenton/Fenton-like reactions; second, the highly expressed reducing substances in the TME reduce CDT efficacy (such as glutathione and GSH). Due to its inherent limitations, CDT alone cannot be employed to eliminate malignant tumors. Thus, CDT combined with other therapeutic approaches has been extensively developed.
For example, Tan et al.(Tan et al. 2020) developed a double-targeted nanodrug (iRPPA@TMZ/MnO) by encapsulating TMZ with the cationic liposome surface modification internalizing arginine-glycine-aspartic acid (iRGD), which can help nanoparticles cross the BBB in addition to targeting tumor cells. iRPPA@TMZ/MnO can accumulate in gliomas and release TMZ and O2 under TME stimulation, thus inhibiting the growth of glioma in situ, reducing tumor hypoxia, lowering the drug resistance of glioma, and effectively enhancing the therapeutic effect (Fig. 9a). To combine chemotherapy with CDT to treat glioma, Mu et al. methodically prepared a tumor-specific metal tea polyphenol-based cascade nanoreactor (DOX@MTP/HA-EGCG) for chemodynamic therapy-enhanced chemotherapy (Mu et al. 2021). Based on an in vitro BBB model, investigators discovered that DOX@MTP/HA-EGCG penetrated the BBB and delivered the drug to the tumor. A nanoreactor infiltrates intracellularly via the CD44 receptor and leaves the lysosome to offer sustained release of DOX, Fe3+, and epigallocatechin-3-gallate (EGCG), resulting in chemotherapeutic effects and CDT. The results of in vivo experiments demonstrated that DOX@MTP/HA-EGCG accumulates selectively in CD44-overexpressing GL261 tumor sites and that the continuous release of DOX and Fe3+ leads to various treatment outcomes (Fig. 9b). The combination of CDT therapy can transform the TME’s acidic condition into the primary treatment factor and improve the therapeutic effect on tumors. Additionally, these results showed a viable approach and application prospect of these nanodrugs for enhancing the efficacy of glioma chemotherapy.
Nanomedicine chemotherapy combined with photodynamic therapy
There has been considerable study of PDT as an adjuvant treatment for GBM for more than 35 years, with many clinical trials conducted during this period (Eljamel et al. 2008; Muller and Wilson 2006). Phototherapy is an effective tool in fighting tumors due to its focus, noninvasive nature, and few side effects (Sun et al. 2016; Mao et al. 2016; Wen et al. 2016; Jiang et al. 2017, 2018a, 2018b; Zhang et al. 2016b, 2018; Ge et al. 2014; Idris et al. 2012; Huang et al. 2019). PDT eliminates tumors by producing singlet oxygen through a reaction between photosensitizers that accumulate in tumor cells and specific light wavelengths.(Chu et al. 2022) A long-wavelength NIR-II laser (1000–1700 nm) has deep penetration and high skin exposure and is suitable for intracranial tumors. However, traditional PDT relies on O2 and laser irradiation; hypoxia is a well-known feature of the TME that limits the generation of fatal ROS during PDT treatment (Liu et al. 2020). In a recent study, Zhu et al. combined NIR-II PDT and chemotherapy for GBM treatment and innovatively proposed the construction of the mesoporous nanosystem RBT@MRN-SS-Tf/Apt to improve the therapeutic potential of gliomas (Fig. 10a) (Zhu et al. 2018). The transferrin (Tf) molecule and aptamer AS1411 (Apt) are immobilized on the nanoparticles surface via redox-cleavable disulfide bonds, allowing the nanoparticles to penetrate the BBB and target the glioma effectively. Additionally, the antitumor drug [Ru(bpy)2(tip)]2+(RBT) produces ROS under 808 nm laser irradiation, inducing apoptosis of tumor cells. Furthermore, Tang and colleagues reported a pH-responsive magnetic mesoporous silica-based nanoplatform for synergistic photodynamic/chemotherapy (Fig. 10b) (Tang et al. 2018). Covering the silicon surface with polydopamine molecules prevented the leakage of DOX under physiological circulation conditions (pH 7.4), and the structure revealed continuous release behavior under low pH conditions (consistent with the acidic microenvironment of the tumor). Additionally, this nanoplatform could be guided to the tumor region under magnetic field navigation, resulting in a higher level of accumulation at the tumor.
Thus, chemo-PDT has a synergistic effect, which helps the release of drugs into tumor tissues, improves the therapeutic effect, and generates new approaches for treating gliomas.
Nanomedicine chemotherapy combined with photothermal therapy
Photothermal therapy (PTT) is a hyperthermia approach that uses photothermal agents to generate sufficient heat under NIR light illumination to ablate solid tumors, and it has attracted considerable attention from researchers.(Li et al. 2023b; Ma et al. 2022; Chen et al. 2022) PTT offers a direct and accurate approach to conducting noninvasive local treatment using the tunable and well-focused properties of incident light (Liu et al. 2019; Zeng et al. 2018). Despite some encouraging results, there are still numerous challenges to overcome when applying PTT to the clinic. For example, NIR light has a limited penetration depth, resulting in incomplete ablation of tumors outside its irradiation range.(Ruan et al. 2021; Chen et al. 2020) Furthermore, high-power lasers can cause unavoidable damage to normal tissues near the tumor, whereas low-power lasers may not prevent tumor recurrence.(Wang et al. 2020c) When PTT is combined with chemotherapy, hyperthermia from PTT can enhance the release of chemotherapy drugs and improve the sensitivity to chemotherapy (Zhang et al. 2020b).
