Abstract
Background
Long-term exposure to inorganic arsenic (As) and cigarette smoking has been associated with adverse health effects such as cancer, cardiovascular disease, and respiratory disease. Oxidative stress is one of the most well-known damage mechanisms for both. However, studies on the association with induced 8-hydroxy-2-deoxyguanosine (8-OHdG) in Iran are scarce, with no study on the interactive effect of As exposure and smoking in the literature.
Objective
This stratified cross-sectional study aimed to assess urinary and serum 8-OHdG levels in the relation to As exposure from drinking water, smoking and their interaction effect.
Methods
This study was based on 132 healthy male subjects living in villages of Hashtroud County, Iran (2021). All participants were categorized into four groups: (i) non-As exposed, never smokers (n = 33); (ii) non-As exposed, active smokers (n = 33); (iii) As exposed, never smokers (n = 33); and (iv) As exposed, active smokers (n = 33). Data on demographic and lifestyle factors were collected, and urinary and serum levels of 8-OHdG were determined by enzyme-linked immunosorbent assay (ELISA). The smoker's daily cigarette consumption and the duration of smoking were self-reported data.
Results
Participants consuming drinking water with an As concentration > 10 µg/L had significantly higher urinary total arsenic (U-tAs) concentrations (median 26.96, IQR 21.35–37.17) µg/g Cr compared to the reference group (median 19.33, IQR 17.29–23.26) µg/g Cr. There was a significant difference in the serum concentration of 8-OHdG between groups (i) and (iii), (iv) and also between groups (ii) and (iv). We did not find a significant interaction effect of As exposure and smoking on the both urinary and serum 8-OHdG levels (P > 0.05). Serum 8-OHdG (S-8OHdG) was associated with average daily intake of As (As-ADI) (β = 0.32; 95% CI 0.01, 0.04, P-value = 0.001) and cigarettes smoked per day (β = 0.3; 95% CI 0.00, 0.043, P-value = 0.046). While As-ADI (β = 0.16; 95% CI 0.001, 0.004, P-value = 0.01) and U-tAs (β = 0.6; 95% CI 0.006, 0.009, P-value < 0.001) were associated with increased urinary 8-OHdG (U-8OHdG), moderate physical activity (β = − 0.15; 95% CI − 0.07, − 0.008, P-value < 0.015) inversely decreased biomarker levels.
Conclusion
Our findings suggest that As exposure and smoking are potential risk factors for oxidative DNA damage, and it is strongly recommended to pay more attention to the role of lifestyle factors in future studies.
Similar content being viewed by others
Introduction
Currently, there is a widespread prevalence of environmental toxicant exposure within populations. Environmental pollutants have the ability to cause an array of both acute and chronic health issues, including endocrine disruption, impaired immune system function, neurological disorders, and potentially carcinogenic effects. These pollutants also have impacts on ecosystems [1]. Among the pollutants considered, a noteworthy category is represented by heavy metals, which have raised mounting concerns due to their toxicity. Certain heavy metals/metalloids, such as chromium (Cr), arsenic (As), cadmium (Cd), and lead (Pb), are classified as priority pollutants due to their pervasive occurrence in the environment, prevalent human exposure, and pronounced toxicity even at low concentrations [2].
Arsenic, a ubiquitous metalloid, is extensively distributed in the environment via natural and anthropogenic sources. Chronic exposure to arsenic, primarily from consumption of contaminated drinking water and certain food sources, has been linked to a spectrum of health conditions such as skin diseases, respiratory disorders, peripheral vascular disorders, diabetes mellitus, and diverse cancers [3, 4]. The prevalence of high arsenic concentrations in groundwater potentially endanger 94 to 220 million people worldwide [5]. The ramifications of arsenic exposure exhibit considerable variation and depend on factors such as concentration, speciation, duration of exposure, and individual attributes [6]. Furthermore, the complex pathogenesis of arsenic-induced toxicity is intertwined with multiple determinants, among which oxidative stress stands out as a well recognized and extensively studied mechanism [7]. The oxidative stress stems from an excessive production of reactive oxygen species (ROS), during which the elevated ROS production overwhelms the counteracting antioxidant mechanism [8]. Arsenic contributes to the generation of ROS while methylation is in progress and promotes diverse forms of DNA damage that culminate in cell apoptosis or DNA mutations [9, 10]. The study of oxidative DNA damage holds paramount importance due to the association between DNA impairments caused by oxidative stress and the development of various health issues, such as cancer, cardiovascular diseases, neurodegenerative disorders and aging [11,12,13]. Moreover, understanding the fundamental mechanisms can provide insights into cellular reactions to oxidative stress, DNA repair pathways and, in particular, the development of preventive and therapeutic approaches targeted at combating diseases linked to oxidative stress. An outcome of oxidative DNA damage is the formation of 8-OHdG, which serves as a reliable biomarker for quantifying the extent of such damage in the whole body through its urinary levels [14]. Chung et al. [15] have reported heightened U-8OHdG concentrations even at low levels of urinary arsenic exposure. Notably, biomonitoring studies that use spot urine samples should employ normalization techniques to minimize the effect of differences in the hydration status of participants on urinary biomarker concentrations [16]. Though there is no consensus on the most robust method [17], a review of previous studies indicates that the most common correction measure for U-As and U-8OHdG is creatinine adjustment [18,19,20,21,22].
