Abstract
Background
Various nano-enabled agrochemicals are being extensively used for soil remediation and to boost crop production by increasing the nutrient efficiency of fertilizers. However, understanding of their potential risks on the manure–soil–plant continuum is limited. These nano-agrochemicals can be potentially toxic to soil microbes and their associated functions, such as nitrogen (N) mineralization and decomposition of organic materials. Moreover, the accumulation of nanoparticles (NPs) in edible crops may reduce food quality, and can cause serious threats to human health. Accordingly, here we investigated how zinc (ZONPs) and iron oxide (IONPs) nanoparticles affect the soil microbial communities, their efficiency of decomposition and N mineralization, radish yield, and plant N recovery after soil application of poultry manure (PM). Furthermore, we studied the associated health risks (DIM, HRI) via dietary intake of radish.
Results
Soil application of ZONPs and IONPs significantly (P < 0.05) increased microbial biomass Zn/Fe indicating their microbial utilization. This decreased the colony-forming units (CFU) of bacteria and fungi. For example, the application of PM with ZONPs and IONPs decreased the CFU of bacteria by 32% and 19%, respectively. In case of fungi, the CFU reductions were slightly different (ZONPs: 28% and IONPs: 23%). Consequently, the N mineralization significantly decreased by 62% and 29% due to ZONPs and IONPs, respectively. Which ultimately resulted in the reduction of radish dry matter yield by 22% and 12%. The respective reductions of the apparent N recovery (ANR) were 65% and 39%. Health risk assessment indicated that DIM and HRI values from both the NPs lie under safe limits.
Conclusions
We conclude that both metal oxide nanoparticles (i.e., ZONPs and IONPs) can significantly affect the soil microbial community, their associated functions, and crop yield with the former being relatively more toxic. However, no evidence was found regarding the health risks to humans via dietary radish intake. These toxicological effects imply restricting the widespread production and use of NPs, and developing strategies for their safe disposal to avoid their contact with soil beneficial microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Over the last decades, massive production, use, and abuse of nano-enabled products have raised concerns about their presence in the environment, and detrimental effects on terrestrial and aquatic ecosystems [1,2,3]. Nanoparticles (NPs) are commonly applied in agriculture [4, 5], soil remediation [6, 7], wastewater treatment [8, 9], and biomedicine [10, 11]. Thus, a huge quantity of NPs enters the environment, which mainly ends up in soil directly or through the application of sludge in farming [2, 12, 13]. According to an estimation, landfills and soils receive the largest share of the produced NPs (63–91% and 8–28%, respectively) followed by the aquatic environment (~ 7%), and air (~ 1.5%) [14].
Metal-based nanoparticles have been shown to affect the soil microbial community structure and their associated functions [15,16,17]. Beneficial microbes such as fungi and bacteria play key roles in nutrient cycling, soil remediation and plant growth [18, 19]. Microbe-mediated soil processes are crucial for improving carbon (C) and nitrogen (N) cycling in the ecosystem, specifically in organic agriculture, where the soil microbes are mainly responsible to release nutrients from added organic matter. Metal-based NPs have been reported to significantly reduce the microbial decomposition and mineralization of the added organic material [17, 20].
Metallic NPs can affect the soil microbiome directly by increased toxicity and reduced nutrient bioavailability [21], or indirectly interact with organic toxicants and increase the cumulative effects [22, 23]. The possible mechanism for direct toxicity includes membrane disruption [24, 25], genotoxicity [26], ROS formation [27, 28], and release of toxic constituents [23]. It is suggested that exposure to NPs interacts with the elements of the microbial membrane, resulting in structural changes, disruption of the cell's functionality, and eventually cell death [29, 30]. Recently, Palza [31] reviewed the antimicrobial effects of NPs on different bacterial species, such as Escherichia coli, Citrobacter freundii, Streptococcus mutans, and fungi, such as Aspergillus nidulans. In a microcosm study, Shen et al. [32] elucidated the toxicological effects of ZONPs on soil microbial community in terms of inhibited microbial respiration, ammonification process, and dehydrogenase and fluorescent diacetate hydrolase activities. Enzymatic activities such as catalase, urease, hydrolysis of fluorescein diacetate, and thermogenic metabolism, and the population of Azotobacter can also be affected by ZONPs [33]. Another investigation [20] showed decreased CFUs of bacteria and fungi by up to 40% after the application of IONPs in manure-amended soil. The reduced CFUs remarkably decreased the microbes-mediated soil processes, such as decomposition and mineralization which consequently affect the fertilizer value of the added organic materials.
The addition of organic materials such as plant litter and manure is crucial for carbon sequestration and recycling of plant nutrients, especially in organic agriculture [34, 35]. Effective recycling of plant nutrients has always remained a challenge for resource-poor small land-holder around the globe due to the uncertainty of nutrient release owing to various soil, waste, and environmental factors. Recently, it has been reported that nutrient cycling can be hampered by some emerging pollutants (i.e., metal-based NPs), as they negatively affect soil microbial structure and functioning [20]. Most of the studies conducted so far on toxicity assessment of NPs were either restricted to hydroponics, culture media and/or soil incubation experiments without the presence of plants [17, 36,37,38]. However, studies involving plants for this purpose are very scarce [20]. Accordingly, the present study aimed to explore and compare the effects of IONPs and ZONPs on soil microbial communities (counts), their performance in terms of decomposition, mineralization and plant N recovery after the application of PM. Furthermore, we aim at assessing the radish crop yield and associated health risks (DIM, HRI) via its dietary intake.
