Abstract
Background
Urban streams are characterised by species-poor and frequently disturbed communities. The recovery of heavily polluted urban streams is challenging but the simple community structure makes recolonisation patterns more transparent. Therefore, they are generally applicable model systems for recolonisation of restored streams. Principal questions of stream restoration concern the drivers and patterns of recolonisation processes. Rarely, recolonisation of restored streams is recorded for a sufficient time to observe patterns of habitat and community development in detail. Over 10 years, we monitored benthic habitat changes and macroinvertebrate communities of eight restored sites in an urban stream network that was formerly used as an open sewer and thus, almost uninhabitable for macroinvertebrates prior to restoration. We analysed changes in environmental variables and communities with a selection of multi-variate analyses and identified indicator species in successional stages.
Results
Proportions of stony substrate and conductivity decreased over time since restoration, while the riparian vegetation cover increased along with the amount of sandy substrate. The communities fluctuated strongly after restoration but began to stabilise after around eight years. TITAN analysis identified 9 species, (e.g. the mayfly Cloeon dipterum and the beetle Agabus didymus), whose abundances decreased with time since restoration, and 19 species with an increasing abundance trend (e.g. several Trichopteran species, which colonised once specific habitats developed). Woody riparian vegetation cover and related variables were identified as major driver for changes in species abundance. In the last phase of the observation period, a dry episode resulted in complete dewatering of some sites. These temporarily dried sections were recolonised much more rapidly compared to the recolonisation following restoration.
Conclusions
Our results underline that community changes following urban stream restoration are closely linked to the evolving environmental conditions of restored streams, in particular habitat availability initialised by riparian vegetation. It takes about a decade for the development of a rich and stable community. Even in streams that were almost completely lacking benthic invertebrates before restoration, the establishment of a diverse macroinvertebrate community is possible, underlining the potential for habitat restoration in formerly heavily polluted urban areas.
Similar content being viewed by others
Background
Effects of stream and river restoration on riverine biota are often minor. This is particularly obvious for benthic macroinvertebrates that are strongly affected by anthropogenic stressors and are frequently used to monitor ecological status and restoration success [64]. Numerous studies document the poor response of macroinvertebrates to particularly hydromorphological restoration measures [13, 15, 19, 31, 64], but the reasons remain speculative. In many cases, restoration may have merely improved the hydromorphological conditions, while poor water quality remains to affect biota and prevents sensitive species from entering the system [12, 17, 43, 48]. Furthermore, low dispersal ability of the species, the distance to population sources and their connectivity to restored stream sections or the lack of source populations restrict recolonisation [6, 28, 57,58,60, 68, 71]. Finally, species that have established populations under degraded conditions may inhibit the recovery of sensitive species through competition [4, 37, 66, 73].
Consequently, the understanding of stream restoration effects is strongly linked to the understanding of recolonisation patterns and processes. In general, recolonisation starts once disturbances that deteriorated the original community have been lifted. Relevant disturbances of stream communities include natural events (such as floods and streambed drying) but in particular human-induced pollution and habitat modification [65, 67]. These anthropogenic pressures are especially common in urban streams, which are often channelised to fit the urban structure and are additionally impacted by stormwater runoff, and input from point or non-point sources [5]. Recolonisation after anthropogenic disturbances is often initialised by restoration measures, which amongst others aim to enhance the stream’s biodiversity [44, 65]. Depending on the restoration goals different measures are implemented [55], including wastewater purification and a variety of hydromorphological measures, e.g. removal of bank reinforcements, revegetation of stream banks, and introduction of woody structures (deadwood) into the stream [10, 22,23,24, 62].
For a better understanding on how restoration initiates macroinvertebrate recolonisation, or fails to do this, the process needs to be broken down into its components. Recolonisation processes are guided by the habitats and environmental conditions provided by the restoration measures. In addition, it is impacted by the arrival of species that favour these conditions and by the occupation of niches of early establishing species, i.e. by competition patterns [66]. A direct result of most restoration measures is the presence of new, unoccupied habitats. Water quality improvement provides niches for species depending on high oxygen concentrations, while the removal of bank reinforcements results in more space for the stream, lower current velocities, and consequently the provision of habitats for lentic species [22]. More generally, restored streams develop more heterogeneous flow patterns, which ultimately leads to higher substrate diversity [45] providing niches for additional species. The establishment of woody riparian vegetation at the stream banks initialises natural succession [50, 62] and a change from open to shaded habitats, as the riparian areas mature. Woody riparian vegetation provides different functions to the stream as it increases the input of particulate organic matter and woody debris, which acts as an important food source and habitat for many benthic species, respectively. In addition, it provides shade, thus mitigates water temperatures and reduces primary production, which favours additional species and allows them to settle [10, 24, 39].
These frequently occurring effects of restoration support various threads of succession and recolonisation: From pollution-tolerant to pollution-sensitive species, from the community of a single habitat to communities of more variable habitats, and from communities of unshaded to those of shaded habitats. The new habitats that are created during restoration are quickly colonised by strong dispersers [30, 72]. This process largely depends on the species pool of the near surroundings and the species dispersal ability [53, 59, 60, 71]. The later arriving species, however, must compete for space and food with species already present, making it more difficult for new species to establish a population. Niches that are occupied at first will change during maturation but also following natural events such as floods and streambed drying [27, 35]. In conclusion, the macroinvertebrate community of restored streams is expected to undergo a distinct succession, driven by habitat availability, dispersal, and competition patterns.
However, this process can rarely be studied in the field and therefore remains hypothetical. Investigating patterns of recolonisation requires continuous long-term studies while existing studies merely focus on the first 1–5 years following restoration [14, 31, 69]. Often there are large temporal gaps between sampling, making it difficult to distinguish restoration effects on macroinvertebrate communities from natural variation unrelated to restoration [34, 36]. In addition, the existing studies on recolonisation patterns are impacted by the lack of information on the pre-restoration community, thereby limiting our ability to accurately interpret these patterns.
Here, we investigated an almost unique situation: The Boye stream network that has been completely restored and was used as an open sewer before restoration. The Boye exemplifies the challenges of restoring urban streams that go well beyond those stream restoration endeavours in rural areas: Strong pollution prior to restoration, limited space for habitat development, few recolonisation sources and multiple barriers for recolonising species [71]. The Boye is part of the Emscher catchment (Western Germany), which was for almost a century used as an open sewer channel for the urban hub Ruhr Metropolitan Area (> 5 million people) until it was restored over the last 20 years. Therefore, only few very tolerant organisms were able to survive in the system. This strong degradation offers unique opportunities for disentangling recolonisation patterns: Due to the limited number of species in the system prior to restoration, the majority of available niches will be occupied by newly arriving species. Consequently, the succession of habitats following restoration conditions and the recolonisation with invertebrates following the development of habitats and dispersal processes can be observed without being “disturbed” by a diverse pre-restoration assemblage. An initial analysis of the development of the benthic invertebrate community in the Boye catchment was conducted by Winking et al. [71, 72] and the sampling of the community in a number of restored sites was continued yearly, over a period of 10 years.