Acknowledging the undeniable influence of genetic factors and modifiable lifestyle habits on the oxidative stress response is essential [23]. Smoking, for instance, stands as significant factor in promoting oxidative stress, increasing the risk of DNA damage and mutations in genes associated with tumorigenesis [24]. In addition to carcinogenesis, there is solid evidence indicating that smoking is linked to various cardiovascular, pulmonary and, neurological diseases [25]. While several studies have highlighted elevated levels of 8-OHdG in smokers [26,27,28,29], some have reported inconclusive findings [30,31,32]. Discrepancies can be attributed to variations in toxic and ROS-inducing compounds within cigarette brands, the daily consumption of cigarettes, the duration of smoking, and the sample size of active smokers in the studies.
Notably, the available literature does not definitively determine whether the effect of arsenic exposure on 8-OHdG levels differs between smokers and non-smokers. Specifically, investigations on the interplay between arsenic exposure and smoking in accentuating oxidative stress remain largely unknown.
In Iran, instances of arsenic contamination of groundwater resources have manifested in various regions, particularly in the western, northwestern, southeastern, and northeastern areas [33]. Hashtroud County, situated near the Sahand volcanic mountains in northwestern Iran, has exhibited anomalies in water resources attributed to geogenic origins [34, 35]. Dastgiri et al. [36] found elevated blood pressure and a higher prevalence of skin lesions in residents of one of the arsenic-affected villages compared to the control village of the county.
In relation to the aforementioned, the main objective of this study was to explore the potential interaction effect resulting from the combined exposure to arsenic through drinking water and smoking on oxidative DNA damage. The deleterious effects of arsenic also target the blood among other organs, however the number of studies investigating the elevation of 8-OHdG in serum or plasma in response to arsenic exposure, especially in the healthy population, is much more limited, so the concentration of this biomarker was investigated in both serum and urine specimens in relation to As exposure and smoking. Furthermore, the study sought to analyze the relationship between some lifestyle factors and fluctuations in 8-OHdG concentrations.
Materials and methods
Study area
The present study was conducted in some villages of Hashtroud County, East Azerbaijan Province, northwest of Iran, with an area of 1990 km2 and located at 37° 39′ N and 47° 19′ E (Fig. 1). According to the last census in Iran (2016), the county's population was over 57,000, with 62% of those living in rural areas and the others in urban areas. Based on the Air Quality Index (AQI), air pollution in the villages of the study area is in most cases lower than the known level that has adverse effects on health (AQI < 100) [37]. The AQI is a well-known index that ranges between 0 and 500, whereby values below 100 are generally considered satisfactory [38].
Groundwater is the main source of drinking water in the study area, and Sheikhi et al. [39] recently reported naturally occurring arsenic contamination in 40% of drinking water samples from Hashtroud's rural communities at concentrations well above the WHO guidelines' maximum permissible value (10 μg/L).
Study population
The stratified cross-sectional study was based on 132 healthy male subjects who were invited through the village health worker and recruited if they met the inclusion criteria and signed the consent form to participate. The study was conducted in September 2021, and our inclusion criteria were an age between 18 and 65 years, exclusively drinking water from village groundwater resources for the last 5 years or longer, and did not have any known chronic and infectious diseases. Subjects were excluded from the study if they reported living in the village for less than 5 years, using a water source other than the village drinking water source for drinking, suffering from chronic diseases such as cancer, cardiovascular diseases, hypertension, diabetes, respiratory diseases (chronic obstructive pulmonary disease and asthma), kidney and liver diseases, infectious diseases such as hepatitis, mental-psychological diseases, and subjects currently receiving medical treatment.
The identification of eligible individuals was conducted by considering the study inclusion and exclusion criteria, with the assistance of healthcare personnel of villages and by utilizing electronic health records. Subsequently, the pertinent list was prepared. Individuals were contacted, an explanation was provided regarding the significance of the research and the procedure for involvement, then the volunteers were invited to take part in the study. The participants had similar characteristics in terms of ethnicity, culture and socioeconomic status and were divided into four groups (33 subjects in each group) based on their exposure to As via drinking water and their smoking status. Since we know that oxidative damage can be caused by various other factors, and it is very difficult to control for all of them, we attempted to partially control for some of these confounding factors by including subjects in the study who had similar occupations, work conditions, and dietary habits, with the main difference being the exposure conditions mentioned. In fact, we tried the exposure to other stressors was almost similar in all groups. The subjects in groups (i) and (ii) lived in rural communities with a drinking water arsenic concentrations less than 10 µg/L. They were divided into two categories based on their smoking habits: those who had never smoked (group i) and those who were active smokers (group ii). The following two groups were subjected to drinking water containing arsenic levels exceeding 10 µg/L and comprised the never smokers (group iii) and the active smokers (group iv).
The involvement of the Behvarz was sought to confirm that the never smokers were indeed not passive smokers, since none of their family members smoked. Smoking is an uncommon behavior among females in the study area, and no women reported being smokers. Therefore, our study includes only males.
All participants who were recruited for study demonstrated their agreement by providing written informed consent prior to their involvement. The study protocol and informed consent were approved by the Academic Committee for Research Ethics (Human Studies) of Tabriz University of Medical Sciences (Approval Number: IR.TBZMED.REC.1399.708).
Demographic and lifestyle data collection
Data related to demographic characteristics and some lifestyle factors, including age, level of education, source of drinking water, daily water consumption rate, number of cigarettes smoked per day and smoking duration were collected through a face-to-face interview. In addition, the Persian version of International Physical Activity Questionnaire (IPAQ) was used to estimate habitual practice of physical activities of participants [40]. To measure the total physical activity, the Metabolic Equivalent Task (MET-min/week) was calculated by summing the adjusted durations (minutes × days) for each type of activity using the following values: walking = 3.3 METs, moderate activity = 4.0 METs, and vigorous activity = 8.0 METs. Then, based on the final scores, the total physical activity was divided into three categories: low, moderate and, high. Participants who reported a total activity time > 960 min per day were considered as outlier data and excluded from the study [41].