Methods
To achieve objectives, an outdoor pot experiment was conducted at the research facility of COMSATS University Islamabad (CUI), Vehari campus (latitude 30.0318° N and longitude 72.3145°E). The soil was obtained from the agricultural field of the campus, whereas both the metallic oxide nanoparticles (IONPs and ZONPs) were provided by Sigma-Aldrich (Saint Louis, MO, USA). IONPs had particle size ≤ 50 nm with a particle density of 5.25 ± 0.1 g mL−1. The respective values in case of ZONPs were ≤ 40 nm, and 133 1.7 ± 0.1 g mL−1. Poultry manure (PM), which consisted of poultry droppings and residues of bedding material, was collected from an adjacent broiler farm. Selected characteristics of the PM together with used soil are given in Table 1.
Experimental setup and treatments
The collected soil was properly homogenized after sieving it using a 4 mm sieve to remove plant and debris material. Afterward, 7 kg of soil was filled in each pot with a surface area of 0.063 m2. PM (at a rate of 25 kg N acre−1) and NPs (at a rate of 1 g kg−1 soil) treatments were applied in soil 2 days before seed sowing and thoroughly mixed in the upper three inches of soil. Five seeds of radish (Raphanus sativus) were sown per pot and later thinned into two healthy seedlings after germination. Throughout the experiment, the moisture content of the soil was maintained at 60%.
Sampling and analysis of soil and plant
Representative soil samples were collected before and after the experiment for the analyses of soil organic matter (SOM), pH, electric conductivity (EC), inorganic nitrogen (N) content, total N content, microbial colony forming units (CFU), and microbial biomass Fe/Zn. Soil pH and EC were determined using calibrated pH and EC meter, respectively. The SOM was determined by the loss in the ignition method. Total N and inorganic N (ammonium and nitrate N) were determined using the Kjeldahl method after following the procedures as described by Estefan [39]. For the mineral N solution, the extract was distilled using boric acid (H3BO3) as a receiver and then titrated against 0.01 N H2SO4. Total Zn and Fe contents of PM, soil, and plant samples were quantified using Atomic Absorption Spectrophotometer (AAS, Model Thermo S-Series as described in Rashid et al. [17]. Soil bacteria, as well as fungi CFU, were estimated using the pour plate method [20].
Microbial biomass Zn/Fe
To quantify the microbial biomass Zn/Fe, we used the fumigation-extraction method. For this purpose, samples were extracted with 25 ml 1 M NH4NO3, filtered and acidified with HNO3, and kept stored at 4 °C. Furthermore, the concentration of labile Zn/Fe was determined according to Rashid et al. [17].
Enumeration of soil bacteria and fungi
For the enumeration of bacteria and fungi, we used the pour plate method. Briefly, 1 g soil was suspended in 99 ml of buffered peptone water in a conical flask (250 ml) and mixed for 1 h on an orbital shaker (150 rpm). Subsequently, dilutions were prepared and poured on Sabouraud Dex-trose Agar for fungi (Himedia, USA) and Nutrient Agar Plates for bacteria (HiMedia, USA) and then incubated at 25 ± 1 °C for 5 days and 30 ± 1 °C for 3 days, respectively. After incubation, colony-forming units (cfu mL−1) were counted using a colony counter (ColonyCount V, Gerber Instruments AG, Effretikon, Switzerland).
Plant metal contents
Nitric acid digestion is an effective way to determine heavy metals in the environmental sample, such as organic-rich samples, soil, and plant material. For this purpose, accurately weighed (0.5 g each) samples were placed in 100-mL beakers, subjected to 10 ml of 65% HNO3, and kept overnight at room temperature. The next day, the mixture was boiled over a hot plate at 350 °C, and drops of hydrogen peroxide were added to these samples during heating until a colorless solution was obtained. The filters were reduced to a final volume of 25 mL for the analyses. The samples were properly labeled, packed in plastic bottles, and analyzed on atomic absorption spectrometer.
Plant harvesting
Radish plants were harvested 85 days after seed sowing. At harvest, the soil around the plant was loosened to facilitate the pulling of complete radish. Thereafter, roots and shoots were separately washed with distilled water and dried using tissue paper. The fresh weight (g) of all the roots and shoots was recorded after harvest (within 30 min.) using a digital weighing balance. Thereafter, representative samples were air-dried in an oven at 70 °C for 48 h to estimate dry matter yield. Thereafter, representative samples were analyzed for total N by the Kjeldahl method [39]. Total Zn or Fe content was determined using atomic absorption spectrophotometer (AAS, Model Thermo S-Series).
By considering total plant biomass together with their N content, total N recovery (TNR) by the plant was determined according to the procedure described by Shah et al. [40]:
where TNUPM and TNUcontrol are the total N uptake by radish in PM applied and controlled pots, respectively. TNapplied represents the amount of applied N in pots through PM.
