Abstract
Background
Bisphenol A (BPA) is currently one of the most widely used synthetic chemicals in the production of a wide range of plastics. Due to its diverse endocrine disrupting potential alternative bisphenols, also referred to as analogues, have been developed. Although the toxicity of BPA is well studied, the (eco)toxicological effects of the bisphenol analogues are largely unknown. The similar molecular structure of the analogues suggests comparable toxicological effects. This study aims to extend the (eco)toxicological knowledge on the bisphenol analogues by evaluating eleven bisphenol analogues compared to the reference substance BPA in in vitro bioassays. The examined endpoints are endocrine potential on three nuclear receptors in recombinant yeast cells of Saccharomyces cerevisiae, baseline toxicity (also referred to as non-specific toxicity, describing the minimal toxicity of a chemical) in the luminescent bacterium Aliivibrio fischeri, and mutagenicity in two strains of Salmonella typhimurium.
Results
Bisphenol A showed estrogenic and anti-androgenic activity at EC50 concentrations of 0.516 mg/L (2.26 × 10–6 M) and 1.06 mg/L (4.63 × 10–6 M), respectively. The assays confirmed notable estrogenic and anti-androgenic activity for the vast majority of analogues in comparable, and often higher, efficacies to BPA. Some analogues showed anti-estrogenic instead of estrogenic activity in a range from 0.789 mg/L (1.45 × 10–6 M; TBBPA) to 2.69 mg/L (2.46 × 10–6 M; BADGE). The baseline toxicity of the analogues revealed a similar tendency of comparable to more prominent effects compared to BPA, ranging from 5.81 mg/L (1.73 × 10–5 M; BPAF) to 39.1 mg/L (1.56 × 10–4 M; BPS). There was no evidence of mutagenicity found.
Conclusion
The examined bisphenol analogues prove to be equally, if not more, problematic in endocrine activities than the reference bisphenol A. Based on these results, the tested bisphenols cannot be regarded as safer alternatives and reinforce the notion of bisphenol analogues being considered as regrettable substitutions.
Similar content being viewed by others
Background
Bisphenol A (BPA) is predominantly used as an industrial raw material in the manufacture of polycarbonate and epoxy resin polymers, commonly referred to as plastics [1,2,3]. The polymers have a wide range of applications in day-to-day life, for instance in food containers and drinking bottles, internal protective coatings of food cans, water pipes or their inner linings, paints, casings of electronics, automobiles, or medical and sports equipment, as well as in less tangible utilization in thermal papers or flame retardants [1, 2, 4]. The global demand for BPA in 2015 amounted to 7.7 million metric tonnes and was predicted to surpass 10 million tonnes by 2022 [5, 6]. Bisphenol A is, therefore, considered a high production volume chemical and generally one of the most produced chemicals worldwide [7]. However, BPA has been the target of heavy criticism due to low-dose effects and diverse modes of action as an endocrine disruptor, taking into account the ubiquitous exposure of consumers. In 2017, the European Chemicals Agency (ECHA) unanimously agreed to identify bisphenol A as a substance of very high concern (SVHC) on account of its endocrine disrupting and toxic to reproduction properties [8]. SVHCs are required to progressively be substituted by less dangerous substances if technically and economically feasible alternatives are available.
The regulations prompted a rapidly growing portfolio and market for BPA alternatives. Products based on the alternatives are frequently promoted under the label “BPA free”. This conveys the impression of safer products, although in the past alternatives for concerning chemicals often did not undergo sufficient testing before market release [9]. Due to the similarities in molecular structure, the alternatives suggest a comparable toxicological relevance, especially in regards to endocrine activity [10,11,12,13]. Some of the bisphenol analogues are already identified or suspected to act as endocrine disruptors and/or toxic to reproduction, e.g., BPS and BPB [14, 15], or as carcinogenic, e.g., TBBPA [15]. In contrast to BPA, the knowledge base for toxicological effects of bisphenol analogues has been poorly assessed so far. Despite the lack of information about (eco)toxicological relevance, the production and application is steadily increasing [10, 11, 13, 16]. The focus of research has thus far been on endocrine potential, predominantly evaluating agonistic estrogenicity [17]. Many studies report similar estrogenic potencies to bisphenol A [10,11,12, 18] with potencies four to six orders of magnitude lower than the natural hormone 17β-estradiol (E2) [10, 12]. Ten of 17 bisphenol analogues evaluated in a review by Pelch et al. [17] were estrogenically active in one or more assays. Among these are BPS, 4,4-BPF, BPAF, BPB, BPE, BPC, BPZ, BPAP, TMBPA, and BPP. BPAF is considered one of the most potent analogues, with a higher potency than BPA itself [17]. BPS, on the other hand, is the least estrogenically potent [17]. There were few to no antagonistic effects observed at the estrogen receptor for most analogues [19, 20]. The bisphenols (BPs) TMBPA [19], TBBPA [19, 21], BADGE, BPBP [21], BPAF and BPP [20], however, did show anti-estrogenic effects. Numerous BPs reportedly act as antagonists at the androgen receptor, including BPB, BPE, BPF, BPZ, BPP, BPAP, BPAF, TMBPA, TBBPA, and TCBPA [11, 13, 22]. Molina-Molina et al. [22] also describe BPS and BPA as weak androgens. A study about mixture effects of the bisphenol derivatives BPA, BPS, and BPF by Park et al. [13] demonstrated stronger estrogenic as well as anti-androgenic effects of bisphenol mixtures compared to the individual bisphenols. Furthermore, some analogues interact with other hormonal receptors, such as the thyroid hormone receptor (TR) [19], the glucocorticoid receptor (GR) or the pregnane X receptor (PXR) [20]. Additionally examined in vitro endpoints are, for instance, the activity at the aryl hydrocarbon receptor [11, 13], DNA damage and toxicity [11, 23] or oxidative stress [24]. In vivo endpoints, such as neurological or behavioural endpoints, remain poorly assessed [17].
In this study, we evaluate to what extent the eleven selected bisphenol analogues BPB, BPE, BPF, BPS, BPZ, BPAF, BPAP, BPBP, BADGE, TBBPA, and TCBPA are suitable replacements for BPA. To answer this question, we conducted an in vitro test battery, which assessed the toxicological endpoints of endocrine activity in five different yeast reporter gene assays, baseline (non-specific) toxicity via the Microtox assay, and mutagenicity in the Ames fluctuation test. It is hypothesized that the bisphenol analogues show comparable effects to BPA in the in vitro assays due to their similar molecular structure, especially with respect to the estrogen and androgen receptor. The chosen test battery intends to extend the scope beyond endocrine disruption by including the additional endpoints of baseline toxicity and mutagenicity for a broader effect profile and hazard assessment. We selected these eleven bisphenol analogues based on their industrial relevance and steadily increasing economic use (i.e., BPF, BPS, BPAF, BPB, BPAP), as well as their structural diversity.