Based on the successional processes described above, we hypothesize: (1) The inter-annual within and between site variability of the communities’ species composition decreases with time since restoration. (2) After restoration, there will be a continuous development of habitats caused by gradual maturation of the sampling sites, which drives community development. (3) Many species that firstly colonised the restored stream reaches vanish quickly due to the ongoing changes in habitat availability, water quality and the arrival of new (competing) species. (4) Over time, the natural succession of riparian vegetation will lead to an increase in shade levels, thereby creating favourable habitat for the establishment of species that depend on such conditions. The species that increase in abundance over time are therefore positively associated with shade.
Materials and methods
Study area
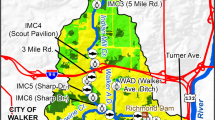
The Boye catchment is located in the Ruhr Metropolitan Area in Western Germany. It is part of the Emscher catchment, which drains into the river Rhine. It has a size of 77 km2 and a total stream length of 90 km (Fig. 1). The downstream parts of the catchment are situated in a highly urbanized area, while the upstream sections are mainly surrounded by agricultural land and forests. In the beginning of the twentieth century, the Emscher and large parts of its tributaries, including most of the Boye network, were transformed into concrete channels to transport domestic wastewater [71, 72]. Between 1993 and 2021 the aboveground streams were restored by building underground sewers to transport the wastewater. The concrete bed and bank reinforcements were removed, the streambeds widened and changed from straightened to sinuate or semi-meandering. Woody riparian vegetation developed naturally.
In spring 2012, several restored sites were selected for monitoring of benthic invertebrates, eight of which were used for our study (Table 1). We selected sites of a similar stream size (i.e. 1st to 2nd order streams) that were restored not more than 14 years ago. Compared to Winking et al. [71] we excluded two sites that were restored 19 years ago and were already in an advanced succession stage when the investigations started, and two sites in sections of larger streams (i.e. the main stem of the Boye thus 2nd to 3rd order streams), which community is hardly comparable. The selected sites differ in time since wastewater was removed and in time since hydromorphological restoration was conducted. Therefore, the first year and years 11 to 14 after restoration are only represented by two sites (Natt, Voun and Kirun, Kirob, respectively). All sites cover the period from 5 to 10 years after restoration. In accordance with our hypotheses, we related the occurrence of individual species and their change in abundance to different parameters: years since wastewater removal and hydromorphological restoration, water quality parameters, the proportion of different microhabitats and shade level.
Sampling, sorting and identification
From 2012 to 2021, the eight sites (Table 1) were sampled yearly in March or April. At each site, standardised multi-habitat-sampling was performed [18] and the following water quality parameters were measured: pH, temperature (°C), conductivity (µS/cm), and oxygen (mg/l). The cover of microhabitat types was estimated in 5% steps and only microhabitats with more than 5% cover were included in the composite sample. Reflecting the estimated cover of microhabitats, 20 sampling units were taken using a hand net (25 × 25 cm, 500 µm mesh size). One sampling unit represented 5% of all present microhabitats. The samples were pooled and preserved in ethanol (96%). The pooled samples were sorted in the laboratory using a standardised subsampling procedure [33]. The specimens were identified according to the operational taxa list for Germany [18], if possible, to species level, except for Chironomidae (tribe level), Diptera (family level) and Oligochaeta (family level). Species counts were standardised to abundance (Ind/m2). The resulting taxalist was adjusted prior to analysis, to account for varying identification levels of different larval instars [41].
Shade level
For the determination of the shade level, percentage riparian cover was used as a proxy. Satellite images and orthophotos were analysed in ArcGIS (version 7.0) to identify the riparian cover in a 500 m long und 20 m wide upstream corridor of each sampling site according to a modified procedure from Kail et al. [24]. Instead of the automated object-based image analysis, woody vegetation was marked manually. The proportions of shaded and unshaded areas within the aforementioned buffer were calculated in Rstudio (version 4.1.2). Satellite images of the Boye catchment were available from Tim-Online (https://www.tim-online.nrw.de/tim-online2/, 9th Aug 2021) for every third year from 2012 to 2018. For 2020, orthophotos were available from ELWAS-WEB [29]. The percentage riparian cover of missing years was complemented with the moving average of the previous and next known values. Hereafter, the percentage riparian cover will be referred to as ‘shade’.
Data analysis
All statistical analyses were conducted using Rstudio v4.1.2 [51]. All figures were created with the package “ggplot2” (v3.3.5, [70]).
Hypothesis (1) (community variability decreases with time since restoration) was addressed by first calculating the number of taxa that occurred in every sampling site per year and plotting them as a function of the time since restoration (TsR [years]). Here, a generalized linear model (GLM) with Poisson error distribution and identity link function was used. The independent variable (TsR) was log transformed. Due to the different years in which the streams were restored, the first year after restoration is only represented by two sampling sites (sites Natt and Voun). From year 11 onward, data were only available from two other sampling sites: Kirun and Kirob.
The patterns of community assembly were investigated with non-metric multidimensional scaling (NMDS). This was based on the Bray–Curtis dissimilarity index and applied on log(x + 1) transformed community data. Differences between the assemblages of different years since restoration were tested with a permutational multivariate analysis of variance (perMANOVA). To investigate the variability of the assemblages within each stream over time, the Jaccard dissimilarities were compared as a function of TsR. A GLM with Beta error distribution and logit link function was fit to the data. Here, presence/absence data was used instead of abundances, because merely the change in species composition was of interest. For these analyses, the “vegan” package (v2.5–7, [42]) was applied.
The second hypothesis (habitat development after restoration and impact on community succession) was tested by analysing the relationship between explanatory variables and communities. The following variables were addressed: 1) water quality variables: conductivity [µS/cm], O2 [%], pH; 2) coverage of substrates [%]: gravel/stones, sand/sludge, loam, particulate organic matter (POM, fine and coarse), macrophytes (emergent and submergent), living parts of terrestrial plants (LPTP), algae (according to the microhabitat distribution from the benthic invertebrate field protocol); 3) level of shade [%]; 4) time since wastewater removal (TsW [years]); 5) time since restoration (TsR [years]).
Correlations between environmental variables were checked on forehand with the “cor” function of the “stats” package (v4.1.2 [51]). The variable “time since wastewater removal” (TsW) was highly correlated (r = 0.93) with the TsR and was therefore excluded from further analysis. All other correlations were below 0.6, thus, no other variables were excluded (Additional file 1: Table S1).