N mineralization (\(\mathrm{Nmin})\) from applied organic N was calculated considering the total radish N uptake (root and shoot) and residual soil mineral N as described earlier by Shah et al. [35]:
Associated health risks assessment
Daily oral intake for Fe and Zn (mg day−1) through consumption of radish was calculated using the following equation (Eq. 3, [41]):
where Cmetal is the Zn/Fe content (mg kg−1) in the edible part of the radish, IR is the vegetable ingestion rate (mg kg−1), ED represents the exposure duration, ED shows exposure frequency, BD indicated the body weight, and AT represents average life expectancy.
Average radish Zn/Fe content (mg kg−1) was determined after analyzing the plant samples for Zn and Fe content as described earlier.
The health risk index (HRI) for humans through radish consumption was calculated following the equation (Eq. 4 [41]):
where DIM is the daily intake of metal through the consumption of radish (kg day−1), C metal is the concentration of metals in the radish (mg kg−1), RD is the oral reference dose for the metal (mg kg−1 of body weight day−1). RD for Zn and Fe was used as 0.300 and 0.700 mg kg−1 day, respectively [42].
Data analyses
Data were analyzed using analysis of variance (ANOVA) in STATISTIX 8.1 software. When the main effect was significantly different, the treatment means were further compared using the least significant difference (LSD) test at a 5% probability level. Furthermore, sole and/or combined effects of IONPs and ZONPs on colony farming units, microbial biomass Fe/Zn, N mineralization and radish N recovery, and radish Fe/Zn content were examined by principal component analysis on correlation matrices using a package of CANOCO 5.0 (Microcomputer Power 281 Inc., Ithaca, NY),
Results
Microbial biomass Fe/Zn contents
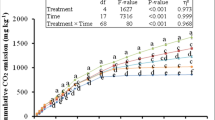
Total microbial biomass Fe/Zn contents from treatments with and without the addition of IONPs and/or ZONPs are presented in Fig. 1a. Unfertilized control and the sole PM treatments had a very low level of microbial biomass Fe/Zn content. However, the contents were significantly greater in soil that received metallic nanoparticles (IONPs and ZONPs). Overall, the results indicated low Fe and Zn contents in non-fumigated than the fumigated soil. In the later soil, IONPs, PM + IONPs, ZONPs, PM + ZONPs, ZONPs + IONPs and PM + ZONPs + IONPs showed 31, 45, 40, 67, 24 and 18 mg kg−1 of Fe and/or Zn contents, respectively (P < 0.001, DF = 7, F value = 14.16). However, in case of non-fumigated soil, the respective values were only 2.9, 3.8, 26.3, 37.3, 4.4 and 5.2 mg kg−1 (P < 0.001, DF = 7, F value = 7.74).
Microbial colony-forming units
The bacterial CFU increased significantly with the application of PM than the unfertilized soil (53 × 104 vs. 33 × 104 g−1 soil; P < 0.05). However, the CFU values were decreased by 20%, 30%, and 10% from PM treatments applied with IONPs, ZONPs, and IONPs + ZONPs, respectively. Likewise, IONPs and ZONPs reduced the fungal CFU in the unfertilized control as well as PM treatments (P < 0.05, Fig. 1b). Note that the addition of IONPs and ZONPs in PM applied soil showed tenfolds higher microbial biomass Fe and/or Zn contents in fumigated compared to non-fumigated soils.
Mineralization from PM with and or without nanoparticles, and total nitrogen uptake
The N mineralization values were the highest from PM alone and least in the case of PM with ZONPs and IONPs (Table 2). Of the organic N applied to radish, about 52%, 18%, 35%, and 31% were mineralized during the experimental period from PM, PM + ZONPs, PM + IONPs, and PM + ZONPs + IONPs.
In the case of N uptake in radish, PM treatment resulted in maximum N uptake, i.e., 6.62 g m−2 as compared to other treatments (Table 2). As compared to sole PM application, addition of NPs decreased TNU by 44% (6.24 vs. 3.45 gm−2; P < 0.05) in ZONPs, 3% (6.62 vs. 6.4 gm−2; P < 0.05) in IONPs and 22% (6.62 vs. 5.15 gm−2; P < 0.05) in ZONPs + IONPs treatments. There was no difference in plant N uptake in unfertilized soil with and/or without ZONPs and/or IONPs.
Radish yield and nitrogen recovery
Soil addition of PM significantly increased (P < 0.05) the radish dry matter over unfertilized control (571 vs. 323 gm−2 of DM). However, application of IONPs, ZONPs and IONPs + ZONPs in PM amended soil considerably reduced the DM yield by 22 (448 vs. 571 gm−2; P < 0.05), 12 (505 vs. 571 gm−2; P < 0.05) and 10% (514 vs. 571 gm−2; P < 0.05), respectively. The soil addition of both the IONPs and ZONPs in unfertilized control have no effects on plant dry matter yield (Fig. 2a). The results showed that 66% of the applied N via PM ended up in radish plants. However, the IONPs, ZONPs and IONPs + ZONPs decreased the radish apparent N recovery fractions from PM by 39% (66.5 vs. 40.62; P < 0.05), 65% (66.5 vs. 22.96; P < 0.05) and 47% (66.5 vs. 35.32; P < 0.05), respectively (Fig. 2b).