Wang et al. developed a multifunctional bionic nanoplatform to deliver targeted photothermal–chemotherapy treatment for gliomas using a photothermal–chemotherapy device to overcome the inefficiency of traditional chemotherapy treatments caused by drug tolerance, nontargeting, and the blood–brain barrier (Ren et al. 2022). The photosensitizer graphene quantum dots (GQDs) and DOX encapsulated in a homotypic cancer cell membrane (CCM) make up the nanoplatform (GQDs/DOX@CCM). The outer membrane of GQDs/DOX@CCM can be destroyed in response to external laser stimulation, resulting in the fast release of DOX and GQDs in glioma cells for chemo-photothermal therapy. In addition to their ability to convert near-infrared light into heat, GQDs also improved chemotherapy by releasing DOX, and synergistic tumor elimination effect was achieved with good biosafety (Fig. 11a). Lu et al. developed a multicomponent self-assembled nanocomposite with an extremely high drug loading capacity to achieve synergistic chemotherapy–photothermal treatment of intracranial glioblastoma (Fig. 11b) (Lu et al. 2021). By electrostatic interaction, positively charged Angiopep-2-modified PEGylated poly-L-lysine (Ang-PEG-g-PLL) was coated on negatively charged two-component nanoparticles, which enhanced BBB penetration and tumor targeting. Furthermore, the nanocomposite shows good biocompatibility and high accumulation ability. Related experimental results revealed that glioma growth is significantly suppressed both in vitro and in vivo after treatment with the nanocomposite.

Adapted from ref. (Ren et al. 2022) with permission from PMC copyright 2022; adapted from ref. (Lu et al. 2021) with permission from Elsevier Ltd. copyright 2021
Chemotherapy combined with photothermal therapy. a Design and application of the GQDs/DOX@C for tumor-targeted chemo-photothermal combination therapy of glioma (Ren et al. 2022). b Illustration of self-assembled nanomaterials for orthotopic glioblastoma chemotherapy and photothermal therapy (Lu et al. 2021).
Overall, the results obtained above demonstrate that combining chemotherapy with PTT may offer novel treatment approaches for chemoresistant tumors and that the development of photothermal therapy combined with chemotherapy will be a potential treatment for glioma.
Nanomedicine chemotherapy combined with magnetic hyperthermia therapy
The oxygen supply in tumor tissue is insufficient due to the poor development of vessels and nerves, resulting in poor heat dissipation function, and it is more sensitive to temperatures of 41℃–45℃, whereas normal cells can tolerate higher temperatures. Magnetic hyperthermia is an emerging approach employed for treating deep tumors by implanting magnetic nanomaterials into tumor tissue and increasing the local temperature of tumor tissue (above 42℃) using an external alternating magnetic field, which has been studied since the 1950s (Gilchrist et al. 1957; Gordon et al. 1979). Superparamagnetic nanoparticles are single-domain magnetic nanostructures with good magnetic susceptibility and are usually employed as magnetic response nanocarriers that can be guided and targeted by an external magnetic field. Superparamagnetic iron oxide nanoparticles are magnetic nanostructures with outstanding biocompatibility. Based on these nanostructures, Attilio et al. developed a multifunctional nanocarrier for drug delivery and hyperthermia therapy to eliminate GBM cells (Marino et al. 2019). Guided by the external magnetic field, the multifunctional nanoplatform is targeted and accumulates at the tumor site. With chronic alternating magnetic field stimulation, the increase in temperature can slow the release of TMZ, resulting in controlled release and effective tumor cell elimination (Fig. 12a). Additionally, Afzalipour and coworkers developed a new approach based on MH and chemotherapy synergistic treatment of glioma. A modified multiple-emulsion approach was employed to synthesize folic acid-loaded TMZ-coated magnetic polymer nanoparticles (TMZ/MNPs-FA) (Afzalipour et al. 2021). In vivo investigations with C6 tumor-bearing rats revealed that combining AFM hyperthermia with thermosensitive TMZ/MNPs-FA significantly influenced tumor suppression during the treatment process, thus prolonging the survival time of tumor-bearing mice after treatment (Fig. 12b). Thus, magnetic particles are heated up due to magnetic loss, causing a hyperthermia effect that can be used to heat tumors evenly and achieve tissue-targeted hyperthermia without being affected by the tumor’s size and location, which has a promising application when combined with chemotherapy.
Nanomedicine chemotherapy combined with starvation therapy
Warburg's effect suggests that tumor cells require glucose for growth, so tumor starvation therapy blocks the flow of oxygen and nutrients through tumor blood vessels, inhibiting tumor growth (Yang et al. 2019; Huo et al. 2017; Wang et al. 2020d). Regulating glucose levels, specifically, is increasingly considered a powerful means of starving cancer cells (Zhang et al. 2017; Liu et al. 2016). Thus far, numerous approaches have been proposed to destroy tumor cells by depriving them of glucose according to this special feature (Koppenol et al. 2011). However, since starvation therapy alone cannot eliminate tumor cells, it must be combined with other interventions, such as chemotherapy and radiotherapy, to enhance its effectiveness. Among them, the starvation-chemotherapy approach has been extensively investigated because of its exceptional efficacy. Gu et al. developed fibronectin extra domain B (EDB)-targeted peptide APTEDB-modified PEG-PLA nanoparticles (APT-NPs) for PTX-loaded nanoparticles (APT-NP-PTX), which have tumor angiogenesis and tumor cell dual-targeting functions and achieve starvation-chemotherapy for glioma (Gu et al. 2014). According to the findings, APT-NP-PTX could effectively inhibit tumor angiogenesis and enhance the apoptosis-inducing activity of PTX (Fig. 13a). Similarly, in Ke's research, a multifunctional bioreactor for starvation-chemotherapy was constructed by embedding glucose oxidase (GOx) and DOX in metal–organic framework nanoparticles modified by targeting ligands (RGD) (referred to as RGD-mGZD) (Ke et al. 2022). With the uptake of RGD-mGZD by tumor cells, GOx performs fast glycolysis, consuming glucose and producing a hyperacidic microenvironment. The bioreactor’s decomposition could be accelerated under acidic conditions to improve the release of chemotherapy drugs (Fig. 13b). The growth of subcutaneous glioma is considerably suppressed in vivo after treatment with RGD-mGZD. Overall, this synergistic therapeutic approach shows potential for treating various cancers and provides a novel avenue for developing multimodal cancer treatment in a clinical setting.
