Abstract
Evidence about associations of phthalates metabolites with increased serum uric acid (SUA) levels in pregnant women remains unknown. To address this, we conducted a cross-sectional population-based study including 851 pregnant women from Zunyi birth cohort in southwest China. Multiple linear regression models were used to explore single relationships between ten urinary phthalate metabolites with SUA and estimated glomerular filtration rate (eGFR). And then, the overall relationship of phthalate mixture with SUA and eGFR were determined by principal component analysis (PCA) and quantile g-computation (Q-g) analysis. The multivariable linear regression showed that mono-butyl phthalate (MBP), mono-octyl phthalate (MOP) and mono-benzyl phthalate (MBzP) were positively associated with SUA, while mono (2-ethylhexyl) phthalate (MEHP) and mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) were associated with increased eGFR level. Moreover, PCA analysis suggested that phthalate mixture was positively associated with SUA, and MOP, MBzP and MEHP appeared to be the major contributors. Furthermore, Q-g regression showed that each quantile increase in phthalate mixture was associated with 3.27% higher SUA (95% CI 0.21%, 6.41%). Our results imply that phthalate metabolites were associated with higher SUA in late pregnant women, and MBP, MBzP and MOP might be the major drivers. So, a health perinatal duration should be seriously taken to counteract the environment-related dysregulated kidney function.
Similar content being viewed by others
Introduction
In recent years, phthalates, commonly used as plasticizers in personal care products, food packaging, and other consumer goods, have raised health concerns. The National Health and Nutrition Examination Survey (NHANES) of 2015–16 revealed that over 90% of women of childbearing age had detectable levels of at least one urine phthalate metabolite, highlighting widespread exposure [1]. These metabolites have also been found in various bodily secretions and excreta [2], prompting increased concern among pregnant women due to potential risks to fetal health. Epidemiological studies have linked pre-gestational and perinatal exposure to phthalates with adverse birth outcomes, underlining the threat they pose to maternal and infant well-being by disrupting essential biological functions [3,4,5].
Animal studies suggest that phthalates contribute to renal dysfunction by inducing oxidative stress and apoptosis, leading to renal fibrosis [6,7,8, 28]. Similarly, epidemiological research has identified potential negative impacts on renal function [9]. Kang et al. found positive associations between phthalate and urinary albumin creatinine among Korean female population [10]; Similarly, the positive relationships between phthalate metabolites and parameters of renal dysfunction: albumin creatinine ratio (ACR), β2-microglobulin (B2M), and N-acetyl-β-d-glucosaminidase (NAG) were also uncovered [11]. However, there has been no consistent results about the effect of phthalates on eGFR, which is the surest indicators for renal function. Some studies showed a positive correlation between eGFR and phthalates [12, 13], but others found their negative association [14, 15].
Given the altered glomerular hyperfiltration and profound physiological changes during pregnancy, pregnant women’s renal function is expected to be greater susceptibility to phthalates exposure, especially for late pregnant women. Therefore, maintaining balanced renal function is important for the health of the mother and delivery. However, to our best knowledge, only two studies explored the influence of phthalate on the renal function of pregnant women: One study found that exposure to phthalate increased the level of microalbumin and NAG in the third trimester [16]; And another one reported that it is more likely to cause an increased renal injury indicators when pregnant women during the third trimester are exposed to both phthalates and melamine simultaneously [17]. Despite the recognition of microalbumin and NAG as early markers of renal injury, routine prenatal screenings seldom include these tests. Instead, serum uric acid (SUA) and serum creatinine levels, which are commonly measured during pregnancy, may offer alternative indicators of phthalate exposure effects for kidney injury. Considering that elevated SUA has been associated with an increased risk of renal dysfunction during pregnancy [19, 20], highlighting the need to identify potential influencing factors to safeguard maternal and fetal health.