Biological samples
Subsequently, biometric data were recorded, and the "clean catch" of urine samples after the removal of the first few ml of the first morning urine were collected in 50 ml disposable polyethylene bottles between 9:00 and 10:00 am. Participants were also asked to fast for at least 8 h overnight before blood sampling. About 2.5 ml peripheral venous blood samples were obtained by venipuncture with a syringe and serum separator tube on the same morning. The samples were centrifuged at 3500 rpm for 10 min, then the serum was aspirated.
Biological samples were immediately transported in a portable cooler with cooling packs to the laboratory for further analysis. In the laboratory, a 5-ml aliquot was separated from each urine sample to measure urinary creatinine concentration within 24 h. The residue was divided into aliquots and then stored with the serum samples at − 70 °C until biomarker analysis.
Drinking water sampling and As determination in water and urine
A total of 21 drinking water samples (at least two or three samples from each well and spring in each community serving the source population) were collected in acid-washed plastic containers for further analysis.
In the laboratory, all glassware was washed with dilute HNO3 and rinsed with ultrapure water. Total As content in drinking water and urine samples was analyzed by the hydride generation method with an atomic absorption spectrometer (Perkin-Elmer AAnalyst 600) coupled to the hydride generation system (Perkin-Elmer FIAS 400).
For urine samples, a microwave digestion system (Multiwave GO, Anton-Paar, Graz, Austria) was used to digest 5 ml sample with 5 ml HNO3 and 2 ml H2O2 for 10 min at 100°C. After digestion, the samples were adjusted to a final volume of 25 ml with ultrapure water.
For quality control, blanks, duplicates, and spiked pooled urine samples were analyzed to confirm the accuracy of the analysis. Spike recovery values ranged from 81 to 118%. The LOD was 0.1 μg/L and the LOQ averaged 1 μg/L.
Urinary creatinine (U-Cr) was determined by a routine clinical method based on the Jaffe reaction for all urine samples according to the method by Butler [42]. The obtained concentrations of As were normalized by U-Cr concentrations.
Determination of As-ADI
The average daily intake of As through drinking water ingestion was calculated using the following equation [43]:
where ADI is expressed as average daily intake of As (mg/kg/day), DWAs is drinking water arsenic concentration (mg/L), IR is daily ingestion rate (L/day) (self-reported by participant), and BW is body weight (kg) (measured during study).
Determination of urinary and serum 8-OHdG levels
The 8-OHdG content in urine and serum samples was determined using an ELISA kit (ZellBio GmbH, Germany; kit cat. no. ZB-11436C-H9648). The kit uses an enzyme-linked immunosorbent assay based on the double antibody sandwich technique [44]. The ELISA kit is influenced by macromolecular components such as protein. Therefore, pretreatment with Amicon Ultra centrifugal filters (Fisher Scientific) was performed prior to serum 8-OHdG measurements.
According to the manufacturer's protocol, the standards, test samples, 8-OHdG antibody, and streptavidin–horseradish peroxidase (HRP) conjugate reagent were added to the wells, mixed, and incubated for 60 min. Then, the unbound conjugates were washed five times with a wash buffer. The chromogen solution was used to produce a blue color, which changed to a yellow color after the acidic stop solution was added. The density of the yellow color and the concentration of 8-OHdG are positively correlated. Finally, the optical density (O.D.) of each well was measured within 10 min using a microplate reader set to 450 nm, and then the concentration was calculated by comparison with a standard curve. Each sample was analyzed in duplicate.
The sensitivity of the method was 0.5 ng/mL, and the procedure had an intra- and inter-assay coefficient of variation of 8.3 and 10.9%, respectively.
Urinary 8-OHdG concentrations were adjusted for U-Cr values. No adjustment for hydration status is required for serum concentrations because blood dilution is relatively stable [45].
Statistical analysis
Data were expressed as mean ± standard deviation (SD) when normally distributed, whereas non-normal data were expressed as the median and interquartile range (IQR): 1st and 3rd quartiles (Q1–Q3). The normality test was performed using Kolmogorov–Smirnov test. The Levene test was employed to assess the homogeneity of variances.
Comparison between study groups in age, water consumption, As-ADI, U-Cr, and Cr-adjusted concentrations of U-tAs and U-8OHdG was performed using the Kruskal–Wallis test. If there was a statistically significant difference between the medians of four independent groups, we conducted a post hoc test, Dunn's test, to determine exactly which groups are different.
In addition, weight, BMI (body mass index), and S-8OHdG were compared between groups using a one-way ANOVA. The comparison of mean differences between the four groups was then carried out using Fisher's LSD post hoc analysis. Categorical data were analyzed using Pearson's Chi-square test.
Using Spearman's rank correlation, degrees of associations between U-tAs and U-8OHdG, U-tAs and S-8OHdG and U-8OHdG and S-8OHdG were investigated.
General linear model (GLM) was applied to explore the interactive effect of As exposure and cigarette smoking on the urinary and serum 8-OHdG levels.
The multiple linear regression (MLR) model was applied to to investigate the association between 8-OHdG concentrations and As-ADI, U-tAs, smoking duration, number of cigarettes smoked per day and physical activity. The model for U-8OHdG was run with log-transformed values, and both models (U-8OHdG, S-8OHdG) were adjusted for age and BMI as covariates. The regression coefficients and 95% confidence intervals (CIs) for each independent variable were determined.
All analyses were performed with SPSS version 21 (IBM software) and GraphPad Prism, version 9 (GraphPad Software, La Jolla, California).