The main gradients influencing the taxonomic composition were identified via redundancy analysis (RDA) (package “vegan”, v2.5–7, [42]). Prior to analysis, the explanatory variables were scaled. The effect of the explanatory variables on changes in species abundance was tested with an analysis of variance (perMANOVA). The variables that best explained the changes in abundance were identified using the forward selection method (“ordiR2step” function, package “vegan”, v2.5–7, [42]) of the RDA applied to log(x + 1) transformed community data.
To test the third hypothesis (early colonising species vanish with habitat succession), first the species most responsible for temporal community changes within and between the sampling sites were identified. We applied the TITAN analysis [2] that is included in the package “TITAN2” (v2.4.1, [3]). Time since restoration was used as a gradient to identify indicator species that show a negative (z-), i.e. decreasing, or positive (z +), i.e. increasing, trend over time. Before the analysis, taxa with less than three occurrences across all samples were excluded, resulting in 77 taxa. Only species with purity and reliability levels above 0.9 were considered as indicators. The value of 1000 replicates was chosen for bootstrap resampling. For the resulting indicator species, their frequency of occurrence across sampling sites per year since restoration was calculated.
Finally, to test the fourth hypothesis (impact of riparian vegetation), the relationship of the explanatory variables on the indicator species excluding TsR was identified via another forward selection of the RDA. The resulting variables were displayed together with the indicator species abundance gradients in a multi-factorial analysis (MFA), created within the package “Factoshiny” (v2.4, [63]).
Results
H1: Community variability decreases with time since restoration
Over the 10 years of sampling, 130 taxa were identified across all sampling sites. The number of taxa per sampling site increases over time. The greatest increase in taxa number was found four years after restoration (Fig. 2). The regression coefficient is significantly different from zero p < 0.001 (mean = 3.4, 2.5% = 1.8, 97.5% = 4.93).
The NMDS of the log-transformed community data shows that the communities change along a temporal gradient at all sites (Fig. 3) (stress = 0.167). The differences between communities of different times since restoration were confirmed by a perMANOVA (F = 11.53, p < 0.05). While the communities at sites sampled in the first years after restoration (2012–2014) are very dissimilar to each other, communities at sites sampled in 2021 are very similar. Thus, the communities move along the gradient of time since restoration, becoming more similar over time.
NMDS of log-transformed community data of eight restored sampling sites in the Boye catchment for 10 consecutive years (stress = 0.167). The points are coloured according to the time since restoration (TsR), measured in years. The numbers behind the sampling site Ids depict the sampling year (e.g. 12 = 2012). TsR was added as an overlay and is displayed by the arrow. Figures per sampling site are shown in Additional file 1: Figure S1
The generalised linear regression model shows that Jaccard dissimilarities between samples of a given sampling site decline with the time since restoration (Fig. 4). The regression coefficient is significantly different from zero p < 0.001 (mean = − 0.12, 2.5% = − 0.15, 97% = − 0.08). The dissimilarity between communities decreases with time. Within the first eight years, the dissimilarity decreases by 30–50% at some sites. The model explains 43% of the variance within the data. The summer 2018 was unusually dry, causing some of the study streams to dry out (Additional file 3: Table S1). The communities that were sampled following this dry period (2019–2021) are displayed in grey (Fig. 4). The outliers in the years 13 and 14 after restoration are part of these communities. After the dry period, the Jaccard dissimilarity increases at two sampling sites.
H2: Habitat development after restoration and impact on community succession
The relationship between environmental variables and overall community variability was analysed, using a RDA on the log(x + 1) transformed community matrix (Fig. 5). The permutation test shows that the environmental variables included in the model have a significant effect on the community composition (F = 2.8, p < 0.05). Most of the variance is explained by the time since restoration (RDA1 = − 0.97), followed by conductivity (RDA1 = 0.47) and the percentage of shade (RDA1 = − 0.43). The correlation matrix reveals that none of the environmental parameters are highly correlated with each other (r > 0.7, Additional file 1: Table S1). The highest correlation coefficient is observed for the coverages of algae and loam (r = 0.58). All other correlation coefficients are below 0.5. The gradients in the RDA show that the proportion of sand and sludge and the percentage of shade increase with time since restoration, while the percentage of loam, algae, POM, as well as pH and conductivity decrease over time.
RDA on Bray–Curtis dissimilarities of log(x + 1) transformed community data. The sampling sites are oriented along the environmental gradients, which mostly influence their community composition. Figures per sampling site are shown in Additional file 1: Figure S2
The forward selection of the RDA identified six explanatory variables to be most important for the changes in species abundances: TsR (p = 0.002), shade (p = 0.002), the proportion of gravel/stones (p = 0.002), sand/sludge (p = 0.002), POM (p = 0.006) and loam (p = 0.022) (Table 2).
For individual sampling sites, the community composition changes along different gradients (Additional file 2: Figure S2). For example, the community composition of the the Haarbach (Haun, Haob) moves along the gradient of gravel/stones. Conductivity and pH decrease with time since restoration. In the first years after restoration, conductivity is especially high in the Nattbach and Haarbach, while pH is high in the Vorthbach (Voun) and the up- and downstream sites of the Wittringer Mühlenbach (Wiob). The percentage of shade and the proportion of sand/sludge cover increase with time, in particular in the Wittringer Mühlenbach (Wiun, Wiob), the Haarbach (Haun) and the Vorthbach (Voun). The communities of the Kirchschemmsbach (Kiob, Kiun) mainly change along the temporal gradient of time since restoration.
The samples in streams that completely fell dry in the year 2018 are marked in red in Fig. 5. This was the case for three of the sampling sites (Natt, Voun and Wiob). Their communities appear to have moved a step backward along the gradient of time since restoration (TsR), compared to other sites, e.g. Haob.
H3: Early colonising species vanish with habitat succession
The main species responsible for the temporal changes and therefore successional processes were identified using the TITAN analysis with time since restoration as a gradient. Nine species were identified, which abundance decreases with time since restoration (Fig. 6a). Five of these mainly occur immediately after restoration, four of which belong to the order of Coleopterans. However, the other species all belong to different taxonomic groups. Cloeon dipterum (cloedipt) for example is an Ephemeroptera and was only found the first two years following restoration. Radix balthica (radibalt), belonging to the class of Gastropoda, is always present, but its abundance decreases with succession, which is also true for the Trichoptera Hydropsyche angustipennis angustipennis (hydrangu).
a TITAN Analysis of community data with time since restoration (TsR) as gradient with a reliability and purity cut-off of 0.9 (bootstraps = 1000). On the left-hand side, taxa with a decreasing trend in abundance are given (black dots, continuous line, z− ). On the right-hand side, taxa with an increasing trend in abundance are shown (white dots, dashed line, z +). The size of the dots shows the z scores. Higher z scores result in larger dots and demonstrate a larger indicator potential. b Heat map of frequency of occurrence across sampling sites per year since restoration. Black boxes = 100%, white boxes = 0%. For explanations of the species abbreviations see Additional file 4: Table S1
In total, 19 species show an increasing abundance trend. Most of these species belong to the order of Diptera, as for example Prodiamesa olivacea (prodoliv) and Eloeophila sp. (eleosp). In addition, different Trichopterans, e.g. Athripsodes bilineatus (athrbili) and Glyphotaelius pellucidus (glyppell) and Ephemeropterans, e.g. Ephemera danica (ephedani) and Baetis rhodani (baetrhod) belong to this second group.