Although some evidences have implied that phthalates exposure associates with increased SUA levels in the general population, whether this association also exists in pregnant women has yet been studied. This study, therefore, seeks to examine the relationship between urinary concentrations of phthalate metabolites and kidney function among late-stage pregnant women in the Zunyi birth cohort. Considering the high multicollinearity among phthalate metabolites, we employed Quantile-g computation (Q-g) to assess the potential adverse effects of chemical mixtures. Additionally, we conducted subgroup analyses to determine if lifestyle factors might influence the relationship between phthalate exposure, SUA, and eGFR, aiming to identify particularly susceptible populations. Our findings aim to enrich the understanding of how phthalate metabolites affect kidney function during pregnancy, providing crucial insights for future research and public health policies.
Methods
Study population
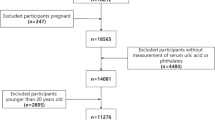
The Zunyi Birth Cohort study was carried out in four hospitals within the Zunyi region of Southwest China, including the First and Second Affiliated Hospitals of Zunyi Medical University, People’s Hospital of Meitan County, and People’s Hospital of Xishui County, starting from May 2020 and April 2022. The primary objective of this cohort is to assess the impact of environmental factors on pregnant women and their fetuses, with detailed methodologies previously described [21]. Women aged 18 to 45 years, experiencing a singleton pregnancy, were initially recruited. After excluding individuals lacking comprehensive data on uric acid, creatinine, phthalate metabolites, covariates, and chronic kidney disease, 851 participants were selected for further analysis, as shown in flowchart (Additional file 1: Figure S1). All participants provided informed consent, with the study receiving ethical approval from Zunyi Medical University (Ethics Approval No. KLL-2019-006).
Measurement of phthalate metabolites
High-performance gas chromatography mass spectrometry (GC-MS/MS, Agilent 7010b, Santa Clara, CA, USA) was used to quantify ten urinary phthalate metabolites in samples, stored at − 80 °C until analysis, from 851 participants. These included mono-methyl phthalate (MMP), mono-ethyl phthalate (MEP), mono-isobutyl phthalate (MiBP), mono-butyl phthalate (MBP), mono-octyl phthalate (MOP), mono-benzyl phthalate (MBZP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), and mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP). Detailed quality control and procedure has been listed in a prior publication [21]. We presented the retention time, ion pairs, collision energy, recovery rate, precision, determination coefficient, and detection limit in Additional file 1: Table S2.
Renal function measurements
Maternal renal function was evaluated by detecting SUA and creatinine concentrations. Because the most of those participants’ age were less than 40, we estimated the eGFR by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI 40) (Björk et al., 2020): eGFR = 144 × (0.993)Age × (Scr/62)−0.320, if the Scr is less than 62 mol/L; eGFR = 144 × (0.993)Age × (Scr/62)−1.209, if the Scr is greater than 62 mol/L. Scr represents the measured serum creatinine, and MA means age at the time of blood sample collection. The unit of eGFR is showed in ml/min/1.73 m2. Well-trained technicians utilized full-automatic biochemical analyzer (BECKMAN-AU5800) to test serum creatinine and uric acid, and used a fully automatic urine analysis workstation to test urine creatinine (URIT-1600).
Covariates
Based on the published literature and the possible biological mechanism, we included the following covariates: Maternal age, pregestational body mass index (BMI), maternal educational level, parity, household income, seasons for sample collection, urine creatinine, and gestational age; Regular physical exercise was defined as exercising at least once a week for more than 30 min; exposure to smoking is defined as passive smoking for at least half an hour, ethnicity, the diagnosis of gestational diabetes, and gestational hypertension were obtained from the electronic medical records.
Statistical analysis
Continuous data with normal and skewed distribution were described as means ± SD and medians (interquartile range) (IQR), respectively, and categorical variables were described as frequency (percentage). Urinary phthalate metabolites and SUA with skewed distribution were natural ln-transformed. Phthalate metabolites concentrations below the limit of detection (LOD) were assigned the value of LOD/√2. Given the link between eGFR and creatinine, we included urine creatinine instead of the adjustment of specific gravity of urine in models for reducing collider problem [14, 23]. Spearman correlation coefficients among ten phthalate metabolites were counted and visualized in heat map by using the “ggcorrplot” and “corrplot” R package.