Methods
Reagents and sample preparation
4,4ʹ-isopropylidenediphenol (bisphenol A, BPA, CAS 80-05-7), 4,4ʹ-(1-methylpropylidene)bisphenol (bisphenol B, BPB, CAS 77-40-7), 4,4ʹ-ethylidenebisphenol (bisphenol E, BPE, CAS 2081-08-5), 4,4ʹ-methylenediphenol (bisphenol F, BPF, CAS 620-92-8), 4,4ʹ-sulphonyldiphenol (bisphenol S, BPS, CAS 80-09-1), 4,4ʹ-cyclohexylidenebisphenol (bisphenol Z, BPZ, CAS 84-55-0), 2,2-bis-(4-hydroxyphenyl)-hexafluoropropane (bisphenol AF, BPAF, CAS 1478-61-1), 1,1-bis(4-hydroxyphenyl)-1-phenylethane (bisphenol AP, BPAP, CAS 1571-75-1), 4,4ʹ-dihydroxytetraphenylmethane (bisphenol BP, BPBP, CAS 1844-01-5), 2,2ʹ-[(1-methylethylidene)bis(4,1-phenyleneoxymethylene)]bisoxirane (bisphenol A diglycidyl ether, BADGE, CAS 1675-54-3), and 3,3ʹ,5,5ʹ-tetrabromo-4,4ʹ-isopropylidenediphenol (Tetrabromobisphenol-A, TBBPA, CAS 79-94-7) were purchased from Sigma–Aldrich® (Taufkirchen, Germany). An aliquote of 2,2ʹ,6,6ʹ-tetrachloro-4,4ʹ-isopropylidenediphenol (Tetrachlorobisphenol-A, TCBPA, CAS 79-95-8) was generously provided by Dr. Manfred Frey (Steinbeis Innovation Center for Cell Culture Technology, Mannheim, Germany). Reagents for sample preparation were the purest grade available.
Individual stock solutions of the selected bisphenols were prepared by dissolving accurately weighed amounts of analytical standard in dimethyl sulfoxide (DMSO, CAS 67-68-5). Nominal concentrations of the stock solutions were determined to achieve the necessary in-test dilution of the substance. The stock solutions were stored in amber glass vials in the dark at room temperature.
In vitro test procedure
Endocrine activity
The endocrine activity was determined with five different yeast-based reporter gene assays, based on an ISO standard procedure [25], as previously described by Abbas et al. [26] with minor modifications. The bisphenols were tested for the Yeast Estrogen Screen (YES, human estrogen receptor α), Yeast Anti-Estrogen Screen (YAES), Yeast Androgen Screen (YAS, human androgen receptor), Yeast Anti-Androgen Screen (YAAS), and Yeast Dioxin Screen (YDS, aryl hydrocarbon receptor).
In brief, individual cultures of genetically modified strains of Saccharomyces cerevisiae were dissolved in growth medium and incubated at 30 °C with shaking at 450 rpm overnight. Cell densities of the overnight cultures were adjusted to 250 ± 25 (YES/YAES), 500 ± 50 (YAS/YAAS), and 1000 ± 100 (YDS) formazine attenuation units (FAU) to account for different growth times of the strains. The assays were carried out in 96-well microtiter plates with one column per treatment (n = 8 technical replicates). Each plate included a negative control and a solvent control (DMSO). After a 20-h incubation period of the plates at 30 °C with shaking at 1200 rpm, the cell densities were checked photometrically at 595 nm (Multiskan Ascent Microplate Reader, Thermo Fisher Scientific, Braunschweig, Germany) for cytotoxicity. If cytotoxicity > 20% occurred, results were not used. Subsequently, a chlorophenol red (CPRG, CAS 99792-79-7) lyticase (CAS 37340-57-1) buffer solution was added to each well to quantify the endocrine activity via a chromogenic reaction. The colour change was recorded photometrically at 540 nm (Multiskan Ascent Microplate Reader) in 5–20 min intervals (YES: 5 min, YAS/YAES/YAAS: 10 min, YDS: 20 min) over a period of 20–40 min. To determine antagonistic activity, we added the corresponding endogenous agonist to activate the receptor (1.44 M 17β-estradiol or 0.048 M testosterone). The reference compounds 17β-estradiol (CAS 50-28-2, YES), 4-hydroxytamoxifen (CAS 68047-06-3, YAES), testosterone (CAS 58-22-0, YAS), flutamide (CAS 133-11-84-7, YAAS), and β-naphthoflavone (CAS 6051-87-2, YDS) were used as positive controls. Along with each assay and experiment, concentration–response curves for the respective reference compound were generated (EC50 values are reported in Additional file 2).
A preliminary limit test with 10 mg/L was performed for each bisphenol to confirm the actual occurrence of endocrine activity, to take note of possible cytotoxic effects, and adjust the highest tested concentration accordingly. A subsequent dilution series was designed based on the results of the limit test. The YES dilution series was carried out with a highest tested concentration of 10 mg/L and a dilution factor of 4. To attain distinct concentration–response curves in the YAAS and YAES the maximum concentration was raised to 40 mg/L and the dilution factor was adjusted to 2. In the cases of TBBPA and TCBPA, a lower maximum concentration of 2.5 to 3 mg/L was chosen for all tests due to cytotoxicity (Additional file 1: Table S1). Each assay was independently conducted three to six times.
Baseline toxicity
The baseline toxicity of the bisphenols was assessed in the Microtox assay with the luminescent bacterium Aliivibrio fischeri. The test is based on an ISO standard procedure [27] with modifications by Escher et al. [28] to utilize 96-well microtiter plates using 3,5-dichlorophenol (CAS 591-35-5) as a positive control. In brief, an A. fischeri cryo culture was diluted with growth medium and incubated at 20 °C in the dark for 60 min. Negative and solvent controls, positive control, and bisphenol extracts were serially diluted (1:2) in saline buffer. 100 µL of each treatment were added to 50 µL of A. fischeri solution. Luminescence inhibition was determined photometrically (Spark® 10 M, Tecan Austria GmbH, Grödig, Austria) after a 30-min incubation period at 20 °C in the dark. The samples were considered toxic if the luminescence inhibition exceeded 20% at the highest tested concentration. Three independent experiments were conducted with two technical replicates per substance and a twofold dilution ranging from 40 mg/L to 312.5 µg/L.
Mutagenicity
The mutagenic potential of the bisphenols was evaluated with the Ames fluctuation test. Two genetically modified strains of Salmonella typhimurium (YG1041 and YG1042), unable to synthesize the essential amino acid histidine, were used to detect point mutations (frameshift mutations and base-pair substitutions, respectively). In the absence of mutagenic compounds, the histidine deficiency mutants do not grow in histidine-free medium. A reverse mutation in genes involved in histidine biosynthesis enables histidine production, and thus bacteria growth. Mutations are detected via a pH change in the assay medium, indicated by a colour change from purple to yellow. The test is based on an ISO standard method [29] and the OECD guideline 471 [30]. In brief, the cell density of the suspended bacteria culture is determined photometrically at a wavelength of 595 nm (VWR® V-1200 Spectrophotometer, VWR International, Radnor, PA, USA). The required amount of bacteria culture is centrifuged at 4400 rpm for 5 min. The bacteria pellet is resuspended in 15 mL of histidine minimal medium to ensure the initial survival of the bacteria at the beginning of the test. 490 µL medium per well and 10 µL sample extract are distributed to a 24-well microtiter plate. To test for metabolic activation of the samples, 17 µL rat liver homogenate mix (S9) is added per well. Mutagenic reference compounds were used as positive controls (without S9: 2-nitrofluorene (CAS 607-57-8), with S9: 2-aminoanthracene (CAS 613-13-8)). The optical density at 595 nm is measured in a microplate reader (Spark® 10 M, Tecan Austria GmbH, Grödig, Austria) before and after a 100-min incubation period at 37 °C in the dark with shaking at 220 rpm to avoid sedimentation. If the optical density decreased after incubation, cytotoxicity occurred and the samples were excluded from further analysis. Immediately after measuring, 2.5 mL reversion medium is added to each well. The bacteria-medium mixture is aliquoted into 384-well microtiter plates with 48 technical replicates per treatment and incubated at 37 °C for 72 h. The mutagenic activity was determined photometrically at a wavelength of 420 nm (Spark® 10 M) by counting the number of wells with a colour change from purple (negative) to yellow (positive).