Plant yield after the application of poultry manure alone and in combination with ZONPs and IONPs (a). N recovery of radish plant from PM with or without NPs (b). The error bars represent standard errors ( ±) of the mean values. Bars with different letters are significantly different at a 5% probability level
Health risk assessment of heavy metals
Total Zn and Fe contents in the radish plant are expressed in Table 3. Daily intake of metals (Zn or Fe) was estimated based on metal concentrations found in edible parts of vegetable, i.e., roots of the radish. Thereafter, the health risk index was calculated using the DIM and the reference dose of the corresponding metal. Results revealed daily intake of Zn value 0.077, 0.079, 0.072, 0.097, 0.107, 0.143, 0.120 and 0.164 mg kg−1 via dietary intake of radish root grown in control, PM, ZONPs, PM + ZONPs, IONPs, PM + IONPs, IONPS, PM + IONPs, ZONPs + IONPs and PM + ZONPs + IONPs (Table 3). The respective values in case of Fe were 0.297, 0.452, 0.281, 0.477, 0.552, 0.612, 0.561, and 0.631. HRI value for Zn as well as Fe remained below 1 for all the treatments (Table 3).
Principal component analysis
According to the output of PCA, the first two axes explained most of the variations (65%) in the data. A positive association between ZONPs and IONPs was found with DIM and HRI parameters. However, both these metallic nanoparticles show negative associations with bacterial CFU, fungal CFU, N mineralization, plant yield and total N recovery (Fig. 3).
Principal component analysis (PCA) of residual soil mineral N, mineralized organic N, colony forming units of bacterial and fungi, microbial biomass Fe and Zn, total dry matter (TDM) yield, total N uptake (TNU), total N recovery (TNU), daily intake of metal (DIM), health risk index (HRI), Plant Fe and Zn content from PM exposed with or without ZONPs and/or IONPs. Treatments are represented by the use of various markers, i.e., open circles (unfertilized control), filled circles (PM), open rectangles (IONPs), filled rectangles (IONPs + PM), open triangles (ZONPs), filled rectangles (ZONPs + PM), open diamond (ZONPs + IONPs) and filled diamonds (ZONPs + IONPs + PM). Most of the variations in the data are explained by PC1 (45.09%) and PC2 (19.58%) as given in the statistics table (inset), and the individual scores are unitless
Other parameters such as Fe/Zn content in plants, and soil microbial biomass Fe/Zn (fumigated or non-fumigated soil) were closely linked with the integrated application of these metallic nanoparticles (IONPs/ZONPs) with PM. PCA results also revealed a positive and strong correlation of PM application with various soil (SOM, Nmin, bacterial and fungal CFU) and plant parameters (plant DM yield, N uptake, and Total N recovery).
Discussion
Results of the current study showed that soil microbial biomass Fe/Zn contents after PM application were below the detection limits of the AAS (Fig. 1a); however, it was complemented by 2–3 times greater numbers of heterotrophic bacterial and fungal colony forming units (Fig. 1b). This might have been due to its contribution in building-up soil carbon pool that provides favorable environments (i.e., food and energy) for growth and multiplication of microorganism. Such results are endorsed by some previous studies, which indicated that the population of bacteria and fungi can be increased by utilizing organic resources [43,44,45]. Our findings corroborate the opinion of Yazdanpanah et al. [46], who reported an increase in soil microbial activities with the addition of organic material. Kamran et al. [20] also reported an increase in viable microbial colony-forming units after the application of farmyard manure. However, the addition of metal-enabled nanoparticles (IONPs and ZONPs) to PM treatment reduced both the bacterial and fungal viable counts as compared to the lone PM treatment. Literature reveals that both these NPs can dissolute quickly once exposed to soil and Zn2+ and Fe2+ are released [47, 48]. When exposed to soil microbes, these metallic ions directly attach to the microbial cell by electrostatic attraction and damage their cell wall by disrupting the hydrophobic tails of lipid bilayers [1]. Such alteration in membrane structure results in NPs penetration into the cell and stimulates the internal signaling cascade leading to protein oxidation and leakage of the electrolyte and thereby cell death [49]. Furthermore, NPs can have an indirect effect by the dissolution of toxic elements and production of reactive oxygen species (ROS) that persuade oxidative stress and damage the DNA and protein [50, 51]. Dissolution results in the release of toxic ions from metal oxide NPs which are absorbed by the microbial cell membrane leading to direct association with amino (-NH), carboxyl (-COOH), and mercapto (-SH) functional groups of protein and nucleic acids. Moreover, the toxic ions may form an association with the genetic material and/or phospholipids of the exposed microorganism. All these associations alter the cellular structure as well as enzymatic activities and thereby results in oxidative stress and production of ROS, i.e., hydrogen peroxide, superoxide anion, singlet oxygen, and hydroxyl ion. These elevated ROS oxidize the bases and deoxyribose of DNA, resulting in DNA damage and cell death [52]. We argue that the direct effect of IONPs or ZONPs would have been dominant in our case, since the increase in microbial biomass Fe/Zn was found after the application of IONPs and ZONPs as reflected in Fig. 1a. As a result, viable colony-forming units of bacterial as well as fungi were considerably reduced. For instance, in PM treatments, a decrease of 20% (43 × 104 vs. 26 × 104), 30% (36 × 104 vs. 23 × 104), and 10% (40 × 104 vs. 25 × 104) in bacterial colonies were observed after the addition