Multivariable linear regression model was conducted to estimate the association of each urinary phthalate metabolite with SUA and eGFR. The percent changes of SUA were calculated by applying a back transformation of {100 × [exp (β)-1]} to the corresponding regression coefficients. Trend analysis was calculated by taking the median phthalate metabolites of each quartile into multivariable linear regression model. In addition, the overall associations of phthalate mixture with SUA and eGFR were estimated by Q-g and principal component analysis (PCA). The results of Q-g were interpreted as the changes of parameters of kidney function with per increment of quantile of phthalate mixture level. This method can effectively control the multi-collinearity and simultaneously identify the major contributor, and estimate the overall association [24, 25]. In PCA, varimax rotation was used to divide them into factor 1–3, and identified the main components of ten chemicals. The chemicals accounting for more than 70% of the total variance of each factor were retained [14], then multiple linear regressions were used to identify the relationship of each factor with renal function. Stratified analyses were applied to examine whether any factors such as education level, gestational hypertension, regular physical exercise, and exposure to smoking atmosphere could modify the associations of phthalates with SUA levels. A two-sided p-value < 0.05 was regarded statistically significant. Statistical analyses were conducted by SPSS version 25.0 (IBM Corp. Armonk, NY, USA) or R software (version 4.2.2).
Results
Characteristics of study population and urinary chemicals
Sociodemographic characteristics of the enrolled 851 women are summarized in Table 1. The mean of maternal age was 27.22 ± 4.99, their pre-pregnancy BMI was 22.52 ± 3.56 kg/m2, and 73.0% of pregnant women were multiparous. 47.5% of pregnant women participated in this study during winter and 3.6% participated during summer. The prevalence rates of pregnancy hypertension and pregnancy diabetes were 12.7% and 21.3% (Table 1).
The distributions of ten urinary phthalate metabolites concentrations were shown in Table 2. MECPP had the highest median concentration with the value of 71.14 μg/L. Besides, the median concentration of MBzP was the lowest with the value of 0.06 μg/L. Besides, a positive correlation between the majority of phthalate metabolites was found with correlation coefficient ranged from 0.01 to 0.8 (Fig. 1).
The association between individual phthalate metabolites and SUA
Table 3 displayed the association of urinary phthalate metabolites with SUA after adjusting for covariates. A onefold increase in MBP, MOP, and MBZP concentrations were associated with increments of 1.51% (95% CI 0.10, 3.05), 1.92% (95% CI 0.50, 3.25), and 1.41% (95% CI 0.20, 2.74) in SUA, respectively. Moreover, there was a positive trend of MBZP and MOP with SUA (P-trend < 0.05). In addition, MEHP and MEHHP, but not other phthalate metabolites, showed the positive associations with eGFR (Table 4).
Q-g computation and PCA analysis
As shown in Fig. 2, of Q-g regression displayed that with each quartile increase of phthalate mixture, the mean estimated changes in SUA and eGFR were 3.27% (95% CI 0.21%, 6.41%) and 0.0870 (95% CI − 0.8823, 1.0563), respectively. We found that MBP, MBZP, MOP, MEHHP, and MIBP (all weights > 10%) were the dominant phthalate metabolites for the positive association with SUA, while MEP, MEHP and MEOHP (all weights > 10%) mainly contributed to negative associations with SUA (Fig. 2A). No significant association was found between phthalate mixture with eGFR (Fig. 2B).
Estimates the effects of one-quartile increase of phthalate metabolites mixtures in UA (A) and eGFR (B) and scaled weights corresponding to the proportion of the effect for each chemical in Quantile g-computation. Models adjusted for age, BMI, annual household income, education, exposure to smoking, parity, nationality, gestational diabetes mellitus, gestational hypertension, regular physical exercise during pregnancy, sample collection season, gestational week at the time of Uric Acid measurement
The details of varimax-rotated factor loadings were displayed in Additional file 1: Table S1. MMP, MEP, MIPB and MBP were highly loaded in factor 1. MEHP, MOP and MBZP were highly loaded in factor 2, and MEOP, MEHHP and MECPP were highly loaded in factor 3. Both the multiple-factor and single factor model consistently indicated factor 2 was significantly positively associated with SUA. However, no any statistical significance between eGFR and these factors was presented (Table 5).