Other multimodal treatments
Many multimodal therapy strategies have been documented to treat GBM as a result of the rapid advancement of therapeutic techniques for malignancies. Among these, the combination of three or more treatment strategies has emerged as a research hotspot and a means of enhancing the therapeutic efficacy of cancer treatments. Shuming Dong et al. developed an upconversion-mediated nanoplatform with a mesoporous ZnFe2O4 shell (noted as Y-UCSZ) for NIR light enhanced CDT and PDT. Due to the huge internal space of Y-UCSZ, it is highly beneficial for accommodating DOX for chemotherapy (Dong et al. 2019). Their results authenticated that the DOX-loaded nanocomplex has high anticancer effectiveness. Although HeLa cells are utilized in therapeutic and dialectical experiments, this also provides a concept for the treatment of glioma.
Conclusion and prospects
In the past few years, the treatment of brain tumors has improved tremendously due to the advancement of health care and treatment technology. However, the presence of the BBB and the high heterogeneity of brain tumors results in the insensitivity of brain tumors to chemotherapy drugs and poor treatment effects. The emergence of nanoparticle drug delivery systems offers a new approach to treating brain tumors. Unlike traditional therapies, polymer nanomedicine can improve chemotherapy by precisely adjusting a nanomedicine's size, shape, and surface-targeted ligand to overcome the blood–brain barrier, and nanomedicine achieves effective delivery. In the future, nanomaterials are expected to outperform existing cancer treatments, including surgery, chemotherapy, and radiation therapy. However, nanomaterials are still in the early stages of clinical application for brain tumors, and there is still a long way to go, since nanomaterials’ degradation behavior and excretion pathways are still unclear, and there are numerous differences among patients and difficulties in reproducing results.
In the clinical transformation process, nanodrugs still face challenges. For example, they must show good characteristics, biocompatibility, and biodegradability, be water-soluble or form colloids in water, extend cycle half-life, and keep up with FDA requirements. However, the innovative development of multifunctional brain-targeted nanomedicines with high BBB penetration and tumor selectivity remains a promising avenue. Nanomaterials’ controllable functional structures and precisely adjustable properties enable the integration of other therapeutic approaches, helping to develop new brain tumor drugs combined with chemotherapy, radiotherapy, photodynamic therapy, and gene therapy. Nanomedicine’s functional integration offers a viable idea for realizing multitarget synergistic therapy and enhancing the therapeutic effect on brain tumors. Additionally, for further research on nanodrugs against brain tumors, researchers must combine basic research and biosafety considerations when investigating and developing anti-brain tumor nanodrugs employing biodegradable materials that do not induce immunogenicity or biological toxicity. To ensure clinical efficacy and provide guarantees for the proper treatment of brain tumors, a unified preparation method was developed to make the obtained nanodrugs more uniform, stable, and controllable.
In conclusion, research on BBB-crossing nanomedicine for improving chemotherapy has shown that these nanomedicines have broad application prospects. However, there is still a long way to go for further clinical transformation in the future.
Availability of data and materials
Not applicable.
References
Afshari AR, Motamed-Sanaye A, Sabri H, Soltani A, Karkon-Shayan S, Radvar S, Javid H, Mollazadeh H, Sathyapalan T, Sahebkar A (2021) Neurokinin-1 receptor (NK-1R) antagonists: potential targets in the treatment of glioblastoma multiforme. Curr Med Chem 28(24):4877–4892
Afshari AR, Sanati M, Mollazadeh H, Kesharwani P, Johnston TP, Sahebkar A (2022) Nanoparticle-based drug delivery systems in cancer: a focus on inflammatory pathways. Semin Cancer Biol 86(Pt 2):860–872
Afzalipour R, Khoei S, Khoee S, Shirvalilou S, Raoufi NJ, Motevalian M, Karimi MY (2021) Thermosensitive magnetic nanoparticles exposed to alternating magnetic field and heat-mediated chemotherapy for an effective dual therapy in rat glioma model. Nanomedicine 31:102319
Alli S, Figueiredo CA, Golbourn B, Sabha N, Wu MY, Bondoc A, Luck A, Coluccia D, Maslink C, Smith CJ (2018) Brainstem blood brain barrier disruption using focused ultrasound: a demonstration of feasibility and enhanced doxorubicin delivery. J Control Release 281:29–41
Ameratunga M, Pavlakis N, Wheeler H, Grant R, Simes J, Khasraw M (2018) Anti-angiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008218.pub4
Amin MR, Ali DW (2019) Pharmacology of medical cannabis. Adv Exp Med Biol 1162:151–165
Arcella A, Palchetti S, Digiacomo L, Pozzi D, Capriotti AL, Frati L, Oliva MA, Tsaouli G, Rota R, Screpanti I, Mahmoudi M, Caracciolo G (2018) Brain targeting by liposome-biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells. ACS Chem Neurosci 9(12):3166–3174