Results
Characteristics of the study participants
The information on the participant's characteristics and As exposure status of the study groups are presented in Table 1. The median age of the study participants was 46 years and the mean BMI was 26.33 ± 3.64 kg/m2. More than 85% had at least a primary school education level. In addition, all respondents reported never drinking alcohol, and most of them worked as farmers (about 90%). Smoker participants reported an average of 14.06 cigarettes per day and smoked for an average of 20.35 (2–40) years. 48.5% had moderate and vigorous physical activity for 150 min or more per week. No statistically significant differences were between the four groups in terms of age, weight, BMI, water consumption and physical activity (P > 0.05).
Drinking water and urinary As concentrations
Arsenic levels in drinking water ranged from 1.1 to 155 µg/L, and the mean concentration in drinking water samples from villages with As contamination (groups (iii) and (iv)) was about 20 times higher than the other villages (groups (i) and (ii)). The participants in groups (i) to (iv) had an average arsenic concentration in their drinking water of 3.9 ± 0.23, 2.5 ± 0.2, 70.2 ± 5.3, and 66.8 ± 6.6 μg/L, respectively. According to Table 1, no statistically significant difference in daily water consumption was found among the study groups, while groups (iii) and (iv) had significantly higher As-ADI compared to groups (i) and (ii) (P < 0.05), which is attributed to the higher As concentrations in their drinking water.
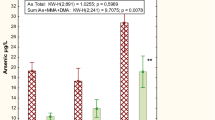
The median concentration of urinary As for overall subjects was 22.63 µg/g Cr and ranged from 10.46 to 76.25 µg/g Cr. The Kruskal–Wallis test followed by Dunn's post-test revealed a significant difference in U-tAs concentration between group (i) and both groups (iii) and (iv) (P < 0.05), which was also the case for group (ii) (Fig. 2). There was no significant difference between smokers and non-smokers within the arsenic-exposed and non-exposed individuals.
Urinary 8-OHdG concentrations
The 8-OHdG concentrations in the urine samples of the participants from the groups with As exposure ((iii) and (iv)) (median = 4.52, 1thQ–3thQ = 3.8–5.53 µg/g Cr) were significantly higher than in the other groups (median = 5.5, 1thQ–3thQ = 4.65–6.92 µg/g Cr) (P < 0.001).
It is noteworthy that the Kruskal–Wallis test showed no significant difference between the four study groups before the creatinine adjustment. However, after adjustment, the significant difference was observed only between group (i) and all other groups. Because group (i) had a significantly higher urinary creatinine concentration, which decreased the adjusted concentration of U-8OHdG compared to others (Fig. 3a–c). Such cases may lead to a misleading interpretation. Figure 4 illustrates a comparison of Cr-adjusted U-8OHdG levels across different circumstances of arsenic exposure and smoking.
The strong, significant correlation was established between urinary arsenic and 8-OHdG concentrations (Spearman's rho = 0.7; p < 0.001) (Fig. 5a).
Serum 8-OHdG levels and correlation with U-8-OHdG
We investigated the sensitivity of serum 8-OHdG to detect an increase in oxidative damage induced by arsenic exposure and/or cigarette smoking. The mean concentrations of the biomarker in serum specimens of subjects in groups (iii) and (iv) were 1.71 ± 0.57 and 1.91 ± 0.66 ng/mL, respectively. For groups (i) and (ii), the mean (SD) of S-8-OHdG levels were 1.34 (0.51) and 1.58 (0.48) ng/mL, respectively (Fig. 6). A comparison between the study groups using one-way ANOVA and Fisher's LSD post hoc analysis revealed significantly higher S-8OHdG concentrations in both groups (iv) and (iii) compared to group (i) (P < 0.05), as is evident in Fig. 7. In addition, a significant difference was also found between group (iv) and group (ii).
Regarding the correlation of 8-OHdG concentrations between serum and urine, we found a positive and relatively weak relationship (r = 0.43, p < 0.001) (Fig. 5b). There was another weak significant correlation between U-tAs and S-8OHdG concentrations as shown in Fig. 5(c) (r = 0.38, p < 0.001).
Interaction or independent effect of arsenic exposure and cigarette smoking on 8-OHdG
The results of the GML analysis on urinary and serum levels of the effect biomarker of DNA damage are summarized in Table 2. In both cases, arsenic exposure (F(U-8OHdG) = 19.37, P(U-8OHdG) < 0.001), (F(S-8OHdG) = 13.22, P(S-8OHdG) < 0.001) and smoking (F(U-8OHdG) = 4.13, P(U-8OHdG) = 0.044), (F(S-8OHdG) = 3.97, P(S-8OHdG) = 0.048) were independently significant predictors of 8-OHdG, but the interaction of the joint exposure was not significant (P > 0.05). The F value for the covariate age was significant, indicating that age is a significant predictor of U-8OHdG.
Multiple linear regression model
Table 3 presents the results of the MLR analysis to explore the association between 8-OHdG and independent variables affecting its urinary and serum levels. Both urine and serum concentrations were significantly associated with As-ADI (β (U-8OHdG) = 0.16, P (U-8OHdG) = 0.01), and (β (S-8OHdG) = 0.32, P (S-8OHdG) = 0.001). While U-8OHdG level was negatively associated with moderate physical activity (β (U-8OHdG) = − 0.15, P(U-8OHdG) = 0.015), significant positive associations were found with U-tAs (β (U-8OHdG) = 0.6, P(U-8OHdG) < 0.001). The number of cigarettes smoked per day was significantly associated with higher levels of S-8-OHdG (β (S-8OHdG) = 0.3, P (S-8OHdG) = 0.046).