Subgroup analyses
The results of subgroup analysis of phthalate metabolites and SUA are presented in Table 6. For pregnant women without regular exercise, onefold increase in MOP and MBzP were associated with 2.43% and 1.71% increase of SUA; for pregnant women who are exposed to smoking for ≥ 6 days per week, onefold increase in MBP and MOP were associated with 1.82% and 2.12% increase of SUA. The results of subgroup analysis of phthalate metabolites and eGFR presented in Additional file 1: Table S3.
Sensitivity analysis
We performed a sensitivity analysis by excluding pregnant women with hyperuricemia and abnormal blood creatinine levels, and the associations of MBP, MOP, and MBZP with SUA (Additional file 1: Table S4) and the association of MEHP with eGFR were still robust (Additional file 1: Table S5).
Discussion
Our findings reveal significant associations between increased SUA levels and higher concentrations of mono-butyl phthalate (MBP), mono-octyl phthalate (MOP), and mono-benzyl phthalate (MBzP) in third-trimester pregnant women. Both PCA and Q-g regression underscore the positive correlation of mixed phthalate metabolites with SUA, highlighting MBP, MBzP, and MOP as primary contributors. This evidence supports the hypothesis that phthalate metabolites may adversely affect kidney function. Notably, the detectable rates of ten phthalate metabolites in our cohort ranged from 61.7 to 100%, indicating widespread exposure among pregnant women in Southwest China and underling the need for further research to evaluate its safety implications for perinatal health.
To date, limited research has focused on the impact of phthalates on renal function in pregnant women. Our study aligns with two prior epidemiological investigations identifying phthalates as nephrotoxic agents during pregnancy. One cohort study linked urine phthalate levels with increased ACR and NAG among late-stage pregnant women in Taiwan [16]. Another study observed elevated levels of NAG and ACR due to combined exposure to phthalates and melamine, compared to exposure to either agent alone [17]. Urinary NAG is a sensitive marker of renal tubular injury [32], and the definition of microalbuminuria is urine ACR higher than 3.5 mg/mmol. While urinary NAG is a recognized marker for renal tubular injury, routine prenatal screenings seldom include NAG and microalbumin tests. Instead, SUA and serum creatinine, which are regularly measured, offer an alternative means to detect potential kidney injury earlier.
Previous studies suggest that more than 70% of urate excretion occurs via the kidneys, with insufficient uric acid salt excretion being a primary cause of hyperuricemia [33]. Previous studies speculated that phthalate metabolites affect the excrete of SUA by decreasing eGFR or interfering organic anion transporters of the proximal tubular epithelial cell membrane [27]. Our results suggest that phthalates may elevate SUA levels by diminishing eGFR or disrupting organic anion transport across proximal tubular epithelial cell membranes. Notably, MEHP and MEHHP showed positive correlations with eGFR, without a clear negative association with other metabolites.
Comparatively, research on a general American adult population found positive correlations between MECPP, MEHHP, MBzP, and MiBP with SUA levels [27]. Our study corroborates these findings for MBzP and introduces moderate associations with MBP and MOP. Given that evidence from Western populations has limited applicability for exploring the association between phthalate metabolites and SUA levels among Chinese individuals. And, the southwestern region of China is an economically underdeveloped region with weak supervision over the use of plasticizers in pregnant women’s specialized products. This variation emphasizes the unique environmental and regulatory landscape in Southwest China, necessitating region-specific studies to inform local public health policies. The present study, based on pregnant women in southwest China, reflected that phthalate metabolites were associated with higher SUA. However, the causal relationships between phthalates metabolites and SUA levels are still needed to be clarified by prospectively designed and multicenter studies with larger sample sizes. This study may provide reference for the future development of standards for pregnant women's products in southwest China.