Arnone CM, Polito VA, Mastronuzzi A, Carai A, Diomedi FC, Antonucci L, Petrilli LL, Vinci M, Ferrari F, Salviato E, Scarsella M, De Stefanis C, Weber G, Quintarelli C, De Angelis B, Brenner MK, Gottschalk S, Hoyos V, Locatelli F, Caruana I, Del Bufalo F (2021) Oncolytic adenovirus and gene therapy with EphA2-BiTE for the treatment of pediatric high-grade gliomas. J Immunother Cancer. https://doi.org/10.1136/jitc-2020-001930
Beauchesne P (2012) Fotemustine: a third-generation nitrosourea for the treatment of recurrent malignant gliomas. Cancers (basel) 4(1):77–87
Beckta JM, Bindra RS, Chalmers AJ (2019) Targeting DNA repair in gliomas. Current Opinion Neurology 32(6):878–885
Bisch SP, Sugimoto A, Prefontaine M, Bertrand M, Gawlik C, Welch S, McGee J (2018) Treatment tolerance and side effects of intraperitoneal carboplatin and dose-dense intravenous paclitaxel in ovarian cancer. J Obstet Gynaecol Can 40(10):1283-1287.e1
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Bucci MK, Maity A, Janss AJ, Belasco JB, Fisher MJ, Tochner ZA, Rorke L, Sutton LN, Phillips PC, Shu HK (2004) Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: results from a single center in the magnetic resonance imaging era. Cancer 101(4):817–824
Caffery B, Lee JS, Alexander-Bryant AA (2019) Vectors for glioblastoma gene therapy: viral & non-viral delivery strategies. Nanomaterials (basel) 9(1):105
Chen Y, Liu L (2012) Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev 64(7):640–665
Chen H, Zhang W, Zhu G, Xie J, Chen X (2017) Rethinking cancer nanotheranostics. Nat Rev Mater. https://doi.org/10.1038/natrevmats.2017.24
Chen TC, da Fonseca CO, Schonthal AH (2018) Intranasal perillyl alcohol for glioma therapy: molecular mechanisms and clinical development. Int J Mol Sci 19(12):3905
Chen B, Zhang C, Wang W, Chu Z, Zha Z, He X, Zhou W, Liu T, Wang H, Qian H (2020) Ultrastable AgBiS2 hollow nanospheres with cancer cell-specific cytotoxicity for multimodal tumor therapy. ACS Nano 14(11):14919–14928
Chen W, Liu C, Ji X, Joseph J, Tang Z, Ouyang J, Xiao Y, Kong N, Joshi N, Farokhzad OC, Tao W, Xie T (2021) Stanene-based nanosheets for beta-elemene delivery and ultrasound-mediated combination cancer therapy. Ange Chem Int Ed 60(13):7155–7164
Chen S, Chu Z, Cao L, Xu L, Jin Q, Liu N, Chen B, Fang M, Wang W, Qian H, Shao M (2022) Antibacterial mechanism and transcriptomic analysis of a near-infrared triggered upconversion nanoparticles@AgBiS2 for synergetic bacteria-infected therapy. Nano Res 15(10):9298–9308
Cheng W, Zeng X, Chen H, Li Z, Zeng W, Mei L, Zhao Y (2019) Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 13(8):8537–8565
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. New England Journal of Medcine 370(8):709–722
Chu Z, Tian T, Tao Z, Yang J, Chen B, Chen H, Wang W, Yin P, Xia X, Wang H (2022) Upconversion nanoparticles@ AgBiS2 core-shell nanoparticles with cancer-cell-specific cytotoxicity for combined photothermal and photodynamic therapy of cancers. Bioact Mater 17:71–80
Cohen K, Weizman A, Weinstein A (2019) Positive and negative effects of cannabis and cannabinoids on health. Clin Pharmacol Ther 105(5):1139–1147
Dai X, Shao Y, Tian X, Cao X, Ye L, Gao P, Cheng H, Wang X (2022) Fusion between glioma stem cells and mesenchymal stem cells promotes malignant progression in 3D-bioprinted models. ACS Appl Mater Interfaces 14(31):35344–35356
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Dilruba S, Kalayda GV (2016) Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol 77(6):1103–1124
Ding S, Khan AI, Cai X, Song Y, Lyu Z, Du D, Dutta P, Lin Y (2020) Overcoming blood-brain barrier transport: advances in nanoparticle-based drug delivery strategies. Mater Today (kidlington) 37:112–125
Dong S, Xu J, Jia T, Xu M, Zhong C, Yang G, Li J, Yang D, He F, Gai S, Yang P, Lin J (2019) Upconversion-mediated ZnFe(2)O(4) nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem Sci 10(15):4259–4271
Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, McAllister LD, Bubalo JS, Kraemer DF, Fortin D, Nixon R, Muldoon LL, Neuwelt EA (2000) Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 88(3):637–647
Eljamel MS, Goodman C, Moseley H (2008) ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci 23(4):361–367
Fox LE (2000) Carboplatin. J Am Anim Hosp Assoc 36(1):13–14
Ganipineni LP, Danhier F, Preat V (2018) Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J Control Release 281:42–57
Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, Jia Q, Niu G, Huang X, Zhou H, Meng X, Wang P, Lee CS, Zhang W, Han X (2014) A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat Commun 5:4596
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. New England Journal of Medcine 370(8):699–708
Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB (1957) Selective inductive heating of lymph nodes. Ann Surg 146(4):596–606
Gordon RT, Hines JR, Gordon D (1979) Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypotheses 5(1):83–102
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, NABTT CNS Consortium (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16(8):2443–2449
Gu G, Hu Q, Feng X, Gao X, Menglin J, Kang T, Jiang D, Song Q, Chen H, Chen J (2014) PEG-PLA nanoparticles modified with APTEDB peptide for enhanced anti-angiogenic and anti-glioma therapy. Biomaterials 35(28):8215–8226
Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H (2011) Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32(31):8010–8020