The indicator species’ frequency of occurrence across sampling sites mirrors their trends in abundance (Fig. 6b). The first species to disappear from all sites is C. dipterum, closely followed by the Coleopteran species, while R. balthica and Asellus aquaticus remain in the system during the complete sampling period but are found at less sites over time. On the other hand, B. rhodani and E. danica only enter the system five and six years after restoration, respectively. Gammarus pulex was present at one site starting the first year after restoration and at all sites from the seventh year onward.
Next to the indicator species, a set of species was identified, that was found every year at nearly all sampling sites without exhibiting a negative or positive trend over time. In total, six taxa occurred in at least 50% of all samples, however, five of these were only identified to higher taxonomic levels: Ceratopogoninae gen. sp., Chironomidae gen. sp., Chironomini gen. sp., Tanypodinae gen. sp., Limnephilini gen. sp., Limnephilus lunatus. Thus, the taxa with increasing or decreasing abundance trends are embedded into a matrix of constantly present taxa.
H4: Impact of riparian vegetation
The indicator species were put into context with the environmental variables via a multi-factorial analysis (MFA) (Fig. 7). The environmental variables that best explained their variation in abundance (limited to the indicator species) were identified using a second forward selection that excluded TsR as variable. The most important parameters influencing changes in indicator species abundance were identified to be shade (p = 0.002), conductivity (p = 0.002), the proportion of gravel/stones (p = 0.002), sand/sludge (p = 0.002), loam (p = 0.002) and pH (p = 0.020) (Table 3). Only these environmental variables were used in the MFA. Species abundances were displayed as gradient arrows since the direction of change in abundance was of major interest. The “increasing” species are clearly separated from the “decreasing” species, pointing to the left and the right side of the MFA, respectively. The majority of the “increasing” species is positively correlated with the gradient of shade level and, as a group, 40% of the variance is explained (Dim.1 = 0.40). On the other hand, the majority of the “decreasing” species is positively correlated with conductivity and 67% of the variance is explained (Dim.1 = 0.67). According to the correlation matrix (Additional file 5: Table S1), shade has the highest positive correlation with P. olivacea (prodoliv) (r = 0.37) and G. pulex (gammpule) (r = 0.34). R. balthica (radibalt) has a weak negative correlation with shade (r = − 0.27). Conductivity is positively correlated with C. dipterum (cloedipt) (r = 0.49) and Agabus didymus ad. (agabdiad) (r = 0.34) and weakly negative with G. pulex (gammpule) (r = − 0.19) and Hemerodromia sp. (hemesp) (r = − 0.16). The highest positive correlation with gravel/stone was found for H. angustipennis (hydrangu) (r = 0.48) and Agabus sp. lv. (agabsp) (r = 0.44).
Discussion
H1: Community variability decreases with time since restoration
Our first hypothesis was confirmed. The results revealed a temporal gradient of community development. Species numbers increase mostly within the first five years after restoration. As time progresses, the distance between communities, thus the variation in species assemblages decreases. The initial distance between communities is likely the result of the sites being recolonised from different population sources and at different speeds. As the sampling sites mature, species can establish more populations and disperse across all tributaries of the Boye. Previous studies addressing successional processes in ponds, temporary wetlands and lakes [7, 26, 52] observed similar patterns and described initial colonisation after restoration to be fast, while the habitat specific assemblages and higher taxa diversity developed later.
Over time, dissimilarities between sampling years decrease, with the largest decrease between years one and eight after restoration. Thereafter, community variability remains at a lower degree. Two major outliers of high variability more than 10 years after restoration are striking: dissimilarity of communities sampled in year 12/13, and 13/14 was high at sites along the Kirchschemmsbach. Increased community dissimilarity in the sampling period past 2018 was also observed for other sites, albeit to a lower degree. These observations are related to the very warm and dry summer 2018, the hottest summer in Germany since 2003, with 75 summer days above 25 °C [11]. As a result, many streams fell (partly) dry, which caused especially hololimnic species to vanish. They spend their complete life cycle in the water column and therefore rely on a constant flow of water. The conditions for the subsequent recolonisation, however, have greatly improved compared to the time 15 years ago, as benthic invertebrate populations have meanwhile colonised most of the Boye catchment.
In contrast to communities of temporary streams, which are adapted to unstable conditions, seasonal streambed drying can have detrimental effects on the community of usually permanent streams. One of the known consequences is the reduction of aquatic diversity, due to the loss of ill-adapted taxa to drying [56]. For example, Iversen et al. [21] found G. pulex and many Trichoptera species to disappear from stream sections that dried out for several months. Species abundance and richness were found to decline in restored and near-natural low mountain range streams of North Rhine-Westphalia following streambed drying but also extreme floods [30]. With climate change and anthropogenic water abstraction, the number of streams undergoing drying events and the duration of such events are expected to increase in the future [16]. These changes will undoubtedly affect biological communities that lack adaptations to such conditions. We conclude that while communities establish a certain degree of stability eight years after restoration, they remain subject to natural variation, which can be greatly increased by extreme heat and streambed drying [1, 30]. Previous research predicted restoration impacts on the invertebrate community about five years following restoration [38, 72]. While the timeframe for community recovery is dependent on various factors, the results highlight the need for continuous data to distinguish restoration effects on macroinvertebrate communities from natural variation unrelated to restoration [30, 34, 40].
H2: Habitat development after restoration and impact on community succession
Our second hypothesis that hydromorphological restoration initiates the development of substrate diversity, was confirmed as well. Once the streams were not transporting wastewater anymore, the removal of bank reinforcements was a major restoration measure conducted at all Boye tributaries. Furthermore, the stream channels were changed from straightened to sinuate or semi-meandering. Consequently, flow velocities were reduced. This causes a change in substrate proportions as the stream matures, with an increase in the proportion of sand, which characterizes lowland streams in the area [49]. Indeed, our results demonstrate that the proportion of sand/sludge increases with time since restoration. The proportion of stones is negatively correlated with the proportion of sand, which suggests that sand aggregated on top of the stones that were used as a replacement of the former concrete bed. Accordingly, the community composition changed from stone-preferring to sand-preferring species, e.g. Ephemera danica.