While exploring the relationship between phthalates and eGFR has yielded mixed results, our analysis indicates a discernible, and positive association between certain metabolites and eGFR. The significance of eGFR as a renal function marker, especially considering physiological changes during pregnancy such as glomerular hyperfiltration, merits cautious interpretation of these findings. As MEHP was positively correlated with higher eGFR in a research of 9989 people in the United States [12]; Another study of 538 American adolescents aged 0–17 showed a positive association between low molecular weight phthalates and eGFR [13]. More evidences also observed their negative correlation [14, 15]. In our study, although mixed effects analysis (Q-g and PCA) showed no obviously significant relationship between phthalate metabolites and eGFR, but univariate analysis displayed a positive association of MEHP and MEHHP with eGFR. Thus, the impact of phthalate on eGFR cannot be ignored. In addition, for the general population, eGFR is thought as the most useful marker to reflect the renal function [34]. However, the glomerular hyperfiltration is considered to be the hallmark for the profound physiological change during pregnancy peroid. Since renal vasodilation increases renal plasma flow which further leads to a greater than 50% rise in glomerular filtration rate during pregnancy [35, 36]. Previous studies have found that the optimal range of eGFR in the second trimester is 120–150 ml/min/1.73 m2. It should be noteworthy that the prevalence of premature delivery and low birth weight infants was significantly higher in pregnant women with an eGFR higher than 150 ml/min/1.73 m2 [37]. Therefore, the positive correlation between MEHP, MEHHP and eGFR should not be directly interpreted as a potential protective effector.
The health benefits of physical exercises are widely known and recognized. There are a lot of evidence that physical exercise improves the health of pregnant women [38, 39]. In addition, our study highlights lifestyle factors, such as physical activity and exposure to smoke, that may influence the relationship between phthalate exposure and SUA levels. This underscores the importance of addressing modifiable lifestyle factors to mitigate potential health risks. This may be due to the higher renal blood flow of people who exercise regularly [40]. Previous studies on this population showed that the urine concentration of phthalate metabolites in passive smokers was significantly higher [21]. Whether passive smoking and phthalates jointly cause the increased uric acid in pregnant women deserves further studies. These results may indicate that future efforts should be made to improve the unhealthy lifestyles of pregnant women.
This investigation, while robust, is not without limitations. Firstly, its cross-sectional nature precludes causal inferences between phthalate exposure and SUA levels. Additionally, the reliance on single urine samples may not fully capture variations in phthalate exposure. Nevertheless, to our certain knowledge, considering our study population has relatively regular lifestyle habits and a stable living environment, we may think that phthalate exposure levels are relatively steady. Thirdly, previous researches have exhibited that purine rich dietary habit has a substantial effect on serum uric acid levels [41]. However, we failed to conduct a complete diet questionnaire in this study. Notably, considering uric acid is not the specific biomarkers of renal function, the positive association between phthalate metabolites and SUA observed in this study couldn’t entirely illustrate the significant relationship of phthalate metabolites with renal function. Besides, covariate-adjusted creatinine standardization, which controls for potential confounding by kidney function, was usually employed to adjust urine dilution. Unfortunately, in our population there is no significant correlation between age, BMI, eGFR, and ln transformed urinary creatinine creativity concentration, leading to failure of modeling, so the covariate-adjusted standardization may not be applicable to our study. In addition, due to the limitations of cross-sectional study, the positive association between eGFR and MEHP, MEHHP found in this study might originate from the reverse causation, more prospective studies are needed.
Despite these constraints, our application of PCA and Q-g computation to analyze the data helps overcome issues related to collinearity and multiple comparisons, providing a clearer representation of real-world phthalate exposure scenarios. Besides, we firstly conducted the above association at pregnant women in southwestern region of China, contributing to fill this knowledge gap regarding phthalate exposure positively associated with dysregulated kidney function under the Southwest China human geography scenario. In summary, our study contributes significantly to the understanding of phthalate exposure’s impact on kidney function among pregnant women in Southwest China, filling a critical gap in the literature and laying the groundwork for future longitudinal and interventional research to elucidate causal relationships and develop targeted public health interventions.