Guo Q-L, Dai X-L, Yin M-Y, Cheng H-W, Qian H-S, Wang H, Zhu D-M, Wang X-W (2022a) Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: current progress and future perspectives. Mil Med Res 9(1):26
Guo Q, Yin M, Fan J, Yang Y, Liu T, Qian H, Dai X, Wang X (2022b) Peroxidase-mimicking TA-VOx nanobranches for enhanced photothermal/chemodynamic therapy of glioma by inhibiting the expression of HSP60. Mater Des 224:111366
Guo W, Wang T, Huang C, Ning S, Guo Q, Zhang W, Yang H, Zhu D, Huang Q, Qian H, Wang X (2023) Platelet membrane-coated C-TiO2 hollow nanospheres for combined sonodynamic and alkyl-radical cancer therapy. Nano Res 16(1):782–791
Guzman M (2003) Cannabinoids: potential anticancer agents. Nat Rev Cancer 3(10):745–755
Harvey KA, Xu Z, Saaddatzadeh MR, Wang H, Pollok K, Cohen-Gadol AA, Siddiqui RA (2015) Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines. J Neurosurg 122(3):547–556
Hsu JF, Chu SM, Liao CC, Wang CJ, Wang YS, Lai MY, Wang HC, Huang HR, Tsai MH (2021) Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: an update. Cancers Basel 13(2):195
Huang Q, Zhang S, Zhang H, Han Y, Liu H, Ren F, Sun Q, Li Z, Gao M (2019) Boosting the radiosensitizing and photothermal performance of Cu2- xSe nanocrystals for synergetic radiophotothermal therapy of orthotopic breast cancer. ACS Nano 13(2):1342–1353
Huo M, Wang L, Chen Y, Shi J (2017) Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun 8(1):357
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology 220(3):640–646
Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y (2012) In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nature Medicne 18(10):1580–1585
Iyer PG, Albini TA (2021) Drug-related adverse effects of antivascular endothelial growth factor agents. Curr Opin Ophthalmol 32(3):191–197
Jackson CM, Lim M, Drake CG (2014) Immunotherapy for brain cancer: recent progress and future promise. Clin Cancer Res 20(14):3651–3659
Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, Kumeria T, Ziegler DS, Kavallaris M, Mazzieri R, Popat A (2021) Frontiers in the treatment of glioblastoma: past, present and emerging. Adv Drug Deliv Rev 171:108–138
Ji X, Ge L, Liu C, Tang Z, Xiao Y, Chen W, Lei Z, Gao W, Blake S, De D, Shi B, Zeng X, Kong N, Zhang X, Tao W (2021) Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat Commun 12(1):1124
Jia L, Tian Y, Chen Y, Zhang G (2018) The silencing of LncRNA-H19 decreases chemoresistance of human glioma cells to temozolomide by suppressing epithelial-mesenchymal transition via the Wnt/beta-Catenin pathway. Onco Targets Ther 11:313–321
Jiang X, Zhang S, Ren F, Chen L, Zeng J, Zhu M, Cheng Z, Gao M, Li Z (2017) Ultrasmall magnetic CuFeSe2 ternary nanocrystals for multimodal imaging guided photothermal therapy of cancer. ACS Nano 11(6):5633–5645
Jiang X, Han Y, Zhang H, Liu H, Huang Q, Wang T, Sun Q, Li Z (2018a) Cu-Fe-Se ternary nanosheet-based drug delivery carrier for multimodal imaging and combined chemo/photothermal therapy of cancer. ACS Appl Mater Interfaces 10(50):43396–43404
Jiang Y, Li J, Zhen X, Xie C, Pu K (2018b) Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Adv Mater 30(14):e1705980
Jiang X, Tan J, Wen Y, Liu W, Wu S, Wang L, Wangou S, Liu D, Du C, Zhu B, Xie D, Ren C (2019) MSI2-TGF-beta/TGF-beta R1/SMAD3 positive feedback regulation in glioblastoma. Cancer Chemother Pharmacol 84(2):415–425
Karakatsani ME, Wang S, Samiotaki G, Kugelman T, Olumolade OO, Acosta C, Sun T, Han Y, Kamimura HA, Jackson-Lewis VJ (2019) Amelioration of the nigrostriatal pathway facilitated by ultrasound-mediated neurotrophic delivery in early Parkinson’s disease. J Control Release 303:289–301
Ke R, Zhen X, Wang HS, Li L, Wang H, Wang S, Xie X (2022) Surface functionalized biomimetic bioreactors enable the targeted starvation-chemotherapy to glioma. J Colloid Interface Sci 609:307–319
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Kuang Y, Lackay SN, Zhao L, Fu ZF (2009) Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res 144(1–2):18–26
Lara-Velazquez M, Alkharboosh R, Norton ES, Ramirez-Loera C, Freeman WD, Guerrero-Cazares H, Forte AJ, Quinones-Hinojosa A, Sarabia-Estrada R (2020) Chitosan-based non-viral gene and drug delivery systems for brain cancer. Front Neurol 11:740
Lee H, Kim H, Han H, Lee M, Lee S, Yoo H, Chang JH, Kim H (2017) Microbubbles used for contrast enhanced ultrasound and theragnosis: a review of principles to applications. Biomed Eng Lett 7(2):59–69
Li J, Zhang Z, Zhang B, Yan X, Fan K (2023a) Transferrin receptor 1 targeted nanomedicine for brain tumor therapy. Biomaterials Science. https://doi.org/10.1039/D2BM02152H
Li G, Wu S, Chen W, Duan X, Sun X, Li S, Mai Z, Wu W, Zeng G, Liu H, Chen T (2023b) Designing intelligent nanomaterials to achieve highly sensitive diagnoses and multimodality therapy of bladder cancer. Small Methods 7(2):2201313
Liao W, Fan S, Zheng Y, Liao S, Xiong Y, Li Y, Liu J (2019) Recent advances on glioblastoma multiforme and nano-drug carriers: a review. Curr Med Chem 26(31):5862–5874
Liu H, Kurtoglu M, Leon-Annicchiarico CL, Munoz-Pinedo C, Barredo J, Leclerc G, Merchan J, Liu X, Lampidis TJ (2016) Combining 2-deoxy-D-glucose with fenofibrate leads to tumor cell death mediated by simultaneous induction of energy and ER stress. Oncotarget 7(24):36461–36473
Liu Y, Bhattarai P, Dai Z, Chen X (2019) Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev 48(7):2053–2108