Verdonschot et al. [64] found that in lowland streams an increase in sand cover has a positive effect on the diversity of Ephemeroptera, Plecoptera and Trichoptera (EPT). Though sand supports low species richness and abundance, it still maintains a unique macroinvertebrate species assembly [74]. In general, the species assemblage is closely related to the available substrates. This is due to the number of niches available and the amount of ecosystem functions that need to be filled by various species. Thus, several studies have shown that species diversity increases with habitat and food source heterogeneity [17, 25, 46, 64].
The level of conductivity was a major driver for the species abundances. Conductivity decreases at least at some sites over time. High conductivity is an analogue for high salinity and is commonly found in urban streams, due to their high nutrient input [9]. Salts that settled in the stream’s sediment, while wastewater was still transported within the stream, causing the high conductivity in the beginning of our study. Few species can cope with such conditions and others will only settle once conductivity is reduced [8]. In addition, during restoration, extra amounts of salts may have entered the streams due to the construction works. Once succession starts, the salts are constantly washed out from the watercourse and the sediment, causing conductivity to decrease over time.
H3: Early colonising species vanish with habitat succession
The third hypothesis was confirmed. We identified 28 indicator species as either increasing or decreasing with time since restoration. Only 9 species decrease, compared to 19 species that increase. This additionally demonstrates the general increase of diversity and taxa richness, as several species successfully establish populations. Less species are lost over time as stable populations develop. The decreasing species include C. dipterum (Ephemeroptera), several Coleoptera species and the Odonata Pyrrhosoma nymphula. All these species are active fliers with a good dispersal ability in their adult stage and thus colonised the newly created habitats quickly. In addition, the open habitat conditions of freshly restored streams are well suited for these species. The lack of riparian vegetation causes macrophytes to grow and water temperatures to increase, depicting a suitable habitat for C. dipterum, which prefers warm water and feeds on periphyton [54]. The Coleoptera species and P. nymphula also favour open landscapes and are predominantly predators. They benefit from quickly colonising r-strategists adapted to unstable conditions, e.g. Chironomidae. Westveer et al. [69] showed that r-strategists are the first to colonise restored streams, while k-strategists arrive later. Hence, certain species are specialized on settling in freshly restored habitats and leave as habitats mature [72]. Some subfamilies of Chironomidae larvae were found in more than 50% of all samples, not exhibiting an either increasing or decreasing trend over time. They are generally high abundant in streams and many species are tolerant to varying water conditions [32]. Limnephilini gen. sp. and one species from this taxa group, Limnephilus lunatus, were also abundant across most of the samples. This underlines the good dispersability of the species and hints on its generalist character; these factors potentially increase the chance on successful colonisation of new niches. The fact that only few taxa were found in more than half of the samples highlights the pronounced differences between stream communities.
The disappearance of some of the first colonising species in maturing streams is likely to be caused by two factors that add to each other. First, once riparian vegetation has established, streams are increasingly shaded, water temperatures decrease and input of particulate organic material (POM) into the stream increases. Consequently, the habitat becomes unsuitable for many of the first colonisers, e.g. grazing species because higher shading reduces biofilms growth. Instead, new niches become available, which can be occupied by a larger number of other species and many of the late colonisers are accordingly associated to shaded sites (Fig. 7). In addition, late colonisers may compete for space and food with the pioneer species. For example, A. aquaticus may have survived under the harsh conditions prevailing in polluted water, as it was present in the streams directly after restoration [20, 21], while other shredders like G. pulex had to immigrate afterwards. As they colonise similar substrates and share the same food source, they are likely to compete for space and food. This could explain the decrease of A. aquaticus and the increase of G. pulex.
Another tolerant species that is present from the first year after restoration onward is R. balthica (Gastropoda). Due to its low dispersal capabilities, the species may have survived the harsh conditions within the stream and was present prior to restoration. Another theory for its early occurrence is that the eggs were carried to the restored sites via other vectors, e.g. waterfowl [7, 61]. Over time, the abundance of R. balthica decreases, which demonstrates that the habitats become increasingly unsuitable for grazing species.
Many of the increasing species belong to the orders Diptera, Ephemeroptera and Trichoptera, which depend on the presence of suitable habitats. As the proportion of shade and sand increases, the abundance of sand-burrowing, active filter feeding species, e.g. E. danica increases as well. The growing riparian vegetation provides additional food sources, adding coarse particulate organic matter, e.g. leaves, to the stream, favouring for example G. pellucidus. The species has spread to most of the sampling sites in the ninth year following restoration, likely due to the increase in available food sources and riparian vegetation that is needed for egg laying.
The strong overall increase in species richness and the change of indicator species over time reflects the current maturation state of the restored streams, from which water managers could judge the progress towards good ecological status.
Urban streams pose particular challenges to restoration, ranging from continuous pollution to the absence of recolonisation sources. Consequently, and in contrast to our findings, Stranko et al. [58] observed the number of mayflies and other intolerant macroinvertebrate species to decline in urban restored sites within 10 years of monitoring and eventually no effect of restoration actions on community composition. However, our findings show that if dispersal capacities and the creation of suitable habitats are permitted to guide local development, biodiversity can be improved and restored, albeit slowly, in urban streams.
H4: Impact of riparian vegetation
In line with our fourth hypothesis, the percentage of shade increases with time since restoration, which shows the succession of riparian forest. Trees stabilize the riverbanks and provide a source for coarse organic material and deadwood [50, 62]. The presence of deadwood increases habitat heterogeneity and thereby supports a higher species diversity [23], while shading decreases water temperatures [10, 24]. This allows especially heat sensitive species to colonise respective sites and is generally important to protect the community from extreme heat. We identified the percentage shade to be an important driver for species abundance. In general, the presence of woody riparian cover has a strong effect on riverine macroinvertebrate communities [47]. Although the authors found effects of woody riparian cover to be greater in rural than in urban streams, 100% woody riparian cover still improved the ecological status of urban streams. Shredders benefit from the input of particulate organic matter, while primary production decreases, which reduces the abundance of grazers [39].
Conclusion
We disentangled the recolonisation process of restored urban streams. It took almost a decade after wastewater was removed and hydromorphological restoration was completed to develop a stable macroinvertebrate community. While the communities underlie continuous shifts, the size of these variations peaks in the first eight years following restoration, when niches develop and are stepwise occupied by invading species. The time since restoration is central for the development of these niches. At restored sites, new instream habitats become available, largely triggered by the succession of the stream’s surroundings, i.e. the growth of woody vegetation on the stream banks. Thus, habitat development as a result of maturation over time is a key driver for successful recolonisation of restored streams.