Conclusion
In conclusion, our study establishes a clear association between elevated exposure to both individual and combined phthalate metabolites and increased SUA levels. This relationship underscores the potential adverse impact of phthalate exposure on renal health during pregnancy. To substantiate these findings and elucidate the underlying biological mechanisms by which phthalates influence SUA levels, future research should employ longitudinal study designs.
Availability of data and materials
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author, [author initials], upon reasonable request.
References
Pacyga DC, Gardiner JC, Flaws JA, Li Z, Calafat AM, Korrick SA et al (2021) Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ Int 155:106676
Gao H, Zhu Y-D, Xu Y-Y, Zhang Y-W, Yao H-Y, Sheng J et al (2017) Season-dependent concentrations of urinary phthalate metabolites among Chinese pregnant women: repeated measures analysis. Environ Int 104:110–117
Santos S, Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral M-P et al (2021) Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes a population-based prospective cohort study. Environ Int. https://doi.org/10.1016/j.envint.2021.106443
Zhang Y, Mustieles V, Williams PL, Wylie BJ, Souter I, Calafat AM et al (2021) Parental preconception exposure to phenol and phthalate mixtures and the risk of preterm birth. Environ Int 151:106440
Chang C-H, Tsai Y-A, Huang Y-F, Tsai M-S, Hou J-W, Lin C-L et al (2022) The sex-specific association of prenatal phthalate exposure with low birth weight and small for gestational age: a nationwide survey by the Taiwan Maternal and Infant Cohort Study (TMICS). Sci Total Environ 806(Pt 3):151261
Amara I, Salah A, Timoumi R, Annabi E, Scuto M, Trovato A et al (2020) Effect of di (2-ethylhexyl) phthalate on Nrf2-regulated glutathione homeostasis in mouse kidney. Cell Stress Chaperones 25(6):919–928
Cheng L, Li J, Cheng J, Wu Z (2019) Dibutyl phthalate-induced activation of ROS and ERK1/2 causes hepatic and renal damage in Kunming mice. Hum Exp Toxicol 38(8):938–950
Wu C-T, Wang C-C, Huang L-C, Liu S-H, Chiang C-K (2018) Plasticizer Di-(2-ethylhexyl)phthalate induces epithelial-to-mesenchymal transition and renal fibrosis in vitro and in vivo. Toxicol Sci 164(1):363–374
Chen J, Shi X, Zhou X, Dong R, Yuan Y, Wu M et al (2020) Renal function and the exposure to melamine and phthalates in Shanghai adults. Chemosphere 246:125820
Kang H, Kim S, Lee G, Lee I, Lee JP, Lee J et al (2019) Urinary metabolites of dibutyl phthalate and benzophenone-3 are potential chemical risk factors of chronic kidney function markers among healthy women. Environ Int 124:354–360
Chen J, Zhou X, Zhang H, Liu Y, Cao C, Dong R et al (2019) Association between urinary concentration of phthalate metabolites and impaired renal function in Shanghai adults. Environ Pollut 245:149–162
Wang Z, Sun Y, Gu L, Zhang T, Liu S, Wang S et al (2022) Association of urinary phthalate metabolites with renal function among 9989 US adults. Ecotoxicol Environ Saf 242:113930
Malits J, Attina TM, Karthikraj R, Kannan K, Naidu M, Furth S et al (2018) Renal Function and exposure to bisphenol a and phthalates in children with chronic kidney disease. Environ Res 167:575–582
Liu M, Zhao L, Liu L, Guo W, Yang H, Chen S et al (2022) Urinary phthalate metabolites mixture, serum cytokines and renal function in children: a panel study. J Hazard Mater 422:126963
Lee I, Park JY, Kim S, An JN, Lee J, Park H et al (2020) Association of exposure to phthalates and environmental phenolics with markers of kidney function: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ Int 143:105877
Tsai H-J, Kuo F-C, Wu C-F, Sun C-W, Hsieh C-J, Wang S-L et al (2021) Association between two common environmental toxicants (phthalates and melamine) and urinary markers of renal injury in the third trimester of pregnant women: the Taiwan Maternal and Infant Cohort Study (TMICS). Chemosphere 272:129925
Chen C-C, Wang Y-H, Wu C-F, Hsieh C-J, Wang S-L, Chen M-L et al (2022) Benchmark dose in the presence of coexposure to melamine and diethylhexyl phthalate and urinary renal injury markers in pregnant women. Environ Res 215(Pt 1):114187
Fathallah-Shaykh SA, Cramer MT (2014) Uric acid and the kidney. Pediatr Nephrol. https://doi.org/10.1007/s00467-013-2549-x