Liu C, Liu B, Zhao J, Di Z, Chen D, Gu Z, Li L, Zhao Y (2020) Nd(3+) -Sensitized upconversion metal-organic frameworks for mitochondria-targeted amplified photodynamic therapy. Angew Chem Int Ed 59(7):2634–2638
Liu D, Dai X, Zhang W, Zhu X, Zha Z, Qian H, Cheng L, Wang X (2023a) Liquid exfoliation of ultrasmall zirconium carbide nanodots as a noninflammatory photothermal agent in the treatment of glioma. Biomaterials 292:121917
Liu D, Dai X, Ye L, Wang H, Qian H, Cheng H, Wang X (2023b) Nanotechnology meets glioblastoma multiforme: emerging therapeutic strategies. Wiley Interdiscip Rev Nanomed Nanobiotechnol 15(1):e1838
Lu L, Wang K, Lin C, Yang W, Duan Q, Li K, Cai K (2021) Constructing nanocomplexes by multicomponent self-assembly for curing orthotopic glioblastoma with synergistic chemo-photothermal therapy. Biomaterials 279:121193
Ma R, Taphoorn MJB, Plaha P (2021) Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry 92(10):1103–1111
Ma Y, Sun Y, Xu L, Li X, Gong D, Miao Z, Qian H (2022) Pseudocatalytic hydrogels with intrinsic antibacterial and photothermal activities for local treatment of subcutaneous abscesses and breast tumors. Adv Healthcare Mater 11(21):2201023
Mao F, Wen L, Sun C, Zhang S, Wang G, Zeng J, Wang Y, Ma J, Gao M, Li Z (2016) Ultrasmall biocompatible Bi2Se3 nanodots for multimodal imaging-guided synergistic radiophotothermal therapy against cancer. ACS Nano 10(12):11145–11155
Marino A, Camponovo A, Degl’Innocenti A, Bartolucci M, Tapeinos C, Martinelli C, De Pasquale D, Santoro F, Mollo V, Arai S, Suzuki M, Harada Y, Petretto A, Ciofani G (2019) Multifunctional temozolomide-loaded lipid superparamagnetic nanovectors: dual targeting and disintegration of glioblastoma spheroids by synergic chemotherapy and hyperthermia treatment. Nanoscale 11(44):21227–21248
Meng Y, Pople CB, Lea-Banks H, Abrahao A, Davidson B, Suppiah S, Vecchio LM, Samuel N, Mahmud F, Hynynen KJ (2019) Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. J Control Release 309:25–36
Messaoudi K, Clavreul A, Lagarce F (2015) Toward an effective strategy in glioblastoma treatment. Part I: Resistance Mechanisms and Strategies to Overcome Resistance of Glioblastoma to Temozolomide, Drug Discov Today 20(7):899–905
Mitchell DA, Batich KA, Gunn MD, Huang M-N, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A (2015) Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519(7543):366–369
Mu M, Chen H, Fan R, Wang Y, Tang X, Mei L, Zhao N, Zou B, Tong A, Xu J, Han B, Guo G (2021) A tumor-specific ferric-coordinated epigallocatechin-3-gallate cascade nanoreactor for glioblastoma therapy. J Adv Res 34:29–41
Muhammad P, Hanif S, Li J, Guller A, Rehman FU, Ismail M, Zhang D, Yan X, Fan K, Shi B (2022) Carbon dots supported single Fe atom nanozyme for drug-resistant glioblastoma therapy by activating autophagy-lysosome pathway. Nano Today 45:101530
Muller PJ, Wilson BC (2006) Photodynamic therapy of brain tumors–a work in progress. Lasers Surg Med 38(5):384–389
Ning S, Dai X, Tang W, Guo Q, Lyu M, Zhu D, Zhang W, Qian H, Yao X, Wang X (2022) Cancer cell membrane-coated C-TiO2 hollow nanoshells for combined sonodynamic and hypoxia-activated chemotherapy. Acta Biomater 152:562–574
Novell A, Kamimura H, Cafarelli A, Gerstenmayer M, Flament J, Valette J, Agou P, Conti A, Selingue E, Badin RA (2020) A new safety index based on intrapulse monitoring of ultra-harmonic cavitation during ultrasound-induced blood-brain barrier opening procedures. Sci Rep 10(1):1–12
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. https://doi.org/10.1093/neuonc/noab200
Pais V, Danaila L, Pais E (2013) Ultrastructural patterns of the activated cell death programs in the human brain. Ultrastruct Pathol 37(2):110–120
Parasramka S, Talari G, Rosenfeld M, Guo J, Villano JL (2017) Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst Rev 7(7):CD011773
Pardridge WM (2015) Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets 19(8):1059–1072
Qian X, Han X, Chen Y (2017) Insights into the unique functionality of inorganic micro/nanoparticles for versatile ultrasound theranostics. Biomaterials 142:13–30
Qiu ZK, Shen D, Chen YS, Yang QY, Guo CC, Feng BH, Chen ZP (2014) Enhanced MGMT expression contributes to temozolomide resistance in glioma stem-like cells. Chin J Cancer 33(2):115–122
Rawluk J, Waller CF (2018) Gefitinib. Recent Results Cancer Res 211:235–246
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr O (2020) Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol 6(7):1003–1010
Ren Y, Miao C, Tang L, Liu Y, Ni P, Gong Y, Li H, Chen F, Feng S (2022) Homotypic cancer cell membranes camouflaged nanoparticles for targeting drug delivery and enhanced chemo-photothermal therapy of glioma. Pharmaceuticals (basel) 15(2):157
Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, Weyerbrock A, Westermaier T, Senft C, Schucht P, Meyer B, Simon M, SN1 study group (2016) Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol 18(1):96–104
Ruan S, Xie R, Qin L, Yu M, Xiao W, Hu C, Yu W, Qian Z, Ouyang L, He Q, Gao H (2019) Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett 19(11):8318–8332
Ruan J, Liu H, Chen B, Wang F, Wang W, Zha Z, Qian H, Miao Z, Sun J, Tian T (2021) Interfacially engineered ZnxMn1–xS@polydopamine hollow nanospheres for glutathione depleting photothermally enhanced chemodynamic therapy. ACS Nano 15(7):11428–11440