The bisphenol solutions were tested at a limit concentration of 10 mg/L for the majority of bisphenols. BADGE and BPBP were tested at a lower concentration of 5 mg/L since the substances were found to be cytotoxic at 10 mg/L. Each substance was tested with metabolic activation through S9 mix and without. The assay was performed twice with 48 technical replicates per treatment.
Data processing
Statistical analyses, correlation analyses, non-linear regressions and estimations of effect concentrations were performed using the software GraphPad Prism® (version 5.03, GraphPad Software Inc., San Diego, CA, USA). Gaussian distribution and homogeneity of variances of the data sets were checked with the D’Agostino and Pearson omnibus normality test and Bartlett’s test for equal variances, respectively. For parametric data sets, a one-way analysis of variance (ANOVA) with Dunnett’s post hoc test was selected to check for possible statistical significance. Non-parametric data sets were analyzed using a Kruskal–Wallis test with Dunn’s post hoc test. A p-value < 0.05 was considered statistically significant. Significant differences in 50% effect concentrations (EC50) of the reference compound BPA and the respective bisphenol analogue were acknowledged if the 95% confidence intervals did not overlap. Correlations were determined using Pearson correlation for normally distributed data. If a data set did not pass the normality test initially, the data set was transformed (ln).
In both the agonistic and antagonistic Yeast Screens, the absorbance was normalized to the combined negative and solvent control (0%) and the maximum activity of the positive control (100%) to calculate relative (anti-)estrogenic and anti-androgenic activities (%). The concentration-response curves of the relative activity were obtained by transforming the x-axis (log) and fitting a non-linear curve to the transformed data. EC50 values were calculated using the non-linear curve fit of the raw, non-normalized and non-transformed data. To adjust for bisphenol curves that did not reach a maximum activity plateau in the tested concentration range, a top constraint was set to the mean maximum activity of the positive control (100%). The maximum relative activity (%) of the bisphenols was obtained through extrapolated top values calculated by GraphPad Prism®. A constraint was set at 0% activity. The concentration–response curves of the Microtox assay were also generated by transforming the x-axis (log) and fitting a non-linear curve to the transformed data. The specific activity (M) of each substance was calculated by dividing the EC50 (mg/L) by the molecular weight (g/mol) of the corresponding bisphenol.
For each tested bisphenol and endocrine assay a baseline specificity ratio (SRbaseline) was calculated according to Escher and Neale [31], if the data were available. The ratio describes how much more potent a chemical is in reporter gene activation (i.e., a specific mode of action) compared to the baseline toxicity. It was calculated by dividing the EC50 of the Microtox assay by the EC50 of the endocrine assay.
Results and discussion
Endocrine activity
This study confirmed the endocrine potential of all twelve tested bisphenols on at least one nuclear hormone receptor in yeast reporter gene assays (Fig. 1). All the bisphenols were able to elicit a response on the human estrogen receptor, with nine compounds acting as agonists and three as antagonists (BPBP, BADGE, TBBPA). Eleven out of the twelve bisphenols showed antagonistic activity on the human androgen receptor, BPS being the sole exception. All bisphenols displayed activity in a concentration-related manner. Two of the five yeast reporter gene assays, namely the YAS and YDS, did not show any endocrine activity above the limit of quantification for the twelve bisphenols at the limit concentration of 10 mg/L (Additional file 1: Figure S1).
Heatmap of the endocrine activity in all five Yeast Screens. Each bisphenol was tested for their estrogenic (YES), anti-estrogenic (YAES), androgenic (YAS), anti-androgenic (YAAS) and dioxin-like (YDS) activity. The colour coding distinguishes between no effect in the tested concentration (green), a significantly higher EC50 (mg/L) compared to BPA (yellow), a similar EC50 as BPA (orange), and a significantly lower EC50 than BPA (red). The significant difference to BPA is determined through non-overlapping confidence intervals (see Tables 1, 2, 3)
The endocrine effects of the bisphenols are likely caused due to their molecular structure resembling the natural hormones 17β-estradiol and testosterone. Binding to the estrogen receptor (ER) as well as androgen receptor (AR) is mainly mediated through the ability to form hydrogen bonds and interactions between the hydrophobic residues on the inner lining of the receptor cavity with the non-polar binding partners [32,33,34]. The two hydroxy groups at the para position of the phenyl rings of the natural hormone 17β-estradiol (E2) are necessary to elicit estrogenic as well as anti-androgenic activity [10, 19, 35]. With two hydroxy groups along with an overall predominantly hydrophobic nature, the chemical structure of the bisphenols makes them suitable to interact with both the ER and AR binding pocket.
Activity at the estrogen receptor
The Yeast Estrogen Screen showed distinct concentration–response curves for the estrogenic activity for nine out of twelve tested bisphenols (Fig. 2). The overall high maximum agonistic activities range from 79.6% (BPS) to 110.9% (TCBPA) (Table 1). Three analogues (BPF, BPAP, TCBPA) elicited a stronger response than the natural ligand of the human estrogen receptor 17β-estradiol (E2, 100%). BPE and BPF show no significant difference in estrogenic efficacy to the reference compound BPA. Whereas BPB, BPAF, and BPZ demonstrate significantly stronger estrogenic activity; BPS, BPAP, and TCBPA show significantly lower activity. The order of magnitude for the specific activity (EC50 (M)) ranges from 10–7 to 10–4 M, with the positive control E2 at 10–10 M.
Yeast Estrogen Screen (YES). Concentration-response curves of the mean estrogenic induction [%] (mean \(\pm\) SEM), relative to the maximum activity of the positive control 17β-estradiol (E2), of the bisphenols BPA, BPB, BPE, BPF, BPS, BPZ, BPAF, BPAP, and TCBPA in the Yeast Estrogen Screen (YES). The bisphenols were tested at concentrations ranging from 38.1 ng/L to 40 mg/L. The order of bisphenols in the legend (top to bottom) follows the ascending order of EC50 values in the figure. A constraint was set at 0% induction, representing the negative control. n = 3 independent experiments. n = 6 for BPA, BPB, and BPF
Modifications of the bridging carbon are presumed to influence the binding of bisphenols to the ER and consequently the estrogenic activity [10, 18, 19, 35]. It has been reported that the length of the alkyl group at the bridging carbon is supposed to enhance estrogenic activity [10]. This would suggest a lower estrogenic activity of BPF (without alkyl) compared to BPA and BPE (methyl group), followed by BPB (ethyl group). The study of Chen et al. [10] confirmed this, ranking the estrogenic potency BPF \(\le\) BPE \(\le\) BPA < BPB. The results of the YES in this study are in accordance with these findings. BPB displayed the highest activity of the four considered bisphenols, followed by the remaining three substances (BPA \(\le\) BPE \(\le\) BPF < BPB). BPA, BPE, and BPF did not differ significantly in their estrogenic activity in the YES. A computational study by Cao et al. [18] combined molecular docking with molecular dynamics simulations and also predicted an enhanced estrogenic activity compared to BPA with smaller-sized substituents on the bridging carbon. Correspondingly, it is postulated that larger substituents, such as the ethyl group of BPB or the additional phenyl group of BPAP, increase the molecular size and thus reduce estrogenic activity [18]. This claim holds true only partially. BPAP does indeed elicit lower estrogenic potential. Nevertheless, measured by the EC50 obtained in the YES, BPB shows significantly higher estrogenic potential compared to BPA. Despite its large cyclohexane group on the bridging carbon, BPZ also contradicted the postulated principle and showed the highest estrogenicity in the YES. Another factor defining the estrogenic potential may be the polarity of the bridging groups. Cao et al. [18] conducted a study with MVLN-cells and demonstrated enhanced estrogenic activity with polar bridging groups, such as BPS (sulfonyl group) and BPAF (trifluoromethyl groups). On the other hand, Kitamura et al. [19] noted that replacing the propane group of BPA with a more hydrophilic group reduced estrogenic activity in MCF-7 cells (advancement of MVLN-cells) remarkably. While the claim of Cao et al. [18] is confirmed for the activity of BPAF in this study, it cannot be confirmed for BPS. In the case of BPS, the assertion of Kitamura et al. [19] of reduced estrogenicity applies since BPS turned out to be the bisphenol with the lowest estrogenic potential by far. The polar bridging group of BPS, contrasted with the other selected bisphenols, possibly elucidates lower binding probability to the ER and as a result, lower estrogenic activity. In silico studies support the statement of lower affinity of BPS to the estrogen receptor [35, 36]. Different studies also report a distinctly lower estrogenic activity of BPS compared to other tested BPs [10, 11]. A study investigating the estrogenic activity of halogenated bisphenol analogues (i.e., TCBPA and TBBPA) found the higher the degree of halogenation the lower the activity at the ER [37, 38]. The brominated analogue TBBPA shows no agonistic effect at the ER [38, 39], while the chlorinated analogue TCBPA has similarly strong estrogenic potential as BPA [37]. Both observations are confirmed by this study. Overall, it is not yet well defined how the chemical specificity at the 3,5-positions of the phenyl rings and the bridging alkyl moiety determine the extent of estrogenic potential of bisphenols. Nevertheless, quantitative structure–activity relationship (QSAR) models can predict ER binding and activity of bisphenol analogues reliably [11, 36].