Our results underline the potential for the restoration of urban streams. Despite numerous challenges including strong pollution prior to restoration, limited space and restricted recolonisation sources, a distinct succession towards a community adapted to stable conditions was observed in all restored sites. Targets for urban stream restoration should therefore be ambitious and key factors for habitat development, in particular the development of riparian vegetation, should be enabled.
Availability of data and materials
The datasets used and analysed during this study are available from the corresponding author on reasonable request.
Abbreviations
- CPOM:
-
Coarse particulate organic matter
- GLM:
-
Generalised linear model
- LPTP:
-
Living parts of terrestrial plants
- MFA:
-
Multi-factorial analysis
- NMDS:
-
Non-metric multidimensional scaling
- perMANOVA:
-
Permutational multivariate analysis of variance
- POM:
-
Particulate organic matter
- RDA:
-
Redundancy analysis
- TsR:
-
Time since restoration
- TsW:
-
Time since wastewater was removed
References
Atkinson CL, Julian JP, Vaughn CC (2014) Species and function lost: role of drought in structuring stream communities. Biol Cons 176:30–38. https://doi.org/10.1016/j.biocon.2014.04.029
Baker ME, King RS (2010) A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol Evol 1(1):25–37. https://doi.org/10.1111/j.2041-210X.2009.00007.x
Baker ME, King RS, Kahle D (2020) TITAN2: Threshold Indicator Taxa Analysis. R package version 2.4.1. https://CRAN.R-project.org/package=TITAN2
Barrett IC, McIntosh AR, Febria CM, Warburton HJ (2021) Negative resistance and resilience: biotic mechanisms underpin delayed biological recovery in stream restoration. Proc Biol Sci 288:20210354. https://doi.org/10.1098/rspb.2021.0354
Bernhardt ES, Palmer MA (2007) Restoring streams in an urbanizing world. Freshw Biol 52:738–751. https://doi.org/10.1111/j.1365-2427.2006.01718.x
Bond NR, Lake PS (2003) Local habitat restoration in streams: constraints on the effectiveness of restoration for stream biota. Ecol Manag Restor 4(3):193–198. https://doi.org/10.1046/j.1442-8903.2003.00156.x
Cañedo-Argüelles M, Rieradeval M (2011) Early succession of the macroinvertebrate community in a shallow lake: response to changes in the habitat condition. Limnologica 41:363–370. https://doi.org/10.1016/j.limno.2011.04.001
Cockerill K, Anderson WP Jr (2014) Creating false images: stream restoration in an urban setting. JAWRA J Am Water Resources Assoc 50(2):468–482. https://doi.org/10.1111/jawr.12131
Couceiro SRM, Hamada N, Luz SLB, Forsberg BR, Pimentel TP (2007) Deforestation and sewage effects on aquatic macroinvertebrates in urban streams in Manaus, Amazonas, Brazil. Hydrobiologia 575:271–284. https://doi.org/10.1007/s10750-006-0373-z
Davies-Colley RJ, Meleason MA, Hall RMJ, Rutherford JC (2009) Modelling the time course of shade, temperature, and wood recovery in streams with riparian forest restoration. NZ J Mar Freshwat Res 43(3):673–688. https://doi.org/10.1080/00288330909510033
DWD (Deutscher Wetterdienst) (2020) Klimastatusbericht Deutschland Jahr 2018. Geschäftsbereich Klima und Umwelt, Offenbach: 1–23. https://www.dwd.de/DE/leistungen/klimastatusbericht/klimastatusbericht.html
Dos Reis Oliveira PC, Geest HG, van der Kraak MHS, Westveer JJ, Verdonschot RCM, Verdonschot PFM (2020) Over forty years of lowland stream restoration: lessons learned? J Environ Manage. https://doi.org/10.1016/j.jenvman.2020.110417
Feld CK, Birk S, Bradley DC, Hering D, Kail J, Marzin A, Melcher A, et al (2011) From natural to degraded rivers and back again. In: Woodward G (ed) Ecosystems in a human-modified landscape. A European perspective, vol 44. Advances in Ecological Research. Elsevier/Academic Press, London, pp 119–209. https://doi.org/10.1016/B978-0-12-374794-5.00003-1
Friberg N, Kronvang B, Ole Hansen H, Svendsen LM (1998) Long-term, habitat-specific response of a macroinvertebrate community to river restoration. Aquat Conserv Mar Freshwat Ecosyst 8(1):87–99
Friberg N, Lindstrøm M, Kronvang B, Larsen SE (2003) Macroinvertebrate/sediment relationships along a pesticide gradient in danish streams. In: The interactions between sediments and water. Springer, Dordrecht, pp 103–110. https://doi.org/10.1007/978-94-017-3366-3_15
Garner G, Hannah DM, Watts G (2017) Climate change and water in the UK: recent scientific evidence for past and future change. Progress Phys Geography Earth Environ 41(2):154–170. https://doi.org/10.1177/0309133316679082
Haase P, Hering D, Jähnig SC, Lorenz AW, Sundermann A (2013) The Impact of hydromorphological restoration on river ecological status: a comparison of fish, benthic invertebrates, and macrophytes. Hydrobiologia 704(1):475–488. https://doi.org/10.1007/s10750-012-1255-1
Haase P, Lohse S, Pauls S, Schindehütte K, Sundermann A, Rolauffs P, Hering D (2004) Assessing streams in germany with benthic invertebrates: development of a practical standardised protocol for macroinvertebrate sampling and sorting. Limnologica 34(4):349–365. https://doi.org/10.1016/S0075-9511(04)80005-7
Hering D, Aroviita J, Baattrup-Pedersen A, Brabec K, Buijse T, Ecke F, Friberg N et al (2015) Contrasting the roles of section length and instream habitat enhancement for river restoration success: a field study of 20 european restoration projects. J Appl Ecol 52(6):1518–1527. https://doi.org/10.1111/1365-2664.12531
Hervant F and Malard F (2019) Chapter 2 - Adaptations: Low Oxygen. In: White WB, Culver DC, Pipan T (eds) Encyclopedia of Caves, Third Edition. Academic Press, pp 8–15. https://doi.org/10.1016/B978-0-12-814124-3.00002-9
Iversen TM, Wiberg-Larsen P, Hansen SB, Hansen FS (1978) The effect of partial and total drought on the macroinvertebrate communities of three small danish streams. Hydrobiologia 60(3):235–242. https://doi.org/10.1007/BF00011718
Januschke K, Jähnig SC, Lorenz AW, Hering D (2014) Mountain river restoration measures and their success(ion): effects on river morphology, local species pool, and functional composition of three organism groups. Ecol Ind 38:243–255. https://doi.org/10.1016/j.ecolind.2013.10.031
Kail J, Hering D, Muhar S, Gerhard M, Preis S (2007) The use of large wood in stream restoration: experiences from 50 projects in Germany and Austria. J Appl Ecol 44(6):1145–1155. https://doi.org/10.1111/j.1365-2664.2007.01401.x