of IONPs, ZONPs, and IONPS + ZONPs, respectively (Fig. 1b). Whereas in case of fungi, the respective decrement was 56% (06 × 104 vs. 14 × 104 g− 1 soil), 29% (06 × 104 vs. 08 × 104 g− 1 soil) and 62% (43 × 104 g− 1 vs. 17 × 104 g− 1 soil). Our results corroborate with [20] who reported around a 40% decline in bacterial colonies after the application of IONPs to farmyard manure amended soil. Such negative results of metallic nanoparticles are also reported in some other previous studies, i.e., [17, 37, 38, 49, 51, 53]. Palza [31] reported the antimicrobial effect of NPs on various species of bacteria, i.e., Escherichia coli, Citrobacter freundii, Streptococcus mutans, and on fungi, such as Aspergillus nidulans. Interestingly, in the unfertilized soil, these NPs did not significantly reduce the viable microbial counts; however, in the PM fertilized soil, NPs had pronounced effects on these microbial parameters. Possible reasons for this discrepancy would be the difference in bio-availability of metallic ion to soil microbes in both situations. In PM amended treatments the bio-availability of metallic ions of the aforementioned NPs would have been greater, since organic material is known to facilitate the dissolution of metallic compounds. According to He et al. [47], fulvic and humic acids are formed after the application of organic materials which facilitate the ligand exchange reaction with the surface of metallic nanoparticles which favors the release of metallic ions in the aqueous solution. The limited release of metallic ion in the unfertilized soil would have been used by the microbes for their metabolic requirements. This was evident in the more or less similar microbial colony forming units of unfertilized soil with or without the addition of IONPs and/or ZONPs (Fig. 1b). Our arguments were strengthened by He et al. [54] who found no effects of IONPs on soil bacterial communities as well as eukaryotic abundance in unfertilized soil. This logic was also supported by the PCA output which revealed a negative correlation between microbial counts and NPs for PM treatments (Fig. 3). This decrease in the microbial count by the NPs in PM treatments reduced the microbial mediated N mineralization. In this study, net N mineralization from PM was reduced by 65% (52% vs. 18%), 33% (52% vs. 35%), and 40% (52% vs. 31%) by ZONPs, IONPs, and ZONPs + IONPs, respectively. These findings corroborate with Rashid et al. [17, 38] and Kamran et al. [20] who found a reduction in N mineralization from plant/waste material, which they ascribed to the reduced microbial activities as a result of NPs toxicity. According to Jiang et al. [55], another possible reason for reduced net N mineralization would be the formation of nitrogenous polymers via abiotic means attributed to the NPs. However, we believe that this possibility can be ruled out in our case, since both the reduced N mineralization and reduction in microbial colony-forming units were witnessed in the same treatments with ZONPs and/or IONPs. This explanation was further strengthened by the PCA analysis which reflected a close correlation between microbial count and N mineralization. As a result, total plant N recovery from the applied PM was decreased by 33% (67% vs. 44%), 17% (67% vs. 54%), and 23% (67% vs. 51%) by ZONPs, IONPs, and ZONPs + IONPs. This reduced N utilization by plants implies lower nitrogen cycling in the agro-ecosystem which results in some serious challenges for food production by resource-poor farmers all over the world and especially in Africa, where manure is often considered a valuable source of recycling plant nutrients. The recycling of plant nutrients is equally important for some of the Southeast Asian countries, i.e., Pakistan, where the availability of inorganic fertilizer has become a constraint due its increased price and low availability in the market. Therefore, enormous applications of metallic nanoparticles (ZONPs and IONPs) must be restricted for agriculture and soil remediation purposes. Furthermore, their use in industries should be limited and the effluent/sludge must be safely disposed-off in agro-ecosystems to avoid contact of metallic nanoparticles with beneficial soil microorganisms.
Overall, the addition of metallic NPs (ZONPs and IONPs) reduced the plant yield from PM treatments. This reduction could be attributed to the fact that (i) NPs can hamper the bioavailability of N from the applied poultry manure and (ii) negative impact on plant physiology such as reduced seed germination, root and shoot elongation leading to decreased biomass, and/or crop yield [56]. We believe that scenario (i) would have prevailed in this study, since no effect of NPs on germination was noticed and there was no difference in plant metal content from manure applied with and/or without ZONPs and/or IONPs.
Health risks associated with metal-contaminated plant consumption are assessed by DIM and HRI parameters. For all the treatments, DIM of Zn and Fe remained within the reference dose of 0.3 and 0.7 mg kg−1 as communicated by the environmental protection agency of the USA [42, 57]. DIM was used to calculate the HRI value and the metal consumption limit is considered safe when HRI < 1 [58,59,60]. In all the cases HRI values remained below 1 (Table 3) indicating the absence of health risks for human consuming radish through their human life span of 70 years. Since both the Zn and Fe elements are essential nutrients for human, the application of ZONPs and IONPs in the soil can increase their concentration in edible plants and thereby may be helpful to achieving the required nutrition. Therefore, to fulfill the malnutrition, these metallic nanoparticles may be used in soil deficient with Zn or Fe, where inhibition of soil microbial activities is not a matter of concern.