The order of rank of the estrogenic potential, measured with the EC50 values, of all tested bisphenols is as follows: BPS < BPAP < TCBPA < BPA, BPF, BPE < BPB < BPAF < BPZ. The order of magnitude ranges from 10–7 to 10–5 M. These results are generally in agreement with other studies using yeast cells but also other cell lines [12, 19, 20]. Overall, it is reported that most bisphenol analogues show comparable estrogenic potential as BPA in the order of magnitude of 10–6 M [11], with BPA being four to six orders of magnitude less potent than the natural estrogen 17β-estradiol [10, 12, 40, 41]. These findings can be confirmed. Hence, BPA and its analogues can be considered weak estrogens [10, 12, 18]. Additionally, it should be mentioned that some cell lines (e.g., MCF-7 [19], CHO-K1 [20], HepG2 [42]) are more sensitive to estrogenic activity than other cell lines (e.g., Ishikawa [42], HeLa [42], yeast [12]). The more responsive cell lines were generally one order of magnitude more sensitive to bisphenol activity, as well as E2 activity.
The anti-estrogenic yeast reporter gene assay showed no effects at 10 mg/L for the bisphenols that were previously described as ER agonists. BPBP, BADGE, and TBBPA did not display agonistic, but in turn moderate antagonistic activity, unlike the reference BPA (Fig. 3). ER inhibition ranged from 39.7 to 91.3% compared to the partially antagonistic positive control 4-hydroxytamoxifen (Table 2). The antagonistic efficacy is in the same order of magnitude as the positive control (10–6 M). These results confirm that all tested bisphenols are able to bind to the ER but not all of them are able to activate it.
Yeast Anti-Estrogen Screen (YAES). Concentration-response curves of the mean anti-estrogenic induction (%) (mean \(\pm\) SEM), relative to the maximum activity of the positive control 4-hydroxytamoxifen (4-OHT), of the bisphenols BADGE, BPBP, and TBBPA in the Yeast Anti-Estrogen Screen (YAES). The bisphenols were tested at concentrations ranging from 9.75 µg/L to 40 mg/L. The order of bisphenols in the legend (top to bottom) follows the ascending order of EC50 values in the figure. A constraint was set at 0% induction, representing the negative control. n = 3 independent experiments
While there is plenty of conclusive data covering the estrogenic effects of bisphenol analogues, the data for anti-estrogenic effects are comparably scarce. The majority of the tested bisphenols show a distinct pattern of ER agonism in combination with AR antagonism [21]. However, a QSAR study by Conroy-Ben et al. [36] revealed a different binding interaction between BPBP and the ERα binding pocket compared to other bisphenols. While agonistic bisphenols (e.g., BPA, BPB, BPE, BPF), as well as the natural hormone E2, interacted with the Glu353 residue of the ER binding pocket via hydrogen bonding, BPBP did not. Instead, BPBP interacted with residue 524 in a similar manner to the D-ring of E2 [36]. This suggests that ERα binding at Glu353 is an indicator of ER agonism. Binding at a different site (i.e., residue 524) merely blocks the binding pocket and does not lead to ER activity.
The ER antagonism for BPBP, BADGE and TBBPA was already observed in other studies [19, 21]. However, BPAP and BPAF also seem to have low antagonistic properties in addition to agonistic potential at the ER [21, 43, 44]. Despite similar tested concentrations, this effect was not revealed in our study. This might be due to different effects of the bisphenols at the ERα and ERβ. While BPAF acted as a full agonist at ERα, it proved to be a specific antagonist at ERβ in various different cell lines [20, 42, 43]. Another explanation would be that, according to Li et al. [42], BPAF as well as BPA act as ERα- and ERβ-antagonists at concentrations lower than 10 nM (approx. 2–3 µg/L), which were not tested in our work.
Activity at the androgen receptor
The Yeast Anti-Androgen Screen revealed antagonistic activity at the human androgen receptor for each selected bisphenol, apart from BPS (Fig. 4). All of the positively tested bisphenols show a similarly high or higher maximum relative activity than the competitive antagonist flutamide (positive control) in a range from 100 to 138.2% (Table 3). Six analogues demonstrate significantly higher antagonistic efficacies (BPB, BPE, BPF, BPAP, TBBPA, TCBPA), while the three analogues BPZ, BPAF, and BADGE show significantly lower efficacies. The order of magnitude for the specific activity is the same as the positive control (10–6 M).
Yeast Anti-Androgen Screen (YAAS). Concentration-response curves of the mean anti-androgenic induction (%) (mean \(\pm\) SEM), relative to the maximum activity of the positive control flutamide (Flu), of the bisphenols BPA, BPB, BPE, BPF, BPZ, BPAF, BPAP, BPBP, BADGE, TBBPA and TCBPA in the Yeast Anti-Androgen Screen (YAAS). The bisphenols were tested at concentrations ranging from 9.75 µg/L to 40 mg/L. The order of bisphenols in the legend (top to bottom) follows the ascending order of EC50 values in the figure. A constraint was set at 0% induction, representing the negative control. n = 3 independent experiments
The Yeast Anti-Androgen Screen displayed significant effects for eleven of the twelve tested bisphenols, except BPS. These results are consistent with the observation that many environmental estrogens also possess the ability to elicit anti-androgenic activity [45], as well as previous predictions on AR antagonism based on QSAR models [11, 36].
The QSAR study by Conroy-Ben et al. [36] confirmed AR binding ability for the bisphenols tested in their study, including BPA, BPB, BPBP, BPE, BPF, BPS, and BPZ, by demonstrating the hydrogen bonding of a bisphenol hydroxy group with the same two amino acids (i.e., Thr877, Asn705), that bind the natural hormone testosterone and the antagonist standard hydroxyflutamide. Binding affinity can predict potential agonism or antagonism but does not determine how considerable the actual effect is going to be [46]. The weakest binding affinity was observed for BPS [36]. Not being able to bind well to the AR would resolve why BPS did not elicit any anti-androgenic response in our study. Other studies also report either no anti-androgenic activity for BPS at all [20, 22, 47] or notably less activity compared to other tested BPs [11, 13, 36]. A possible explanation for the weaker binding might be the polar sulfonyl bridge, making BPS less able to form hydrophobic interactions with the ligand binding domain.