Kail J, Palt M, Lorenz A, Hering D (2021) Woody buffer effects on water temperature: the role of spatial configuration and daily temperature fluctuations. Hydrol Process 35(1):e14008. https://doi.org/10.1002/hyp.14008
Kemp JL, Harper DM, Crosa GA (1999) Use of “functional habitats” to link ecology with morphology and hydrology in river rehabilitation. Aquat Conserv Mar Freshwat Ecosyst 9(1):159–178
Kuranchie A, Harmer A, Evans B, Brunton DH (2021) Restoration of a pond: monitoring water quality and macroinvertebrate community succession. https://doi.org/10.1101/2021.10.19.465034
Lake PS (2003) Ecological effects of perturbation by drought in flowing waters: effects of drought in streams. Freshw Biol 48(7):1161–1172. https://doi.org/10.1046/j.1365-2427.2003.01086.x
Lake PS, Bond N, Reich P (2007) Linking ecological theory with stream restoration. Freshw Biol 52(4):597–615. https://doi.org/10.1111/j.1365-2427.2006.01709.x
Land NRW, dl-de/by-2–0 (www.govdata.de/dl-de/by-2-0) https://www.elwasweb.nrw.de < August 2021>
Lorenz AW (2020) Continuous riverine biodiversity changes in a 10-years-post-restoration-study—impacts and pitfalls. River Res Appl 37(2):270–282. https://doi.org/10.1002/rra.3729
Louhi P, Mykrä H, Paavola R, Huusko A, Vehanen T, Mäki-Petäys A, Muotka T (2011) Twenty years of stream restoration in finland: little response by benthic macroinvertebrate communities. Ecol Appl 21(6):1950–1961. https://doi.org/10.1890/10-0591.1
Mathuriau C, Chauvet E (2002) Breakdown of leaf litter in a neotropical stream. J N Am Benthol Soc 21(3):384–396. https://doi.org/10.2307/1468477
Meier C, Haase P, Rolauffs P, Schindehütte K, Schöll F, Sundermann A, Hering D (2006) Methodisches Handbuch Fließgewässerbewertung zur Untersuchung und Bewertung von Fließgewässern auf der Basis des Makrozoobenthos vor dem Hintergrund der EG- Wasserrahmenrichtlinie. http://www.fliessgewaesserbewertung.de
Miller SW, Budy P, Schmidt JC (2010) Quantifying macroinvertebrate responses to in-stream habitat restoration: applications of meta-analysis to river restoration. Restor Ecol 18(1):8–19. https://doi.org/10.1111/j.1526-100X.2009.00605.x
Milner AM, Robertson AL, McDermott MJ, Klaar MJ, Brown LE (2013) Major flood disturbance alters river ecosystem evolution. Nat Clim Chang 3(2):137–141. https://doi.org/10.1038/nclimate1665
Muotka T, Paavola R, Haapala A, Novikmec M, Laasonen P (2002) Long-term recovery of stream habitat structure and benthic invertebrate communities from in-stream restoration. Biol Cons 105(2):243–253. https://doi.org/10.1016/S0006-3207(01)00202-6
Murphy JF, Winterbottom JH, Orton S, Simpson GL, Shilland EM, Hildrew AG (2014) Evidence of recovery from acidification in the macroinvertebrate assemblages of UK fresh waters: a 20-year time series. Ecol Indicators Threats Upland Waters 37:330–340. https://doi.org/10.1016/j.ecolind.2012.07.009
Narf R (1985) Aquatic insect colonization and substrate changes in a relocated stream segment. The Great Lakes Entomologist 18(2). https://scholar.valpo.edu/tgle/vol18/iss2/4
Nebgen EL, Herrman KS (2019) Effects of shading on stream ecosystem metabolism and water temperature in an agriculturally influenced stream in central Wisconsin, USA. Ecol Eng 126:16–24
Negishi J, Richardson J (2003) Responses of organic matter and macroinvertebrates to placements of boulder clusters in a small stream of Southwestern British Columbia Canada. Can Fish Aquat Sci 60(3). https://doi.org/10.1139/F03-013
Nijboer RC, Schmidt-Kloiber A (2004) The effect of excluding taxa with low abundances or taxa with small distribution ranges on ecological assessment. In: Hering D, Verdonschot PFM, Moog O, Sandin L (eds) Integrated assessment of running waters in Europe. Springer, Dordrecht
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E and Wagner H (2020) Vegan: Community Ecology Package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan
Omoniyi GE, Piscart C, Pellan L, Bergerot B (2022) Responses of macroinvertebrate communities to hydromorphological restoration of headwater streams in brittany. Water 14(4):553. https://doi.org/10.3390/w14040553
Palmer MA, Bernhardt ES, Allan JD, Lake PS, Alexander G, Brooks S, Carr J et al (2005) Standards for ecologically successful river restoration. J Appl Ecol 42(2):208–217. https://doi.org/10.1111/j.1365-2664.2005.01004.x
Palmer MA, Menninger HL, Bernhardt E (2010) River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshw Biol 55:205–222. https://doi.org/10.1111/j.1365-2427.2009.02372.x
Palmer MA, Poff NL (1997) The influence of environmental heterogeneity on patterns and processes in streams. J N Am Benthol Soc 16(1):169–173. https://doi.org/10.2307/1468249
Palt M, Hering D, Kail J (2023) Context-specific positive effects of woody riparian vegetation on aquatic invertebrates in rural and urban landscapes. J Appl Ecol Accepted Author Manuscript. https://doi.org/10.1111/1365-2664.14386
Poff NL (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J N Am Benthol Soc 16(2):391–409. https://doi.org/10.2307/1468026
Pottgiesser T, Sommerhäuser M (2014) 'Fließgewässertypologie Deutschlands' Handbuch Angewandte Limnologie: Grundlagen - Gewässerbelastung - Restaurierung - Aquatische Ökotoxikologie - Bewertung - Gewässerschutz, 1–61. John Wiley and Sons Ltd. Hobokan. https://doi.org/10.1002/9783527678488.hbal2004005
Purcell AH, Friedrich C, Resh VH (2002) An assessment of a small urban stream restoration project in northern California. Restor Ecol 10(4):685–694. https://doi.org/10.1046/j.1526-100X.2002.01049.x
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ruhí A, Herrmann J, Gascón S, Sala J, Geijer J, Boix D (2012) Change in biological traits and community structure of macroinvertebrates through primary succession in a man-made swedish wetland. Freshwater Sci 31(1):22–37. https://doi.org/10.1899/11-018.1
Sarremejane R, Cid N, Stubbington R, Datry T, Alp M, Cañedo-Argüelles M, Cordero-Rivera A et al (2020) DISPERSE, a trait database to assess the dispersal potential of European aquatic macroinvertebrates. Sci Data 7(1):386. https://doi.org/10.1038/s41597-020-00732-7
Schmidt-Kloiber A, Hering D (2015) www.freshwaterecology.info—an online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol Ind 53:271–282