Discussion
Several studies have been conducted to evaluate the effect of arsenic exposure through drinking water on the status of oxidative stress. However, the number of studies that simultaneously investigate the influence of individual and lifestyle factors is limited. On the other hand, the interaction effect of one of the most significant lifestyle factors, smoking, with arsenic exposure on oxidative stress has not been determined. In this study, these cases were investigated among a rural male population living in the northwest of Iran.
To estimate the internal As doses, we measured U-tAs in the study groups. Note, it is well known that the excretion of organic As in urine is generally associated with the consumption of seafood (e.g., fishes, crustaceans, bivalves, seaweeds) [46], this is while fish and seafood are not stapled food items among Iranian population as the annual per capita fish consumption (about 9.2 kg/year) is well below the world average [47], and also subjects recruited in this study, reported no fish and seafood consumption in the week preceding biospecimens collection and before. Therefore, it can be said that the measured U-tAs can be attributed to the sum of arsenic metabolites (iAs + MMA + DMA). Our measured concentrations were lower compared to other studies conducted in regions with high As drinking water exposure, e.g., Bangladesh (1730.39 µg/g Cr) [48], Peru (Cairani district: 601.61 µg/g Cr, Camilaca district: 30.24 µg/g Cr) [49], Shanxi China (124.93 µg/g Cr) [50], and Cambodia (86.77 µg/g Cr) [51].
We found that despite significantly higher U-tAs concentrations in subjects exposed to arsenic concentrations > 10 μg/L via drinking water, internal As doses were not negligible in non-exposed subjects. Because urinary arsenic is a valid biomarker for integrating exposure from multiple sources [52], it is reasonable to speculate that subjects in villages with low arsenic levels in drinking water may also have been exposed, likely due to dietary intake, direct soil ingestion from cultivated agricultural products, and dust inhalation.
The levels of urinary 8-OHdG in individuals who were subjected to arsenic exposure were found to be significantly increased when compared to those with low or no exposure, exhibiting a median value of 5.51 µg/g Cr in contrast to 4.42 µg/g Cr.
A comparison between the four groups showed that Cr-adjusted concentrations were significantly different between group (i) and the other groups, which could lead to misinterpretation. It may seem that the non-exposure to arsenic and/or non-smoking in group (i), necessarily caused such a significant difference. However, since creatinine-adjusted values are calculated by dividing the unadjusted concentrations (micrograms of 8-OHdG per liter of urine) by the creatinine concentrations (grams of creatinine per liter of urine), the higher U-Cr value in group (i) has led to such a difference. Creatinine is the breakdown product of creatine, which is formed in muscle tissue, released into the bloodstream, and excreted in the urine via glomerular filtration of the kidneys [53]. Owing to concerns about variability in U-Cr values as a function of age, sex, race/ethnicity, and body size, it is recommended that the creatinine-adjusted concentration of an analyte for an individual be compared with a reference value obtained on a similar demographic group (e.g., adults with adults, children with children) [16]. In our study, participants lived in similar villages of the same county, and there were no significant differences in their demographic characteristics. Several reasons can be envisioned for this, including the possibility that individuals in group (i) may have more muscle mass or lean body mass, a gradual decline in U-Cr excretion with age [54] that may have occurred in group (ii), and the possible association between arsenic exposure and U-Cr concentrations (low U-Cr levels in groups ((iii), (iv)), as some studies have reported an association between As metabolism and the relative proportion of di-methyl arsenical species (DMAs) in urine with U-Cr [55,56,57,58]. Given cross-sectional analysis, our ability to draw a causal inference is limited, and further studies are warranted.
As far as the 8-OHdG concentrations in serum was concerned, the comparison of the four study groups showed a significantly higher mean concentration in groups (iv) and (iii) compared to group (i). A significant difference was also found between group (iv) and group (ii). Therefore, it can be said that serum or plasma concentrations of 8-OHdG, when individuals are randomly assigned to different groups based on exposure conditions, may well reveal differences in DNA damage between exposure groups. In general, similar studies on healthy subjects are rare. For example, a birth cohort study conducted in Vietnam reported increased 8-OHdG levels in cord blood serum associated with maternal arsenic exposure [59]. In another study, the mean plasma 8-OHdG concentration was found to be significantly higher in chronically low As-exposed women (As, 11–50 µg/L) than in the control group (As < 10 µg/L), (8-OHdG:122.58 ± 73.53 vs 33.13 ± 12.10 ng/mL) in West Bengal [60].
Although the underlying mechanisms by which arsenic causes such oxidative damage are not yet fully understood, possible mechanisms could include inhibition of antioxidant enzymes such as superoxide dismutase (SOD) and catalase, interface with cellular antioxidant defense systems by reducing levels of intracellular antioxidants such as glutathione, inhibition of DNA repair mechanisms, and alterations in epigenetic processes [7]. The results of the study exhibited that arsenic has the ability to not only cause DNA damage, but also impede the function of a key protein involved in DNA repair. This effect which is observed even at low As concentrations, can further intensify the overall oxidative DNA damage caused by arsenic itself and possibly by other oxidants as well [61]. As in our study, S-8OHdG levels were higher in arsenic-exposed smokers than in unexposed smokers.
According to GLM, the main effects of As exposure and smoking on both U-8OHdG and S-8OHdG concentrations were statistically significant. However, no interaction was found between these factors, suggesting that the effect of As exposure on 8-OHdG levels in smokers was not significantly different from that observed in non-smokers. This may potentially be attributed to the limited sample size of As-exposed subjects in our study, which consequently resulted in insufficient statistical power for the interaction analysis. In addition, it is possible that the biological mechanisms underlying the interaction between arsenic and smoking are complex and multifactorial, making them challenging to address in a single study.