Conclusions
Results of the current study revealed an increased plant DM yield and ANR after the application of PM. However, the application of both agro-nanoparticles (ZONPs and IONPs) decreased viable counts of bacteria and fungi, and consequently reduced the crop yield and N recovery. Nevertheless, HRI values from the application of these metallic NPs remained within safe limits. These findings create toxicological concerns over the entrance of NPs in soil which must be restricted to ensure good soil health and C and N cycling from wastes. Considering the toxic effects of these metallic nanoparticles, this study warrants developing some safe strategies for the disposal of the nanoparticles in agro-ecosystems to avoid their contact with beneficial soil microorganisms. Further in-depth research should focus on the bioavailability of these metallic nanoparticles and their free metal ion in differentially textured soils amended with/without organic wastes, and their interaction with the microbes.
Availability of data and materials
All data generated or analyzed during this study are included in this article. However, any further details are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ANR:
-
Apparent N Recovery
- C:
-
Carbon
- CFU:
-
Colony Forming Units
- DIM:
-
Daily Intake of Metal
- EC:
-
Electric Conductivity
- HEC:
-
Higher Education Commission
- HRI:
-
Health Risk Index
- IONPs:
-
Iron Oxide Nanoparticles
- LSD:
-
Least Significant Difference
- N:
-
Nitrogen
- NPs:
-
Nanoparticles
- PGPR:
-
Plant Growth Promoting Rhizobacteria
- PM:
-
Poultry manure
- SOM:
-
Soil Organic Matter
- ZONPs:
-
Zinc Oxide Nanoparticles
References
Ameen F, Alsamhary K, Alabdullati JA (2021) A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol Environ Saf 213:112027
Bundschuh M, Filser J, Luderwald S, McKee MS, Metreveli G, Schaumann GE, Schulz R, Wagner S (2018) Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur 30(1):6
Lead JR, Batley GE, Alvarez PJJ, Croteau MN, Handy RD, McLaughlin MJ, Judy JD, Schirmer K (2018) Nanomaterials in the environment: behavior, fate, bioavailability, and effects—an updated review. Environ Toxicol Chem 37(8):2029–2063
Wang D, Saleh NB, Byro A, Zepp R, Sahle-Demessie E, Luxton TP, Ho KT, Burgess RM, Flury M, White JC, Su C (2022) Nano-enabled pesticides for sustainable agriculture and global food security. Nat Nanotechnol 17(4):347–360
Ngo QB, Dao TH, Nguyen HC, Tran XT, Van Nguyen T, Khuu TD, Huynh TH (2014) Effects of nanocrystalline powders (Fe, Co and Cu) on the germination, growth, crop yield and product quality of soybean (Vietnamese species DT-51). Advc Natural Sci: Nanoscience and Nanotechnology 5(1):015016
Mueller NC, Nowack B (2010) Nanoparticles for remediation: solving big problems with little particles. Elements 6(6):395–400
Tagliabue M, Reverberi AP, Bagatin R (2014) Boron removal from water: needs, challenges and perspectives. J Clean Prod 77:56–64
Hernandez D, Salas D, Giménez D, Buitrago P, Esquena S, Palou J, de la Torre P, Pernas J, Gich I, de Segura GG (2015) Pelvic MRI findings in relapsed prostate cancer after radical prostatectomy. Radiation Oncol 10(1):262
Singh S, Kumar V, Romero R, Sharma K, Singh J (2019) Applications of nanoparticles in wastewater treatment. In: Prasad R, Kumar V, Kumar M, Choudhary D (eds) Nanobiotechnology in bioformulations. Springer International Publishing, Cham, pp 395–418
Gherasim O, Puiu RA, Birca AC, Burdusel AC, Grumezescu AM (2020) An updated review on silver nanoparticles in biomedicine. Nanomaterials Basel. 10:11
Qin Z, Li Y, Gu N (2018) Progress in applications of prussian blue nanoparticles in biomedicine. Adv Healthc Mater 7(20):e1800347
Keller AA, Adeleye AS, Conway JR, Garner KL, Zhao L, Cherr GN, Hong J, Gardea-Torresdey JL, Godwin HA, Hanna S, Ji Z, Kaweeteerawat C, Lin S, Lenihan HS, Miller RJ, Nel AE, Peralta-Videa JR, Walker SL, Taylor AA, Torres-Duarte C, Zink JI, Zuverza-Mena N (2017) Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7:28–40
McGillicuddy E, Murray I, Kavanagh S, Morrison L, Fogarty A, Cormican M, Dockery P, Prendergast M, Rowan N, Morris D (2017) Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci Total Environ 575:231–246
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanoparticle Res. https://doi.org/10.1007/s11051-013-1692-4
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A-J, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17(5):372–386
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42(12):4447–4453
Rashid MI, Shahzad T, Shahid M, Ismail IM, Shah GM, Almeelbi T (2017) Zinc oxide nanoparticles affect carbon and nitrogen mineralization of Phoenix dactylifera leaf litter in a sandy soil. J Hazard Mater 324(Pt B):298–305