Saija A, Princi P, Trombetta D, Lanza M, De Pasquale A (1997) Changes in the permeability of the blood-brain barrier following sodium dodecyl sulphate administration in the rat. Exp Brain Res 115(3):546–551
Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L (2016) Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 235:34–47
Scaringi C, De Sanctis V, Minniti G, Enrici RM (2013) Temozolomide-related hematologic toxicity. Onkologie 36(7–8):444–449
Scheetz L, Kadiyala P, Sun X, Son S, HassaniNajafabadi A, Aikins M, Lowenstein PR, Schwendeman A, Castro MG, Moon JJ (2020) Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas. Clin Cancer Res 26(16):4369–4380
Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2(9):494–503
Seo YE, Suh HW, Bahal R, Josowitz A, Zhang J, Song E, Cui J, Noorbakhsh S, Jackson C, Bu T, Piotrowski-Daspit A, Bindra R, Saltzman WM (2019) Nanoparticle-mediated intratumoral inhibition of miR-21 for improved survival in glioblastoma. Biomaterials 201:87–98
Shi H, Sun S, Xu H, Zhao Z, Han Z, Jia J, Wu D, Lu J, Liu H, Yu R (2020) Combined delivery of temozolomide and siPLK1 using targeted nanoparticles to enhance temozolomide sensitivity in glioma. Int J Nanomedicine 15:3347–3362
Sidaway P (2017) Brain cancer: Temozolomide improves outcomes. Nat Rev Clin Oncol 14(11):648
Strobel H, Baisch T, Fitzel R, Schilberg K, Siegelin MD, Karpel-Massler G, Debatin K-M, Westhoff M-A (2019) Temozolomide and other alkylating agents in glioblastoma therapy. Biomedicines 7(3):69
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Sun C, Wen L, Zeng J, Wang Y, Sun Q, Deng L, Zhao C, Li Z (2016) One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 91:81–89
Sztriha L, Betz AL (1991) Oleic acid reversibly opens the blood-brain barrier. Brain Res 550(2):257–262
Tam DY, Ho JW, Chan MS, Lau CH, Chang TJH, Leung HM, Liu LS, Wang F, Chan LLH, Tin C, Lo PK (2020) Penetrating the blood-brain barrier by self-assembled 3D DNA nanocages as drug delivery vehicles for brain cancer therapy. ACS Appl Mater Interfaces 12(26):28928–28940
Tan J, Duan X, Zhang F, Ban X, Mao J, Cao M, Han S, Shuai X, Shen J (2020) Theranostic nanomedicine for synergistic chemodynamic therapy and chemotherapy of orthotopic glioma. Adv Sci Weinh 7(24):2003036
Tang PA, Tsao MS, Moore MJ (2006) A review of erlotinib and its clinical use. Expert Opin Pharmacother 7(2):177–193
Tang XL, Jing F, Lin BL, Cui S, Yu RT, Shen XD, Wang TW (2018) pH-responsive magnetic mesoporous silica-based nanoplatform for synergistic photodynamic therapy/chemotherapy. ACS Appl Mater Interfaces 10(17):15001–15011
Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X (2019a) Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev 48(11):2967–3014
Tang Z, Liu Y, He M, Bu W (2019b) Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Ange Chem Int Ed 58(4):946–956
Thangudu S, Cheng FY, Su CH (2020) Advancements in the blood-brain barrier penetrating nanoplatforms for brain related disease diagnostics and therapeutic applications. Polymers (Basel) 12(12):3055
Tomar MS, Kumar A, Srivastava C, Shrivastava A (2021) Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim Biophys Acta Rev Cancer 188616
Valle-Argos B, Gomez-Nicola D, Nieto-Sampedro M (2010) Synthesis and characterization of neurostatin-related compounds with high inhibitory activity of glioma growth. Eur J Med Chem 45(5):2034–2043
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 19:1–12
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Wade M, Li YC, Wahl GM (2013) MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer 13(2):83–96
Wang L, Yuan Y, Lin S, Cheng D, Wang X, Jiang Q, Shuai X (2014) Co-delivery of 5-fluorocytosine and cytosine deaminase into glioma cells mediated by an intracellular environment-responsive nanovesicle. Polym Chem 5(15):4542–4552
Wang X, Yan F, Liu X, Wang P, Shao S, Sun Y, Sheng Z, Liu Q, Lovell JF, Zheng H (2018) Enhanced drug delivery using sonoactivatable liposomes with membrane-embedded porphyrins. J Control Release 286:358–368
Wang X, Zhong X, Bai L, Xu J, Gong F, Dong Z, Yang Z, Zeng Z, Liu Z, Cheng L (2020a) Ultrafine titanium monoxide (TiO1+x) nanorods for enhanced sonodynamic therapy. J Am Chem Soc 142(14):6527–6537
Wang X, Zhong X, Gong F, Chao Y, Cheng L (2020b) Newly developed strategies for improving sonodynamic therapy. Mater Horiz 7(8):2028–2046
Wang W-N, Pei P, Chu Z-Y, Chen B-J, Qian H-S, Zha Z-B, Zhou W, Liu T, Shao M, Wang H (2020c) Bi2S3 coated Au nanorods for enhanced photodynamic and photothermal antibacterial activities under NIR light. Chem Eng J 397:125488
Wang X, Zhong X, Liu Z, Cheng L (2020d) Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 35:100946
Wang Z, Zhang S, Zhang R, Chen X, Sun G, Zhou M, Han Q, Zhang B, Zhao Y, Jiang B, Yang Y, Yan X, Fan K (2021) Bioengineered dual-targeting protein nanocage for stereoscopical loading of synergistic hydrophilic/hydrophobic drugs to enhance anticancer efficacy. Adv Func Mater 31(29):2102004
Weber-Adrian D, Kofoed RH, Chan JWY, Silburt J, Noroozian Z, Kügler S, Hynynen K, Aubert IJT (2019) Strategy to enhance transgene expression in proximity of amyloid plaques in a mouse model of Alzheimer’s disease. Theranostics 9(26):8127
Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 9(5)
Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P (2018) NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Can Res 78(4):1031–1043
Wen L, Chen L, Zheng S, Zeng J, Duan G, Wang Y, Wang G, Chai Z, Li Z, Gao M (2016) Ultrasmall biocompatible WO3- x nanodots for multi-modality imaging and combined therapy of cancers. Adv Mater 28(25):5072–5079
Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L, Wu Y, Daldrup-Link HE (2021) Glioblastoma Multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res 171:105780
Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y (2019) Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization. Adv Mater 31(3):e1802244
Xue X (2019) Nanomedicine in brain diseases: principles and application. Springer, Cham
Yang B, Chen Y, Shi J (2019) Nanocatalytic medicine. Adv Mater 31(39):e1901778
Yin M, Chen X, Guo Q, Xiao L, Gao P, Zang D, Dong J, Zha Z, Dai X, Wang X (2022) Ultrasmall zirconium carbide nanodots for synergistic photothermal-radiotherapy of glioma. Nanoscale 14(40):14935–14949
Yong K, Kaleem S, Ma M, Lian X, Zhang Z (2022) Antiglioma natural products from the marine-associated fungus Penicillium sp. ZZ1750. Molecules 27(20):7099
Younis M, Faming W, Hongyan Z, Mengmeng T, Hang S, Liudi Y (2019) Iguratimod encapsulated PLGA-NPs improves therapeutic outcome in glioma, glioma stem-like cells and temozolomide resistant glioma cells. Nanomedicine 22:102101
Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 86(2):343–349
Zeng X, Luo M, Liu G, Wang X, Tao W, Lin Y, Ji X, Nie L, Mei L (2018) Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv Sci (weinh) 5(10):1800510
Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z, Zhang J, Yao H, Wang Z, Shi J (2016a) Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Ange Chem Int Ed 55(6):2101–2106
Zhang S, Sun C, Zeng J, Sun Q, Wang G, Wang Y, Wu Y, Dou S, Gao M, Li Z (2016b) Ambient aqueous synthesis of ultrasmall PEGylated Cu2-x Se nanoparticles as a multifunctional theranostic agent for multimodal imaging guided photothermal therapy of cancer. Adv Mater 28(40):8927–8936
Zhang C, Ni D, Liu Y, Yao H, Bu W, Shi J (2017) Magnesium silicide nanoparticles as a deoxygenation agent for cancer starvation therapy. Nat Nanotechnol 12(4):378–386
Zhang S, Huang Q, Zhang L, Zhang H, Han Y, Sun Q, Cheng Z, Qin H, Dou S, Li Z (2018) Vacancy engineering of Cu2-xSe nanoparticles with tunable LSPR and magnetism for dual-modal imaging guided photothermal therapy of cancer. Nanoscale 10(7):3130–3143
Zhang Y, Fu X, Jia J, Wikerholmen T, Xi K, Kong Y, Wang J, Chen H, Ma Y, Li Z, Wang C, Qi Q, Thorsen F, Wang J, Cui J, Li X, Ni S (2020a) Glioblastoma therapy using codelivery of cisplatin and glutathione peroxidase targeting siRNA from iron oxide nanoparticles. ACS Appl Mater Interfaces 12(39):43408–43421
Zhang C, Li D, Pei P, Wang W, Chen B, Chu Z, Zha Z, Yang X, Wang J, Qian H (2020b) Rod-based urchin-like hollow microspheres of Bi2S3: facile synthesis, photo-controlled drug release for photoacoustic imaging and chemo-photothermal therapy of tumor ablation. Biomaterials 237:119835
Zhang Z, Conniot J, Amorim J, Jin Y, Prasad R, Yan X, Fan K, Conde J (2022) Nucleic acid-based therapy for brain cancer: challenges and strategies. J Control Release 350:80–92
Zhao S, Duan H, Yang Y, Yan X, Fan K (2019) Fenozyme protects the integrity of the blood–brain barrier against experimental cerebral malaria. Nano Lett 19(12):8887–8895
Zhao M, van Straten D, Broekman MLD, Preat V, Schiffelers RM (2020) Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 10(3):1355–1372
Zhu X, Zhou H, Liu Y, Wen Y, Wei C, Yu Q, Liu J (2018) Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater 82:143–157
Zottel A, VideticPaska A, Jovcevska I (2019) Nanotechnology meets oncology: nanomaterials in brain cancer research, diagnosis and therapy. Materials (basel) 12(10):1588
Zou Z, Dong YS, Liu DD, Li G, Hao GZ, Gao X, Pan PY, Liang GB (2021) MAP4K4 induces early blood-brain barrier damage in a murine subarachnoid hemorrhage model. Neural Regen Res 16(2):325–332
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52202343), Anhui Provincial Natural Science Foundation (2208085MH251, 2208085QC81), the Basic and Clinical Cooperative Research and Promotion Program of Anhui Medical University (2021xkjT028), Anhui Medical University Scientific Research Fund (No. 2021xkj131), Anhui Provincial Quality Engineering Project for Higher Education Institutions (2022jyxm761) and the Research Fund of Anhui Institute of Translational Medicine (2022zhyx-C01). The authors would like to thank the Shiyanjia lab (www.shiyanjia.com) for their help in language polishing.
Author information
Authors and Affiliations
Contributions
DL and XW were responsible for the conception and design of the review. The manuscript was mainly designed by DL and XD, and written through contributions of all authors. DL, HQ, XD, HC, ZT, HZ and WH collected resources. DL, XD, and XW revised the manuscript. All authors contributed to the article and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, D., Dai, X., Tao, Z. et al. Advances in blood–brain barrier-crossing nanomedicine for anti-glioma. Cancer Nano 14, 58 (2023). https://doi.org/10.1186/s12645-023-00211-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12645-023-00211-9