A significant association between As-ADI and 8-OHdG levels in both urine and serum was found by adjusted MLR analysis. The ADI is an As exposure estimation factor that takes into account both DWAs concentration and water ingestion rate and has been largely overlooked in studies on arsenic-induced oxidative stress, although it may have a strong relationship with oxidative damage. It is particularly important in hot climates where daily consumption of drinking water is high, even when arsenic concentration in water is low. It is therefore recommended to take this into account in similar studies. In addition, urinary arsenic was associated with the increased U-8OHdG. This finding is in a good accordance with other studies that have found a similar association [32, 62,63,64], and more recently Wang et al. [65] reported a 41.5% increase in 8-OHdG levels for every 10 µg/g Cr increase in urinary arsenic concentration in a meta-analysis study. Regarding the association between 8-OHdG concentrations and physical activity in healthy individuals, previous studies have shown discordant results [66,67,68]. It can be explained by the hormesis theory, so that while regular and moderate exercise produces beneficial adaptive effects against oxidative stress, vigorous exercise may lead to increased production of free radicals [69], and in our study, moderate physical activity showed a significant inverse correlation with U-8OHdG levels.
We found that increase in serum 8-OHdG concentrations depended not only on the As-ADI but also on the number of cigarettes smoked per day. Although in this case as well, the results of some studies did not show a significant correlation between 8-OHdG levels and smoking habits, Kulikowska-Karpińska and Czerw [70] found such a correlation with the duration of smoking and the number of cigarettes smoked daily.
Some limitations of this study should be pointed out. Firstly, the small sample size, which may increase the random bias. Secondly, the assessment of smoking status was not conducted quantitatively through biochemical measurements. Nevertheless, it relied not only on self-reported data from individuals, but also on data derived from Behvarz and electronic health records.
There may be a relationship between urinary creatinine concentration and arsenic metabolites, and U-Cr may be a marker of arsenic methylation efficiency [55]. However, the urinary arsenic species were not determined due to budget constraints. Finally, exposure to pesticides may also increase the risk of oxidative stress [71]. Although the majority of participants were primarily involved in wheat farming and spraying was predominantly carried out by others with tractors, detailed information on their pesticide exposure was not available.
Conclusions and future directions
This study investigated the association between arsenic exposure, cigarette smoking and oxidative DNA damage measured as 8-OHdG levels in urine and serum samples of male subjects in the northwest of Iran. The conspicuous augmentation of biomarker concentration in both biological fluids in response to arsenic exposure was apparent. However, interaction effect of joint exposures was not significant, and the increment in question did not exhibit a statistically significant disparity when comparing smokers and non-smokers. Furthermore, our findings suggest that some lifestyle factors may introduce confounding or mediating effects on the relationship between the main exposure factor and related changes, which ought to be appropriately considered in the future similar studies.
It is important to acknowledge that although our study has a regional context, the implications of our findings can extend beyond this context and may be of interest to policy makers and public health officials implementing targeted strategies to reduce As exposure from drinking water and promote healthier lifestyle choices on a worldwide level.
For future studies, it is recommended to also investigate the antioxidant levels, as systemic oxidative stress can be influenced by the content of endogenous antioxidants. Moreover, conducting further studies with a larger sample size and gender-balanced participants is advisable for evaluation of the association between S-8OHdG and arsenic species in blood. Incorporating smoking-related biomarkers such as nicotine/cotinine can enhance the understanding of associations, specially possible interactions.
Availability of data and materials
All data analyzed or generated during study are included in this published paper.
Abbreviations
- As:
-
Inorganic arsenic
- 8-OHdG:
-
8-Hydroxy-2-deoxyguanosine
- ELISA:
-
Enzyme-linked immunosorbent assay
- U-tAs:
-
Urinary total arsenic
- U-Cr:
-
Urinary creatinine
- DWAs:
-
Arsenic concentration of drinking water
- As-ADI:
-
Average daily intake of As
- U-8OHdG:
-
Urinary levels of 8-OHdG
- S-8OHdG:
-
Serum levels of 8-OHdG
- ROS:
-
Reactive oxygen species
- IPAQ:
-
International Physical Activity Questionnaire
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- BMI:
-
Body mass index
- GLM:
-
General linear model
References
Sanchez W, Egea E (2018) Health and environmental risks associated with emerging pollutants and novel green processes. Environ Sci Pollut Res 25:6085–6086. https://doi.org/10.1007/s11356-018-1372-0
Paithankar J, Saini S, Dwivedi S et al (2021) Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262:128350
Raju N (2022) Arsenic in the geo environment: a review of sources, geochemical processes, toxicity and removal technologies. Environ Res 203:111782
Rahaman M, Rahman M, Mise N et al (2021) Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut 289:117940
Podgorski J, Berg M (2020) Global threat of arsenic in groundwater. Science 368:845–850