Kumar A, Verma JP (2019) The role of microbes to improve crop productivity and soil health. Ecological wisdom inspired restoration engineering. Springer, Singapore, pp 249–265
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20
Kamran M, Ali H, Saeed MF, Bakhat HF, Hassan Z, Tahir M, Abbas G, Naeem MA, Rashid MI, Shah GM (2020) Unraveling the toxic effects of iron oxide nanoparticles on nitrogen cycling through manure-soil-plant continuum. Ecotoxicol Environ Saf 205:111099
Kraas M, Schlich K, Knopf B, Wege F, Kagi R, Terytze K, Hund-Rinke K (2017) Long-term effects of sulfidized silver nanoparticles in sewage sludge on soil microflora. Environ Toxicol Chem 36(12):3305–3313
Chen F, Wu L, Xiao X, Rong L, Li M, Zou X (2020) Mixture toxicity of zinc oxide nanoparticle and chemicals with different mode of action upon Vibrio fischeri. Environ Sci Eur. https://doi.org/10.1186/s12302-020-00320-x
Dinesh R, Anandaraj M, Srinivasan V, Hamza S (2012) Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 173:19–27
de Planque MR, Aghdaei S, Roose T, Morgan H (2011) Electrophysiological characterization of membrane disruption by nanoparticles. ACS Nano 5(5):3599–3606
Adams J, Wright M, Wagner H, Valiente J, Britt D, Anderson A (2017) Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol Biochem 110:108–117
Vannini C, Domingo G, Onelli E, De Mattia F, Bruni I, Marsoni M, Bracale M (2014) Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol 171(13):1142–1148
Buyukhatipoglu K, Clyne AM (2011) Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A 96(1):186–195
Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R (2009) EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J Phys Chemis C 113(36):15997–16001
Suresh AK, Pelletier DA, Wang W, Moon J-W, Gu B, Mortensen NP, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2010) Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ Sci Technol 44(13):5210–5215
Yoon KY, Hoon Byeon J, Park JH, Hwang J (2007) Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373(2–3):572–575
Palza H (2015) Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 16(1):2099–2116
Shen Z, Chen Z, Hou Z, Li T, Lu X (2015) Ecotoxicological effect of zinc oxide nanoparticles on soil microorganisms. Front Environ Sci Eng 9(5):912–918
Chai H, Yao J, Sun J, Zhang C, Liu W, Zhu M, Ceccanti B (2015) The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull Environ Contam Toxicol 94(4):490–495
Shah G, Rashid M, Shah G, Groot J, Lantinga E (2013) Mineralization and herbage recovery of animal manure nitrogen after application to various soil types. Plant Soil 365(1):69–79
Shah GM, Tufail N, Bakhat HF, Imran M, Murtaza B, Farooq ABU, Saeed F, Waqar A, Rashid MI (2017) Anaerobic degradation of municipal organic waste among others composting techniques improves N cycling through waste-soil-plant continuum. J Soil Sci Plant Nutr 17(2):529–542
Antisari LV, Carbone S, Gatti A, Vianello G, Nannipieri P (2013) Toxicity of metal oxide (CeO2, Fe3O4, SnO2) engineered nanoparticles on soil microbial biomass and their distribution in soil. Soil Biol Biochem 60:87–94
Frenk S, Ben-Moshe T, Dror I, Berkowitz B, Minz D (2013) Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS ONE 8(12):e84441
Rashid MI, Shahzad T, Shahid M, Imran M, Dhavamani J, Ismail IM, Basahi JM, Almeelbi T (2017) Toxicity of iron oxide nanoparticles to grass litter decomposition in a sandy soil. Sci Rep 7(1):41965
Estefan G (2013) Methods of soil, plant, and water analysis: a manual for the West Asia and North Africa region. International Center for Agricultural Research in the Dry Areas (ICARDA), Beirut
Shah GM, Tufail N, Bakhat HF, Ahmad I, Shahid M, Hammad HM, Nasim W, Waqar A, Rizwan M, Dong R (2019) Composting of municipal solid waste by different methods improved the growth of vegetables and reduced the health risks of cadmium and lead. Environ Sci Pollut Res Int 26(6):5463–5474
Shah AH, Shahid M, Khalid S, Natasha, Shabbir Z, Bakhat HF, Murtaza B, Farooq A, Akram M, Shah GM, Nasim W, Niazi NK (2020) Assessment of arsenic exposure by drinking well water and associated carcinogenic risk in peri-urban areas of Vehari Pakistan. Environ Geochem Health 42(1):121–133
Mahmood A, Malik RN (2014) Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore Pakistan. Arabian J Chemis 7(1):91–99
Faissal A, Ouazzani N, Parrado JR, Dary M, Manyani H, Morgado BR, Barragan MD, Mandi L (2017) Impact of fertilization by natural manure on the microbial quality of soil: molecular approach. Saudi J Biol Sci 24(6):1437–1443
Tang H, Li C, Xiao X, Shi L, Cheng K, Wen L, Li W (2020) Effects of short-term manure nitrogen input on soil microbial community structure and diversity in a double-cropping paddy field of southern China. Sci Rep 10(1):13540