Grimaldi et al. [48] observed that the most potent ER agonists turned out to be the most potent AR antagonists and proposed that larger substituent groups on the bridging carbon could cause a reduction in endocrine efficacy. In our study, there is no correlation between ER agonists and AR antagonists (Pearson r = − 0.1125; Additional file 2). A trend of larger side chain groups or molecular size in general causing lowered endocrine potential is also not visible.
The order of rank of the anti-androgenic potential of all seven bisphenols is as follows: (BPS <) BADGE, BPZ \(\approx\) BPAP < BPA, BPAF < BPB, BPBP < BPF < BPE < TBBPA, TCBPA). The ranking corresponds very well with the results of van Leeuwen et al. [21] and is consistent with other studies using various cell systems [11, 20, 47, 49]. However, some studies report a weaker or no anti-androgenic effect for bisphenols that showed a higher efficacy than BPA in our study (e.g., TBBPA, TCBPA, BPBP, BPF) [17, 19, 22, 36, 48, 50]. All the bisphenols that tested positive were antagonistically active in the same order of magnitude as the weak anti-androgen flutamide (10–6 M) [51], classifying them as weak anti-androgens. Mammalian cell lines (e.g., CHO [11], CHO-K1 [20], HeLa [48], NIH3T3 [19], MDA-kb2 [50], PALM [22]) do not seem to be much more sensitive to anti-androgenic activity compared to yeast cells (see [21, 36, 49]).
None of the selected bisphenols showed agonistic effects at the AR at a concentration of 10 mg/L. The results of the YAS are coherent with those of the YAAS, since full antagonists do not convey intrinsic activity. These findings are in agreement with most other studies [13, 20, 21, 36, 46, 49].
Activity at the aryl hydrocarbon receptor
None of the selected bisphenols showed effects in the Yeast Dioxin Screen at the tested limit concentration of 10 mg/L. High affinity to the aryl hydrocarbon receptor (AhR) requires certain structural characteristics, such as non-covalent binding with the binding cavity (van der Waals forces or hydrogen bonds), hydrophobic effects, or planarity (“classic” AhR ligands) [52, 53]. Substances may also bind to the AhR without these structural requirements (“non-classic” ligands), but do not display much affinity and/or intrinsic activity compared to dioxin (TCDD) [52, 54]. Since it is possible for BPs to form hydrogen bonds, they may be able to moderately bind to the AhR. The negative results of the YDS indicate no intrinsic activity, potentially characterising the bisphenols only as partial agonists or antagonists.
The literature on AhR activity of BPs is ambiguous. According to the US National Toxicology Program Tox21, none of the 14 bisphenols tested (including nine of the BPs tested in our study) act as an agonist at the AhR [17]. Rosenmai et al. [11] have reported AhR agonism for BPA, BPE, and BPF, whereas BPB and BPS showed no effect on the AhR in rat hepatoma cells. Park et al. [13] also regard BPA and BPF as AhR agonists in DR-EcoScreen cells, along with BPS. Zenata et al. [55] note negligible activation of the AhR for BPS compared to the positive control TCDD in a cell line derived from HepG2 cells [55]. Bonefeld-Jørgensen et al. [56], however, observed AhR antagonism for BPA in mouse hepatoma cells. The differences in AhR activity might be due to the usage of various transfected mammalian hepatoma cell lines compared to yeast cells, as mammalian cell lines are generally more sensitive than yeasts mainly due to the absence of a cell wall. Furthermore, the concentrations eliciting a response in studies with mammalian cells are mostly above 10 mg/L, which was the limit concentration for the Yeast Dioxin Screen. It is worth noting that even mouse [13] and rat [11] hepatoma cells yield opposing results at the same concentration (25 mg/L BPS).
Baseline toxicity
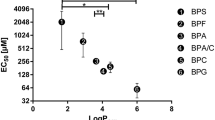
Nine of the twelve selected bisphenols induced baseline toxicity in a concentration-related manner within the analyzed concentration range (Fig. 5). The analogues BPBP, BADGE, and TBBPA did not elicit effects beyond the threshold of 20% luminescence inhibition at the maximum concentration (40 mg/L). Consequently, we were unable to derive EC50 values and omitted the concentration–response curves from the figure. The remaining bisphenols demonstrated a maximum luminescence inhibition of 51.6% (BPS) to 100% (BPB, BPZ, BPAF, BPAP) (Table 4). Merely BPS and BPE displayed lower maximum inhibition compared to the reference compound BPA (89.5%). The efficacies, measured in EC50 values, ranged from 5.81 to 39.1 mg/L (Table 4). BPB, BPF, BPZ, BPAF, and BPAP had a significantly higher effect than BPA. The rank of EC50 values corresponds well to the rank of maximum inhibition efficacy of the substances. The specific activity (EC50 (M)) of the bisphenol analogues (Table 4) is similar, but not identical, to the order of ranks of the EC50. The analogues show specific activity in the same order of magnitude as the reference BPA (10–5 M), indicating similarly strong potential for baseline toxicity.
Microtox assay. The baseline toxicity, expressed as luminescence inhibition (%) (mean \(\pm\) SEM), is displayed as concentration-response curves of the bisphenols BPA, BPB, BPE, BPF, BPS, BPZ, BPAF, BPAP, and TCBPA in the Microtox assay. The bisphenols were tested at concentrations ranging from 313 µg/L to 40 mg/L. The order of bisphenols in the legend (top to bottom) follows the ascending order of EC50 values in the figure. Constraints were set at 0% and 100% luminescence inhibition. n = 3 independent experiments
Baseline toxicity, also referred to as non-polar narcosis or non-specific toxicity, describes the minimal toxicity of a chemical [57, 58]. Although the precise molecular mechanism has not yet been conclusively resolved [59], it is widely accepted as a disturbance of the cell membrane caused by the partitioning of hydrophobic chemicals into the membrane lipids [57] and their accumulation [60], resulting in failing integrity and function of the membrane [59]. Since this phenomenon is strongly correlated with the hydrophobicity of a substance [61], the octanol/water partition coefficient (log KOW) can be useful for interpreting the results.
In theory, the order of ranks of bisphenol baseline toxicity, according to the sequence of experimental/predicted log KOW values obtained from the EPI Suite™ software (EPIWEB 4.1, U.S. Environmental Protection Agency, USA), should be the following: BPS (1.65), BPF (2.91), BPE (3.19), BPA (3.32), BADGE (3.84), BPB (4.13), BPAF (4.47), BPAP (4.86), BPZ (5.0), BPBP (6.08), TCBPA (6.22), TBBPA (6.53). This is generally reflected in the test results. The actual order of ranks was (BPBP, TBBPA, BADGE) < BPS < BPE < TCBPA < BPA < BPB < BPF, BPZ, BPAP < BPAF. It is noticeable that the three analogues with the highest lipophilicity (BPBP, TCBPA, TBBPA) do not also display the strongest baseline toxicity. In turn, BPBP and TBBPA did not surpass the threshold luminescence inhibition of 20% at the highest concentration of 40 mg/L and are, therefore, not considered toxic in this test, despite being clearly lipophilic. Another factor determining the ability to pass through membranes could be the spatial dimensions of the compounds, as proposed by Bruggeman et al. [62]. A larger molecular volume or diameter would, therefore, interfere with membrane passage, which could be plausible for BPBP, BADGE and TBBPA. TCBPA eliciting an effect, but not TBBPA, could be attributed to the smaller effective diameter of chlorine compared to bromine [62]. When excluding TCBPA and the bisphenols with no effect, a significant negative linear correlation of EC50 to log KOW with a Pearson r of − 0.825 and R2 of 0.681 (p = 0.0116) becomes apparent (Additional file 2). This direct relationship was already observed before [63].