Nilkarnjanakul W, Watchalayann W, Chotpantarat S (2023) Urinary arsenic and health risk of the residents association in contaminated-groundwater area of the urbanized coastal aquifer, Thailand. Chemosphere 313:137313
Flora S (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51:257–281
Al-Gubory K (2014) Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod Biomed Online 29:17–31
Ding W, Hudson L, Liu K (2005) Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem 279:105–112
Nesnow S, Roop B, Lambert G et al (2002) DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol 15:1627–1634
Chatterjee N, Walker G (2017) Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58:235–263
Münzel T, Camici G, Maack C et al (2017) Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J Am Coll Cardiol 70:212–229
Bohr V, Ottersen O, Tønjum T (2007) Genome instability and DNA repair in brain, ageing and neurological disease. Neuroscience 145:1183–1186
Guo C, Li X, Wang R et al (2016) Association between oxidative DNA damage and risk of colorectal cancer: sensitive determination of urinary 8-Hydroxy-2′-deoxyguanosine by UPLC–MS/MS analysis. Sci Rep 6:32581
Chung C, Huang C, Pu Y et al (2008) Urinary 8 hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol Appl Pharmacol 226:14–21
Barr D, Wilder L, Caudill S et al (2005) Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113:192–200
Middleton DR, Watts MJ, Polya DA (2019) A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a UK population exposed to arsenic in drinking water. Environ Int 130:104721
Rafiee A, Delgado-Saborit J, Aquilina N et al (2022) Assessing oxidative stress resulting from environmental exposure to metals (Oids) in a middle Eastern population. Environ Geochem Health 44:2649–2668
Normandin L, Ayotte P, Levallois P et al (2014) Biomarkers of arsenic exposure and effects in a Canadian rural population exposed through groundwater consumption. J Expo Sci Environ Epidemiol 24:127–134
Graille M, Wild P, Sauvain J et al (2020) Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int J Mol Sci 21:3743
Abuawad A, Goldsmith J, Herbstman J et al (2022) Urine dilution correction methods utilizing urine creatinine or specific gravity in arsenic analyses: comparisons to blood and water arsenic in the FACT and FOX studies in Bangladesh. Water 14:1477
Bortey-Sam N, Ikenaka Y, Akoto O et al (2018) Association between human exposure to heavy metals/metalloid and occurrences of respiratory diseases, lipid peroxidation and DNA damage in Kumasi, Ghana. Environ Pollut 235:163–170
Dato S, Crocco P, D’Aquila P et al (2013) Exploring the role of genetic variability and lifestyle in oxidative stress response for healthy aging and longevity. Int J Mol Sci 14:16443–16472
An A, Kim K, Park H et al (2019) Association between expression of 8-OHdG and cigarette smoking in non-small cell lung cancer. J Pathol Transl Med 53:217–224
Das SK (2003) Harmful health effects of cigarette smoking. Mol Cell Biochem 253:159–165
Chen H, Liou S, Ho S et al (2007) Oxidative DNA damage estimated by plasma 8-hydroxydeoxyguanosine (8-OHdG): influence of 4, 4’ methylenebis (2 chloroaniline) exposure and smoking. J Occup Health 49:389–398
Joshi B, Singh S, Shrma P et al (2020) Effect of cigarette smoking on selected antioxidant enzymes and oxidative stress biomarkers. J Clin Diagnostic Res 14:19–23
Pilger A, Germadnik D, Riedel K et al (2001) Longitudinal study of urinary 8-hydroxy-2′-deoxyguanosine excretion in healthy adults. Free Radic Res 35:273–280
Seet R, Lee CY, Loke W et al (2011) Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic Biol Med 50:1787–1793
Li J, Fan R, Lu S et al (2015) Exposure to polycyclic aromatic hydrocarbons could cause their oxidative DNA damage: a case study for college students in Guangzhou, China. Environ Sci Pollut Res 22:1770–1777
Sakano N, Takahashi N, Wang D et al (2009) Plasma 3-nitrotyrosine, urinary 8-isoprostane and 8-OHdG among healthy Japanese people. Free Radic Res 43:183–192
Wai KM, Umezaki M, Mar O et al (2019) Arsenic exposure through drinking Water and oxidative stress Status: a cross-sectional study in the Ayeyarwady region, Myanmar. J Trace Elem Med Biol 54:103–109
Nemati-Mansour S, Hudson-Edwards K, Mohammadi A et al (2022) Environmental occurrence and health risk assessment of arsenic in Iran: a systematic review and Meta-analysis. Hum Ecol Risk Assess An Int J 28:683–710
Mosaferi M, Nemati S, Armanfar F et al (2017) Geogenic arsenic contamination in Northwest of Iran; role of water basin hydrochemistry. J Environ Heal Sustain Dev 2:209–220
Nadiri A, Asghari Moghaddam A, Sadeghi F, Aghaee H (2012) Investigation of arsenic anomalies In water resources of Sahand Dam. J Environ Stud 38:16–18
Dastgiri S, Mosaferi M, Fizi MAH et al (2010) Arsenic exposure, dermatological lesions, hypertension, and chromosomal abnormalities among people in a rural community of northwest Iran. J Health Popul Nutr 28:14–22
The Weather channel. https://weather.com/forecast/air-quality/l/cedb8da467d24fdb96d085e03cd3b5f50dc55382ca69d3a4675459bebb861bb7. Accessed 25 Feb 2024
Mohan M, Kandya A (2007) An analysis of the annual and seasonal trends of air quality index of Delhi. Environ Monit Assess 131:267–277
Sheikhi S, Faraji Z, Aslani H (2021) Arsenic health risk assessment and the evaluation of groundwater quality using GWQI and multivariate statistical analysis in rural areas, Hashtroud, Iran. Environ Sci Pollut Res 28:3617–3631
Moghaddam M, Aghdam F, Jafarabadi MA, Allahverdipour H, Nikookheslat S, Safarpour S (2012) The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J 18:1073–1080
Craig C, Marshall A, Sjöström M et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sport Exerc 35:1381–1395
Butler A (1975) The Jaffé reaction. identification of the coloured species. Clin Chim Acta 59:227–232
USEPA (1989) Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A). EPA/540/1-89/002