Nagano S, Takahashi M, Miyai N, Oka M, Utsumi M, Shiba M, Mure K, Takeshita T, Arita M (2017) Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive subjects. Hypertens Res 40(6):620–624
Soltani Z, Rasheed K, Kapusta DR, Reisin E (2013) Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep 15(3):175–181
Liao J, Fang D, Liu Y, Xiong S, Wang X, Tian Y et al (2022) Exposure characteristics of phthalate metabolites among the Zunyi cohort of pregnant women in southwest China. Environ Sci Pollut Res Int 29(39):58869–58880
Björk J, Nyman U, Larsson A, Delanaye P, Pottel H (2021) Estimation of the glomerular filtration rate in children and young adults by means of the CKD-EPI equation with age-adjusted creatinine values. Kidney Int 99(4):940–947
O’Brien KM, Upson K, Cook NR, Weinberg CR (2016) Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect 124(2):220–227
Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ (2020) A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):47004
Lai X, Yuan Y, Liu M, Xiao Y, Ma L, Guo W et al (2022) Individual and joint associations of co-exposure to multiple plasma metals with telomere length among middle-aged and older Chinese in the Dongfeng-Tongji cohort. Environ Res 214(Pt 3):114031
Tsai H-J, Wu C-F, Hsiung CA, Lee C-H, Wang S-L, Chen M-L et al (2022) Longitudinal changes in oxidative stress and early renal injury in children exposed to DEHP and melamine in the 2011 Taiwan food scandal. Environ Int 158:107018
Tan Y, Fu Y, Yao H, Wu X, Yang Z, Zeng H et al (2022) Relationship between phthalates exposures and hyperuricemia in US general population, a multi-cycle study of NHANES 2007–2016. Sci Total Environ 859(Pt 1):160208
Wu CF, Hsiung CA, Tsai HJ, Tsai YC, Hsieh HM, Chen BH et al (2018) Interaction of melamine and di-(2-ethylhexyl) phthalate exposure on markers of early renal damage in children: the 2011 Taiwan food scandal. Environ Pollut 235:453–461
Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L et al (2020) Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environ Int 135:105342
Villanger GD, Drover SSM, Nethery RC, Thomsen C, Sakhi AK, Øvergaard KR et al (2020) Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ Int 137:105509
Harel Z, Park AL, McArthur E, Hladunewich M, Dirk JS, Wald R et al (2020) Prepregnancy renal function and risk of preterm birth and related outcomes. CMAJ 192(30):E851–E857
Siew ED, Ware LB, Ikizler TA (2011) Biological markers of acute kidney injury. J Am Soc Nephrol 22(5):810–820
Lipkowitz MS (2012) Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep 14(2):179–188
Colombo M, McGurnaghan SJ, Blackbourn LAK, Dalton RN, Dunger D, Bell S et al (2020) Comparison of serum and urinary biomarker panels with albumin/creatinine ratio in the prediction of renal function decline in type 1 diabetes. Diabetologia 63(4):788–798
Hui D, Hladunewich MA (2019) Chronic Kidney Disease and Pregnancy. Obstet Gynecol 133(6):1182–1194
Williams D, Davison J (2008) Chronic kidney disease in pregnancy. BMJ 336(7637):211–215
Park S, Lee SM, Park JS, Hong J-S, Chin HJ, Na KY et al (2017) Midterm eGFR and adverse pregnancy outcomes: the clinical significance of gestational hyperfiltration. Clin J Am Soc Nephrol 12(7):1048–1056
Davenport MH, Ruchat S-M, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N et al (2018) Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med 52(21):1367–1375
Connolly CP, Conger SA, Montoye AHK, Marshall MR, Schlaff RA, Badon SE et al (2019) Walking for health during pregnancy: A literature review and considerations for future research. J Sport Health Sci 8(5):401–411
Xiong Z, Zhu C, Qian X, Zhu J, Wu Z, Chen L (2013) Serum uric acid is associated with dietary and lifestyle factors in elderly women in suburban Guangzhou in Guangdong province of south China. J Nutr Health Aging 17(1):30–34
Coelho MOC, Monteyne AJ, Kamalanathan ID, Najdanovic-Visak V, Finnigan TJA, Stephens FB et al (2020) Short-communication: ingestion of a nucleotide-rich mixed meal increases serum uric acid concentrations but does not affect postprandial blood glucose or serum insulin responses in young adults. Nutrients. https://doi.org/10.3390/nu12041115
Funding
This work was supported by the Science & Technology Program of Guizhou Province (QKHHBZ[2020]3002); the Science & Technology Program of Guizhou Province (QKHPTRC-GCC[2022]039-1); the Science & Technology Program of Guizhou Province (QKHPTRC-CXTD[2022]014); and the National Natural Science Foundation of China (81860139).