Li C, Shi L, Tang H, Cheng K, Wen L, Li W, Xiao X, Wang K (2021) Organic manure management increases soil microbial community structure and diversity in the double-cropping rice paddy field of southern China. Commun Soil Sci Plant Anal 52(11):1224–1235
Yazdanpanah N, Pazira E, Neshat A, Mahmoodabadi M, Sinobas LR (2013) Reclamation of calcareous saline sodic soil with different amendments (II): impact on nitrogen, phosphorous and potassium redistribution and on microbial respiration. Agric Water Manag 120:39–45
He S, Feng Y, Gu N, Zhang Y, Lin X (2011) The effect of gamma-Fe2O3 nanoparticles on Escherichia coli genome. Environ Pollut 159(12):3468–3473
Tourinho PS, van Gestel CA, Lofts S, Svendsen C, Soares AM, Loureiro S (2012) Metal-based nanoparticles in soil: fate, behavior, and effects on soil invertebrates. Environ Toxicol Chem 31(8):1679–1692
Rajput VD, Minkina T, Sushkova S, Tsitsuashvili V, Mandzhieva S, Gorovtsov A, Nevidomskyaya D, Gromakova N (2018) Effect of nanoparticles on crops and soil microbial communities. J Soils Sediments 18(6):2179–2187
Rastogi A, Zivcak M, Sytar O, Kalaji H, He X, Mbarki S, Brestic M (2017) Impact of metal and metal oxide nanoparticles on plant: a critical review. Front Chem 5:78
Ameen F, Alsamhary K, Alabdullatif JA, ALNadhari S (2021) A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol Environ Safety 213:112027
Khanna K, Kohli SK, Handa N, Kaur H, Ohri P, Bhardwaj R, Yousaf B, Rinklebe J, Ahmad P (2021) Enthralling the impact of engineered nanoparticles on soil microbiome: a concentric approach towards environmental risks and cogitation. Ecotoxicol Environ Saf 222:112459
Burke DJ, Pietrasiak N, Situ SF, Abenojar EC, Porche M, Kraj P, Lakliang Y, Samia AC (2015) Iron oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int J Mol Sci 16(10):23630–23650
He S, Feng Y, Ni J, Sun Y, Xue L, Feng Y, Yu Y, Lin X, Yang L (2016) Different responses of soil microbial metabolic activity to silver and iron oxide nanoparticles. Chemosphere 147:195–202
Jiang X, Xin X, Li S, Zhou J, Zhu T, Muller C, Cai Z, Wright AL (2015) Effects of Fe oxide on N transformations in subtropical acid soils. Sci Rep 5(1):8615
Moghaddasi S, Fotovat A, Khoshgoftarmanesh AH, Karimzadeh F, Khazaei HR, Khorassani R (2017) Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol Environ Saf 144:543–551
Natasha, Shahid M, Sardar A, Anwar H, Khalid S, Shah SH, Shah AH, Bilal M (2021) Effect of co-application of wastewater and freshwater on the physiological properties and trace element content in Raphanus sativus: soil contamination and human health. Environ Geochem Health 43(6):2393–2406
Abbasi AM, Iqbal J, Khan MA, Shah MH (2013) Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas Pakistan. Ecotoxicol Environ Saf 92:237–244
Cui Y-J, Zhu Y-G, Zhai R-H, Chen D-Y, Huang Y-Z, Qiu Y, Liang J-Z (2004) Transfer of metals from soil to vegetables in an area near a smelter in Nanning China. Environ Int 30(6):785–791
Uriah LA, Shehu U (2014) Environmental risk assessment of heavy metals content of municipal solid waste used as organic fertilizer in vegetable gardens on the Jos Plateau Nigeria. Am J Environ Protection 3(6–2):1–13
Acknowledgements
The authors are grateful to the Higher Education Commission (HEC), Pakistan for the financial support of this study via the NRPU project (9098/Balochistan/NRPU/R&D/HEC/2017). We are thankful to Anam Khalil, Umer Farooq, and Atika Waqar for their help during experimental harvesting and laboratory analysis. We are also thankful to Prof (Rtd.) Dr. Muhammad Aslam for English proofreading of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The funding to execute the research was provided by Higher Education Commission (HEC), Pakistan under the grant program of ‘National research projects for universities NRPU” with project number: (9098/Balochistan/NRPU/R&D/HEC/2017).
Author information
Authors and Affiliations
Contributions
GMS: conceptualization, supervision, funding acquisition, project administration, writing—original draft; MA: methodology, investigation, formal analysis, writing—original draft, IA and MS: methodology, writing—review and editing; SK: methodology, formal analysis, writing—review and editing; MI: methodology, writing—review and editing; MAN: methodology, formal analysis, writing—review and editing; NS: conceptualization, formal analysis, visualization, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shah, G.M., Amin, M., Shahid, M. et al. Toxicity of ZnO and Fe2O3 nano-agro-chemicals to soil microbial activities, nitrogen utilization, and associated human health risks. Environ Sci Eur 34, 106 (2022). https://doi.org/10.1186/s12302-022-00687-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-022-00687-z