Kohl T, Ascoli C (2017) Immunometric antibody sandwich enzyme-linked immunosorbent assay. Cold Spring Harb Protoc 6:pdb.prot093716
Bommarito P, Beck R, Douillet C et al (2019) Evaluation of plasma arsenicals as potential biomarkers of exposure to inorganic arsenic. J Expo Sci Environ Epidemiol 29:718–729
Taylor V, Goodale B, Raab A et al (2017) Human exposure to organic arsenic species from seafood. Sci Total Environ 580:266–282
FAO (2015) Fishery and Aquaculture Country Profiles, The Islamic Republic of Iran. Accessed 27 Mar 2017
Parvez F, Medina S, Santella R et al (2017) Arsenic exposures alter clinical indicators of anemia in a male population of smokers and non-smokers in Bangladesh. Toxicol Appl Pharmacol 331:62–68
Ale-Mauricio DA, Villa G, Gastañaga MDC (2018) Urinary arsenic concentrations in adult population of two districts from the Tacna Region, Peru, 2017. Rev Peru Med Exp Salud Publica 35:183–189
Wang D, Shimoda Y, Wang S et al (2017) Total arsenic and speciation analysis of saliva and urine samples from individuals living in a chronic arsenicosis area in China. Environ Health Prev Med 22:1–9
Chanpiwat P, Himeno S, Sthiannopkao S (2015) Arsenic and other metals’ presence in biomarkers of Cambodians in arsenic contaminated areas. Int J Environ Res Public Health 12:14285–14300
Zheng L, Kuo C, Fadrowski J et al (2014) Arsenic and chronic kidney disease: a systematic review. Curr Environ Heal Reports 1:192–207
Topf J, Inker L (2019) Measurement of glomerular filtration rate. In: Nephrology Secrets, Fourth Edi. pp 22–29
Weinstein J, Anderson S (2010) The aging kidney: physiological changes. Adv Chronic Kidney Dis 17:302–307
Basu A, Mitra S, Chung J et al (2011) Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ Health Perspect 119:1308–1313
Gamble M, Liu X, Ahsan H et al (2005) Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 113:1683–1688
Kile M, Hoffman E, Hsueh Y et al (2009) Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Perspect 117:455–460
Hall M, Liu X, Slavkovich V et al (2009) Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ Health Perspect 117:825–831
Navasumrit P, Chaisatra K, Promvijit J et al (2019) Exposure to arsenic in utero is associated with various types of DNA damage and micronuclei in newborns: a birth cohort study. Environ Heal 18:1–16
Dutta K, Prasad P, Sinha D (2015) Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health 218:564–574
Qin X, Hudson L, Liu W et al (2008) Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol 21:1806–1813
Tsai T, Kuo C, Hsu L et al (2021) Association between arsenic exposure, DNA damage, and urological cancers incidence: a long-term follow-up study of residents in an arseniasis endemic area of northeastern Taiwan. Chemosphere 226:129094
De Loma J, Krais A, Lindh C et al (2022) Arsenic exposure and biomarkers for oxidative stress and telomere length in indigenous populations in Bolivia. Ecotoxicol Environ Saf 231:113194
Kordas K, Roy A, Vahter M et al (2018) Multiple-metal exposure, diet, and oxidative stress in Uruguayan school children. Environ Res 166:507–515
Wang D, Liang Q, Tai D et al (2023) Association of urinary arsenic with the oxidative DNA damage marker 8-hydroxy-2 deoxyguanosine: a meta-analysis. Sci Total Environ 904:166600
Llorente-Cantarero FJ, Aguilar-Gómez F, Leis R, Bueno G et al (2021) Relationship between physical activity, oxidative stress, and total plasma antioxidant capacity in Spanish children from the GENOBOX Study. Antioxidants 10:320
Bloomer R, Fisher-Wellman K (2008) Blood oxidative stress biomarkers: influence of sex, exercise training status, and dietary intake. Gend Med 5:218–228
Nakatani K, Komatsu M, Kato T et al (2005) Habitual exercise induced resistance to oxidative stress. Free Radic Res 39:905–911
Boccatonda A, Tripaldi R, Davì G, Santilli F (2016) Oxidative stress modulation through habitual physical activity. Curr Pharm Des 22:3648–3680
Kulikowska-Karpińska, E Czerw K (2015) Estimation of 8-hydroxy-2’-deoxyguanosine (8-OHdG) concentration in the urine of cigarette smokers. Wiad Lek (Warsaw, Pol 1960) 68:32–38
Tope A, Panemangalore M (2007) Assessment of oxidative stress due to exposure to pesticides in plasma and urine of traditional limited resource farm workers: formation of the DNA-adduct 8-hydroxy-2-deoxy-guanosine (8-OHdG). J Environ Sci Heal Part B 42:151–155
Acknowledgements
The authors are very grateful to all healthcare workers and study participants from the villages of Hashtroud County for their contribution to this study.
Funding
This is a report of the database from a Ph.D. thesis registered at Tabriz University of Medical Sciences, which was supported by the Health and Environment Research Centre (Grant No. 65297) and in part by grants from Iran National Science Foundation (INSF) (No. 99011986), for which we express our sincere gratitude.
Author information
Authors and Affiliations
Contributions
S-NM: formal analysis, investigation, data curation, writing—original draft. MM: conceptualization, funding acquisition, supervision, writing—review and editing. JB: methodology, investigation. AM: study design, methodology, data curation. RD: methodology. LN: methodology, investigation. MM: visualization, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was in accordance with ethical consideration of the Academic Committee for Research Ethics (Human Studies) of Tabriz University of Medical Sciences (Approval Number: IR.TBZMED.REC.1399.708). All participants provided a written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nemati-Mansour, S., Mosaferi, M., Babaie, J. et al. Interplay of arsenic exposure and cigarette smoking on oxidative DNA damage in healthy males. Environ Sci Eur 36, 86 (2024). https://doi.org/10.1186/s12302-024-00915-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00915-8