These results are reasonably conclusive with the study of Owczarek et al. [64], which also found stronger baseline toxicity for BPF and lower toxicity for BPS and BPE compared to BPA. BPZ was reported to be only slightly toxic, edging towards inactivity [64], therefore, not complying with our results and the overall presented trend of increased baseline toxicity with decreased polarity.
Baseline specificity ratio
The baseline specificity ratio (SR) is the ratio of the non-specific baseline toxicity of a substance to its specific mode of action [31], in this case reporter gene activation. It measures the degree of specificity of the effects of bisphenols and can illustrate how much more potent each bisphenol is in its endocrine activity compared to the baseline toxicity. A reliable threshold for specificity is an SR above 10 [31]. Substances with an SR ≤ 1 are non-specific, 1 ≤ SR < 10 is considered moderately specific but with uncertainty, 10 ≤ SR < 100 is specific and an SR above 100 is highly specific [31].
The majority of bisphenols in this study exhibit specific effects at the ER and AR, with specificity ratios between 12.5 (BPA, anti-androgenic activity) and 83.6 (BPE, anti-androgenic activity) (Fig. 6). Some analogues, such as BPS and BPAP, are only moderately specific in reporter gene activation. Both halogenated analogues (i.e., TBBPA and TCBPA) show high specificity for ER and AR antagonism and are the only analogues to reach SR values above 100.
Baseline specificity ratio of each tested bisphenol. The baseline specificity ratio (SR) was calculated for the estrogenic (YES), anti-estrogenic (YAES), and anti-androgenic (YAAS) activity using Eq. 2. SR ≤ 1 is not specific, 1 ≤ SR < 10 is moderately specific (with high uncertainty), 10 ≤ SR < 100 is specific, and 100 ≤ SR is highly specific. “-” indicates that there was at least one EC50 missing for the calculation of the baseline specificity ratio. All EC50 values can be looked up in Tables 1, 2, 3, and 4. The data set calculating the SR includes an EC50 for baseline toxicity of TBBPA with a wide confidence interval (89.17–161.5 mg/L)
As this concept is fairly new and originally devised to assess (unknown) mixture toxicity effects (e.g., in wastewater or surface water samples), only one other study has used it yet to better understand the specificity in single substance testing. Lee et al. [65] used QSAR-predicted 10% nominal baseline toxicity values (IC10,baseline) and compared them to experimentally determined 10% cytotoxicity (IC10) values in four mammalian reporter gene cell lines (AREc32, AhR-CALUX, PPARγ-BLA, and SH-SY5Y) for 392 chemicals. They calculated the toxic ratio (TR = IC10,baseline/IC10) to identify whether chemicals act as baseline toxicants (TR < 10) or indicate enhanced toxicity due to a specific mode of action (TR ≥ 10). They observed the more hydrophobic a chemical is, the more likely it is classified as a baseline toxicant [65]. For TBBPA, they found a TR < 0.1 in SH-SY5Y, suggesting no specific mode of action, markedly contrasting the results of this study. The review of Robitaille et al. [66] mentions that only a very small number of chemicals act as high-potency agonists in hormone receptor transactivation assays. Most show low to medium ER agonistic potency, with specificity ratios below 1000, in no way reaching the high potency of the natural ER agonists (e.g., E2) at SRs of 106–107 [31]. This is conclusive with our data, as all tested bisphenols have SR values for ER agonism below 100. As for AR antagonistic SRs, Escher et al. [67] suggest further research into the mechanisms of antagonistic effects by environmental samples and caution with interpreting the data, as cytotoxicity cannot be differentiated from AR antagonism. Since we performed single substance testing, monitored the cytotoxicity, and discarded measurements exceeding 20% cytotoxicity, there is little uncertainty for false-positive AR antagonism.
Mutagenicity
The assessment of the mutagenic activity of the twelve selected bisphenols did not yield any positive results at the tested concentration of 5–10 mg/L (Additional file 1: Table S2). Neither the parent substances nor their metabolites caused frameshift mutations or base-pair substitutions.
These results are in accordance with the literature. Fic et al. [23] report no mutagenic effects of the bisphenols BPF, BPAF, BPZ, and BPS, as well as for four additional analogues, using the TA98 and TA100 Salmonella strains in the Ames assay with and without metabolic activation. An umu test of the bisphenols BPA, BPF, BPB, and BPS as well as their metabolites also did not show mutagenic activity [10]. However, other genetic modes of action such as genotoxicity have been reported for BPA and some of its analogues [16]. For instance, induction of DNA damage in the form of DNA strand breakage was observed for BPA and BPS in HepG2 cells at concentrations above human exposure levels [23]. TBBPA is reported to promote several cancers in rats [68] and was classified as a probable carcinogen [69]. So far, the connection between carcinogenicity and BPA is still unclear [70].
Conclusion
The work presented in this study focuses on broadening and fortifying the existing knowledge concerning the (eco)toxicological profile of various bisphenol analogues as replacements for the excessively assessed and hazardous substance bisphenol A. The results contribute to the question of to what extent the eleven selected bisphenol analogues, BPB, BPE, BPF, BPS, BPZ, BPAF, BPAP, BPBP, BADGE, TBBPA, and TCBPA, are suitable replacements for BPA. To resolve this, several toxicological endpoints, with a focus on endocrine activity, were assessed in vitro. As most of these BPs have been tested by others, it is widely agreed upon, that bisphenol analogues are equally as potent at the ER and AR in vitro as BPA [11,12,13, 18, 47], with some of them being even more potent [20, 21]. Nine BPs elicited weak estrogenic activity, with the remaining three acting as ER antagonists, and all BPs except for BPS showed weak anti-androgenic activity. These results might help to prioritize BPs for further in vivo testing. The results of the present study suggest that the selected bisphenol analogues are not safe alternatives to the high production volume chemical bisphenol A. Many other studies are in agreement that the continuing substitution of BPA with structurally similar analogues needs to be carried out with caution [9, 11, 18, 71], as they are already deemed emerging contaminants and regrettable substitutions.
Availability of data and materials
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AhR:
-
Aryl hydrocarbon receptor
- AR:
-
Androgen receptor
- BADGE:
-
bisphenol A diglycidyl ether
- BPA:
-
Bisphenol A
- BPB:
-
Bisphenol B
- BPBP:
-
Bisphenol BP
- BPE:
-
Bisphenol E
- BPF:
-
Bisphenol F
- BPS:
-
Bisphenol S
- BPs:
-
Bisphenols
- BPZ:
-
Bisphenol Z
- BPAF:
-
Bisphenol AF
- BPAP:
-
Bisphenol AP
- E2:
-
17β-estradiol
- EC50 :
-
50% effect concentration
- ER:
-
Estrogen receptor
- S9:
-
Rat liver homogenate mix
- SR:
-
Specificity ratio
- TBBPA:
-
Tetrabromobisphenol A
- TCBPA:
-
Tetrachlorobisphenol A
- YAAS:
-
Yeast Anti-Androgen Screen
- YAS:
-
Yeast Androgen Screen
- YAES:
-
Yeast Anti-Estrogen Screen
- YDS:
-
Yeast Dioxin Screen
- YES:
-
Yeast Estrogen Screen
References
Huang YQ, Wong CKC, Zheng JS et al (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99. https://doi.org/10.1016/j.envint.2011.04.010
Im J, Löffler FE (2016) Fate of bisphenol A in terrestrial and aquatic environments. Environ Sci Technol 50:8403–8416. https://doi.org/10.1021/acs.est.6b00877
Qiu W, Zhan H, Hu J et al (2019) The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: a critical review of recent progress. Ecotoxicol Environ Saf 173:192–202. https://doi.org/10.1016/j.ecoenv.2019.01.114
Transparency Market Research (2013) Bisphenol A market for polycarbonates, epoxy resins and other applications—global industry analysis, aize, share, growth and forecast, 2013–2019. Transparency Market Research
Almeida S, Raposo A, Almeida-González M, Carrascosa C (2018) Bisphenol A: food exposure and impact on human health. Compr Rev Food Sci Food Saf 17:1503–1517. https://doi.org/10.1111/1541-4337.12388
Nasello S, Beiguel É, Fitó-Friedrichs G et al (2022) Thermal paper as a potential source of bisphenol A for humans and the environment: migration and ecotoxicological impact. Environ Sci Pollut Res Int 29:53382–53394. https://doi.org/10.1007/s11356-022-19561-9
Vandenberg LN, Ehrlich S, Belcher SM et al (2013) Low dose effects of bisphenol A. Endocr Disruptors. https://doi.org/10.4161/endo.26490
ECHA (2017) MSC unanimously agrees that Bisphenol A is an endocrine disruptor. https://echa.europa.eu/de/-/msc-unanimously-agrees-that-bisphenol-a-is-an-endocrine-disruptor. Accessed 3 Apr 2023.