Author information
Authors and Affiliations
Contributions
Author contributions statement Qifu Hong, Tao Pu (first author): conceptualization, methodology, software, investigation, formal analysis, writing—original draft; Maojie Li: data curation, writing—original draft; Zhongbao Chen: visualization; Rong Zeng: resources; Mingzhe Zhang: software; Lulu Dai: writing—review & editing; Songlin An: investigation; Xubo Shen: supervision; Xuejun Shang: Validation; Kunming Tian, Yuanzhong Zhou (corresponding author): conceptualization, funding acquisition, resources, supervision, writing—review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
After review by the Medical Ethics Committee of Zunyi Medical University, the qualifications and experience of the researchers in this project meet the experimental requirements, and the research plan meets the requirements of scientificity and relevant ethical principles. The research content does not pose potential risks to the subjects and does not constitute harm to them. The recruitment of subjects follows the principles of voluntary and informed consent, and the method of obtaining informed consent is appropriate, which can maximize the protection of subjects’ rights and privacy. The degree of risk that subjects may face is appropriate compared to the expected benefits of the study. There is no conflict of interest between the research content and results. Agree that the research team will carry out relevant experiments according to the research plan of the National Key R&D Plan Task Book (2018YFC1004302) as planned.
Competing interests
The authors declare they have no conflict of interest in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flowchart. Table S1. Varimax-rotated factor loadings of phthalate metabolites in PCA. Table S2. Retention time, ion pair, collision energy, determination coefficient, recovery rate, and detection limit of each metabolite. Table S3. Stratified regression analysis of the association of MEHP and MEHHP with eGFR (n = 851). Table S4. Association of urinary phthalate metabolites with SUA in pregnant women without hyperuricemia and abnormal blood creatinine levels (N=770). Table S5. Association of urinary phthalate metabolites with eGFR in pregnant women without hyperuricemia and abnormal blood creatinine levels (N=770). Table S6. Association of urinary phthalate metabolites with SUA in pregnant women(N=851). Table S7. Association of urinary phthalate metabolites with eGFR in the pregnant women(N=851). Table S8. Association of urinary phthalate metabolites with SUA in pregnant women (N=851). Table S9. Association of urinary phthalate metabolites with SUA in pregnant women (N=851). Table S10. Association of urinary phthalate metabolites with UA in pregnant women (N=851). Table S11. Association of urinary phthalate metabolites with eGFR in pregnant women (N=851). Table S12. Association of urinary phthalate metabolites with eGFR in pregnant women (N=851). Excluded MIBP. Table S13. Association of urinary phthalate metabolites with UA in pregnant women (N=851). Excluded MBP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, Q., Pu, T., Li, M. et al. Association between urinary phthalate metabolites and renal function in late pregnant women. Environ Sci Eur 36, 98 (2024). https://doi.org/10.1186/s12302-024-00909-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00909-6