Shields FD, Copeland RR, Klingeman PC, Doyle MW, Simon A (2003) Design for stream restoration. J Hydraul Eng 129(8):575–584. https://doi.org/10.1061/(ASCE)0733-9429(2003)129:8(575)
Soria M, Leigh C, Datry T, Bini LM, Bonada N (2017) Biodiversity in perennial and intermittent rivers: a meta-analysis. Oikos 126:1078–1089. https://doi.org/10.1111/oik.04118
Stanford JA, Ward JV, Liss WJ, Frissell CA, Williams RN, Lichatowich JA, Coutant CC (1996) A general protocol for restoration of regulated rivers. Regul Rivers: Res Manage 12(4–5):391–413
Stranko SA, Hilderbrand RH, Palmer MA (2012) Comparing the fish and benthic macroinvertebrate diversity of restored urban streams to reference streams. Restor Ecol 20:747–755. https://doi.org/10.1111/j.1526-100X.2011.00824.x
Sundermann A, Antons C, Cron N, Lorenz AW, Hering D, Haase P (2011) Hydromorphological restoration of running waters: effects on benthic invertebrate assemblages. Freshw Biol 56(8):1689–1702. https://doi.org/10.1111/j.1365-2427.2011.02599.x
Sundermann A, Stoll S, Haase P (2011) River restoration success depends on the species pool of the immediate surroundings. Ecol Appl 21(6):1962–1971. https://doi.org/10.1890/10-0607.1
Taylor CM, Duggan IC (2012) Can biotic resistance be utilized to reduce establishment rates of non-indigenous species in constructed waters? Biol Invasions 14(2):307–322. https://doi.org/10.1007/s10530-011-0063-2
Thompson R, Parkinson S (2011) Assessing the local effects of riparian restoration on urban streams. NZ J Mar Freshwat Res 45(4):625–636. https://doi.org/10.1080/00288330.2011.569988
Vaissie P, Monge A, Husson F (2021) Facoshiny: perform factorial analysis from 'FactoMineR' with a shiny application. R package version 2.4. http://factominer.free.fr/graphs/factoshiny.html
Verdonschot RCM, Kail J, McKie BG, Verdonschot PFM (2016) The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 769(1):55–66. https://doi.org/10.1007/s10750-015-2575-8
Verdonschot PFM, Verdonschot RCM (2022) The role of stream restoration in enhancing ecosystem services. Hydrobiologia. https://doi.org/10.1007/s10750-022-04918-5
Vos M, Hering D, Gessner MO, Leese F, Schäfer RB, Tollrian R, Boenigk J et al (2023) The asymmetric response concept explains ecological consequences of multiple stressor exposure and release. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2023.162196
Wallace JB (1990) Recovery of lotic macroinvertebrate communities from disturbance. Environ Manage 14(5):605–620. https://doi.org/10.1007/BF02394712
Ward JV, Stanford JA (1995) Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. Regul Rivers: Res Manage 11(1):105–119. https://doi.org/10.1002/rrr.3450110109
Westveer JJ, van der Geest HG, van Loon EE, Verdonschot PFM (2018) Connectivity and seasonality cause rapid taxonomic and functional trait succession within an invertebrate community after stream restoration. PLoS ONE 13(5):e0197182. https://doi.org/10.1371/journal.pone.0197182
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Winking C, Lorenz AW, Sures B, Hering D (2014) Recolonisation patterns of benthic invertebrates: a field investigation of restored former sewage channels. Freshw Biol 59(9):1932–1944. https://doi.org/10.1111/fwb.12397
Winking C, Lorenz AW, Sures B, Hering D (2016) Start at zero: succession of benthic invertebrate assemblages in restored former sewage channels. Aquat Sci 78(4):683–694. https://doi.org/10.1007/s00027-015-0459-7
Wolff BA, Duggan SB, Clements WH (2019) Resilience and regime shifts: do novel communities impede ecological recovery in a historically metal-contaminated stream? J Appl Ecol 56(12):2698–2709. https://doi.org/10.1111/1365-2664.13503
Yamamuro AM, Lamberti GA (2007) Influence of organic matter on invertebrate colonization of sand substrata in a northern michigan stream. J N Am Benthol Soc 26(2):244–252
Acknowledgements
We acknowledge the Emschergenossenschaft/Lippeverband (Essen) for providing information on the study streams. We appreciate the help of all students and department employees who helped in the field and lab in the past ten years. Many thanks to Christian Feld and Wim Kaijser for their statistical advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. The first 2 years of this study were financed by KuLaRuhr as part of the German Federal Ministry of Education and Research programme “Sustainable land use” (BMBF, project number 033L020A, http://www.kularuhr.de). This paper results from the CRC RESIST, for which funding was provided by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—CRC 1439—Project Number: 426547801.
Author information
Authors and Affiliations
Contributions
AL, SG and DH designed the study; AL organised and conducted sample collection and processing, with contributions by SG; AL collected the data; SG analysed the data and led the writing of the manuscript. All authors contributed to the drafts and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Spearman’s Rank coefficients of all environmental variables.
Additional file 2: Figure S1.
NMDS of log-transformed community data per sampling site, sampling years connected. Time since restoration was added as an overlay (red arrow).
Additional file 3
: Table S1. Records on streambed drying in the Boye catchment between 2018 and 2020. Information on the periods of streambed drying in the Boye were collected unstandardized from the local water board (Emschergenossenschaft) and colleagues from the University of Duisburg-Essen. All the information was recorded in either August or September of the respective years.
Additional file 4: Table S1.
Explanation of species abbreviations (ad adult, lv larvae, sp species, gen genus).
Additional file 5: Table S1.
Spearman’s Rank coefficients of indicator species and major environmental variables (for explanations of the species abbreviations refer to Additional file 4: Table S1).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gillmann, S.M., Hering, D. & Lorenz, A.W. Habitat development and species arrival drive succession of the benthic invertebrate community in restored urban streams. Environ Sci Eur 35, 49 (2023). https://doi.org/10.1186/s12302-023-00756-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00756-x