Rochester JR, Bolden AL (2015) Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123:643–650. https://doi.org/10.1289/ehp.1408989
Chen M-Y, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol A and other bisphenols. Environ Toxicol 17:80–86. https://doi.org/10.1002/tox.10035
Rosenmai AK, Dybdahl M, Pedersen M et al (2014) Are structural analogues to bisphenol A safe alternatives? Toxicol Sci 139:35–47. https://doi.org/10.1093/toxsci/kfu030
Ruan T, Liang D, Song S et al (2015) Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 124:150–155. https://doi.org/10.1016/j.chemosphere.2014.12.017
Park C, Song H, Choi J et al (2020) The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.114036
ECHA (2021) Annex XV report proposal for identification of A substance of very high concern on the basis of the criteria set out in reach article 57.
ECHA (2023) ECHA adds nine hazardous chemicals to Candidate List. https://echa.europa.eu/de/-/echa-adds-nine-hazardous-chemicals-to-candidate-list. Accessed 3 Apr 2023.
Usman A, Ahmad M (2016) From BPA to its analogues: Is it a safe journey? Chemosphere 158:131–142. https://doi.org/10.1016/j.chemosphere.2016.05.070
Pelch K, Wignall JA, Goldstone AE et al (2019) A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology. https://doi.org/10.1016/j.tox.2019.06.006
Cao H, Wang F, Liang Y et al (2017) Experimental and computational insights on the recognition mechanism between the estrogen receptor α with bisphenol compounds. Arch Toxicol 91:3897–3912. https://doi.org/10.1007/s00204-017-2011-0
Kitamura S, Suzuki T, Sanoh S et al (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84:249–259. https://doi.org/10.1093/toxsci/kfi074
Kojima H, Takeuchi S, Sanoh S et al (2019) Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 413:48–55. https://doi.org/10.1016/j.tox.2018.12.001
van Leeuwen SPJ, Bovee TFH, Awchi M et al (2019) BPA, BADGE and analogues: A new multi-analyte LC-ESI-MS/MS method for their determination and their in vitro (anti)estrogenic and (anti)androgenic properties. Chemosphere 221:246–253. https://doi.org/10.1016/j.chemosphere.2018.12.189
Molina-Molina J-M, Amaya E, Grimaldi M et al (2013) In vitro study on the agonistic and antagonistic activities of bisphenol S and other bisphenol A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272:127–136. https://doi.org/10.1016/j.taap.2013.05.015
Fic A, Žegura B, Sollner Dolenc M et al (2013) Mutagenicity and DNA damage of bisphenol A and its structural analogues in HepG2 cells. Arh Hig Rada Toksikol 64:189–200. https://doi.org/10.2478/10004-1254-64-2013-2319
Ullah A, Pirzada M, Jahan S et al (2018) Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere 209:508–516. https://doi.org/10.1016/j.chemosphere.2018.06.089
ISO 19040-1:2018 (2018) Water quality—determination of the estrogenic potential of water and waste water—part 1: yeast estrogen screen (Saccharomyces cerevisiae), 1st edn. International Organization for Standaradization (ISO), Geneva
Abbas A, Schneider I, Bollmann A et al (2019) What you extract is what you see: optimising the preparation of water and wastewater samples for in vitro bioassays. Water Res 152:47–60. https://doi.org/10.1016/j.watres.2018.12.049
ISO 11348-3:2007 (2007) Water quality—determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (luminescent bacteria test)—Part 3: method using freeze-dried bacteria, 2nd edn. International Organization for Standardization (ISO), Geneva
Escher BI, Bramaz N, Mueller JF et al (2008) Toxic equivalent concentrations (TEQs) for baseline toxicity and specific modes of action as a tool to improve interpretation of ecotoxicity testing of environmental samples. J Environ Monit 10:612–621. https://doi.org/10.1039/B800949J
ISO 11350:2012 (2012) Water quality—determination of the genotoxicity of water and waste water—Salmonella/microsome fluctuation test (Ames fluctuation test), 1st edn. International Organization for Standardization (ISO), Geneva
OECD (2020) Test No. 471: bacterial reverse mutation test. OECD
Escher BI, Neale PA (2021) Effect-based trigger values for mixtures of chemicals in surface water detected with in vitro bioassays. Environ Toxicol Chem 40:487–499. https://doi.org/10.1002/etc.4944
Pereira de Jésus-Tran K, Côté P-L, Cantin L et al (2006) Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci 15:987–999. https://doi.org/10.1110/ps.051905906
Yang X, Liu H, Yang Q et al (2016) Predicting anti-androgenic activity of bisphenols using molecular docking and quantitative structure-activity relationships. Chemosphere 163:373–381. https://doi.org/10.1016/j.chemosphere.2016.08.062
Yaşar P, Ayaz G, User SD et al (2017) Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol 16:4–20. https://doi.org/10.1002/rmb2.12006
Grignard E, Lapenna S, Bremer S (2012) Weak estrogenic transcriptional activities of bisphenol A and bisphenol S. Toxicol In Vitro 26:727–731. https://doi.org/10.1016/j.tiv.2012.03.013
Conroy-Ben O, Garcia I, Teske SS (2018) In silico binding of 4,4’-bisphenols predicts in vitro estrogenic and antiandrogenic activity. Environ Toxicol 33:569–578. https://doi.org/10.1002/tox.22539
Riu A, le Maire A, Grimaldi M et al (2011) Characterization of novel ligands of ERα, Erβ, and PPARγ: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci 122:372–382. https://doi.org/10.1093/toxsci/kfr132
Riu A, Grimaldi M, le Maire A et al (2011) Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119:1227–1232. https://doi.org/10.1289/ehp.1003328
Meerts IA, Letcher RJ, Hoving S et al (2001) In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109:399–407. https://doi.org/10.1289/ehp.01109399
Routledge EJ, Sumpter JP (1996) Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem 15:241–248. https://doi.org/10.1002/etc.5620150303
vom Saal FS, Cooke PS, Buchanan DL et al (1998) A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health 14:239–260. https://doi.org/10.1177/074823379801400115
Li Y, Burns KA, Arao Y et al (2012) Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ Health Perspect 120:1029–1035. https://doi.org/10.1289/ehp.1104689
Matsushima A, Liu X, Okada H et al (2010) Bisphenol AF is a full agonist for the estrogen receptor ERα but a highly specific antagonist for ERβ. Environ Health Perspect 118:1267–1272. https://doi.org/10.1289/ehp.0901819
Xiao X, Li J, Yu T et al (2018) Bisphenol AP is anti-estrogenic and may cause adverse effects at low doses relevant to human exposure. Environ Pollut 242:1625–1632. https://doi.org/10.1016/j.envpol.2018.07.115
Sohoni P, Sumpter JP (1998) Several environmental oestrogens are also anti-androgens. J Endocrinol 158:327–339. https://doi.org/10.1677/joe.0.1580327
Wang S, Aarts JMMJG, de Haan LHJ et al (2014) Towards an integrated in vitro strategy for estrogenicity testing. J Appl Toxicol 34:1031–1040. https://doi.org/10.1002/jat.2928
Teng C, Goodwin B, Shockley K et al (2013) Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact 203:556–564. https://doi.org/10.1016/j.cbi.2013.03.013
Grimaldi M, Boulahtouf A, Toporova L, Balaguer P (2019) Functional profiling of bisphenols for nuclear receptors. Toxicology 420:39–45. https://doi.org/10.1016/j.tox.2019.04.003
Fic A, Žegura B, Gramec D, Mašič LP (2014) Estrogenic and androgenic activities of TBBA and TBMEPH, metabolites of novel brominated flame retardants, and selected bisphenols, using the XenoScreen XL YES/YAS assay. Chemosphere 112:362–369. https://doi.org/10.1016/j.chemosphere.2014.04.080
Christen V, Crettaz P, Oberli-Schrämmli A, Fent K (2010) Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere 81:1245–1252. https://doi.org/10.1016/j.chemosphere.2010.09.031
Herath W, Khan IA (2010) Microbial metabolism. Part 11. Metabolites of flutamide. Chem Pharm Bull (Tokyo) 58:562–564. https://doi.org/10.1248/cpb.58.562
Pohjanvirta R (2011) The AH receptor in biology and toxicology. John Wiley & Sons, Hoboken
Gu C, Cai J, Fan X et al (2020) Theoretical investigation of AhR binding property with relevant structural requirements for AhR-mediated toxicity of polybrominated diphenyl ethers. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126554
Denison MS, Nagy SR (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43:309–334. https://doi.org/10.1146/annurev.pharmtox.43.100901.135828
Zenata O, Dvorak Z, Vrzal R (2017) Profiling of bisphenol S towards nuclear receptors activities in human reporter cell lines. Toxicol Lett 281:10–19. https://doi.org/10.1016/j.toxlet.2017.09.006
Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM (2007) Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. https://doi.org/10.1289/ehp.9368
van Wezel AP, Opperhuizen A (1995) Narcosis due to environmental pollutants in aquatic organisms: residue-based toxicity, mechanisms, and membrane burdens. Crit Rev Toxicol 25:255–279. https://doi.org/10.3109/10408449509089890
Massei R, Knapen D, Covaci A et al (2021) Sublethal effect concentrations for nonpolar narcosis in the zebrafish embryo. Environ Toxicol Chem 40:2802–2812. https://doi.org/10.1002/etc.5170
Escher BI, Ashauer R, Dyer S et al (2011) Crucial role of mechanisms and modes of toxic action for understanding tissue residue toxicity and internal effect concentrations of organic chemicals. Integr Environ Assess Manag 7:28–49. https://doi.org/10.1002/ieam.100
Urrestarazu Ramos E, Vaes WHJ, Verhaar HJM, Hermens JLM (1998) Quantitative structure−activity relationships for the aquatic toxicity of polar and nonpolar narcotic pollutants. J Chem Inf Comput Sci 38:845–852. https://doi.org/10.1021/ci980027q
Broderius SJ, Kahl MD, Hoglund MD (1995) Use of joint toxic response to define the primary mode of toxic action for diverse industrial organic chemicals. Environ Toxicol Chem 14:1591–1605. https://doi.org/10.1002/etc.5620140920
Bruggeman WA, Opperhuizen A, Wijbenga A, Hutzinger O (1984) Bioaccumulation of super-lipophilic chemicals in fish. Toxicol Environ Chem 7:173–189. https://doi.org/10.1080/02772248409357024
Ren S, Frymier PD (2002) Estimating the toxicities of organic chemicals to bioluminescent bacteria and activated sludge. Water Res 36:4406–4414. https://doi.org/10.1016/S0043-1354(02)00153-7
Owczarek K, Kudłak B, Simeonov V et al (2018) Binary mixtures of selected bisphenols in the environment: their toxicity in relationship to individual constituents. Molecules. https://doi.org/10.3390/molecules23123226
Lee J, Braun G, Henneberger L et al (2021) Critical membrane concentration and mass-balance model to identify baseline cytotoxicity of hydrophobic and ionizable organic chemicals in mammalian cell lines. Chem Res Toxicol 34:2100–2109. https://doi.org/10.1021/acs.chemrestox.1c00182
Robitaille J, Denslow ND, Escher BI et al (2022) Towards regulation of endocrine disrupting chemicals (EDCs) in water resources using bioassays—a guide to developing a testing strategy. Environ Res 205:112483. https://doi.org/10.1016/j.envres.2021.112483
Escher BI, Aїt-Aїssa S, Behnisch PA et al (2018) Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci Total Environ 628–629:748–765. https://doi.org/10.1016/j.scitotenv.2018.01.340
den Braver-Sewradj SP, van Spronsen R, Hessel EVS (2020) Substitution of bisphenol A: a review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit Rev Toxicol 50:128–147. https://doi.org/10.1080/10408444.2019.1701986
Grosse Y, Loomis D, Guyton KZ et al (2016) Carcinogenicity of some industrial chemicals. Lancet Oncol 17:419–420. https://doi.org/10.1016/S1470-2045(16)00137-6
Jalal N, Surendranath AR, Pathak JL et al (2018) Bisphenol A (BPA) the mighty and the mutagenic. Toxicol Rep 5:76–84. https://doi.org/10.1016/j.toxrep.2017.12.013
Eladak S, Grisin T, Moison D et al (2015) A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 103:11–21. https://doi.org/10.1016/j.fertnstert.2014.11.005
Acknowledgements
We would like to thank Dr. Hansgeorg Jehner (Gerty-Strohm-Stiftung, Frankfurt/Main, Germany) for providing the individual scholarship for the doctoral thesis of Natalie Reininger. We also thank Andrea Dombrowski and Delia Hof for their laboratory assistance with the Ames fluctuation test. We thank the five anonymous reviewers for their constructive feedback to improve this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
N.R. conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and tables, wrote the main manuscript text and implemented reviewer revisions. J.O. conceived and designed the experiments and reviewed drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors consent to the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Column bar graphs of the YAS (Yeast Androgen Screen; A) and YDS (Yeast Dioxin Screen; B) limit tests at a concentration of 10 mg/L of the twelve tested bisphenols. Table S1. Cytotoxicity threshold in each in vitro assay. Table S2. Total number and percentage [%] of revertants in the Ames fluctuation test at a limit concentration of 10 mg/L with Salmonella typhimurium strains YG1041 (A) and YG1042 (B).
Additional file 2.:
Results of the Pearson correlations between EC50 values of the bioassays with positive results (YES, YAES, YAAS, Microtox) and the log KOW, and raw data of the positive controls of each bioassay (YES, YAES, YAS, YAAS, YDS, Microtox assay, Ames fluctuation test).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reininger, N., Oehlmann, J. Regrettable substitution? Comparative study of the effect profile of bisphenol A and eleven analogues in an in vitro test battery. Environ Sci Eur 36, 76 (2024). https://doi.org/10.1186/s12302-024-00900-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00900-1