Abstract
Background
Antibiotics and antibiotic resistance genes (ARGs) threaten ecological sustainability and human health, especially the drinking water sources of the Douhe Reservoir, which have critical significance amid their direct use by people and the ecological hub of flora and fauna. Although antibiotics and ARGs pollution in reservoirs have been reported, it is of no practical significance to only study the changes (increase or decrease) of the abundance and diversity of ARGs, and it is more important to explore the mechanisms of the changes affecting ARGs. Thus, the occurrence and prevalence characterizations of the spatial and seasonal of the ARGs, mobile genetic elements (MGEs) and bacterial communities were comprehensively studied in present study.
Results
263 ARG (nine types of ARGs) and 51 MGE subtypes were detected in 56 samples, and the characteristics of the temporal and spatial distribution of ARGs, MGEs and the composition of bacterial communities were significantly different. Moreover, the correlation among the ARGs, bacterial communities, MGEs and environmental factors were defined, and the co-occurrence patterns associated with ARG subtypes, bacterial genera, and MGE subtypes between water and sediment of the Douhe Reservoir were different.
Conclusions
In summary, ARGs were ubiquitous presence in water and sediment of the Douhe Reservoir, and the multidrug, aminoglycoside and macrolide–lincosamide–streptogramin B (MLSB) were main types of ARGs. Bacterial genera and the environmental factors [such as temperature (T), nitrate–nitrogen (NO– 3–N), total dissolved nitrogen (TDN), and total phosphorus (TP)] significantly affected the distribution pattern of ARGs. Overall, this research revealed the spatiotemporal change and transmission mechanisms of ARGs in the typical drinking water sources of reservoirs, which will supply clues to ensure the safety of water sources.
Similar content being viewed by others
Introduction
Hundreds of antibiotics have been isolated or synthesized and used in clinical medicine, agricultural planting, animal husbandry, aquaculture and other fields ever, since the penicillin was used to treat human infections in 1940s [1]. However, most of the different types of antibiotics used in various fields will eventually be released into the environment in an un-metabolized form through various approaches, such as agricultural and pharmaceutical wastewater, human and animal excrement. The continuous release of antibiotics into the aquatic environment will induce the appearance of antibiotic resistance genes (ARGs) [2,3,4]. For instance, many studies have found that different types of ARGs appeared in fish [5, 6], shrimp [7, 8], pigs [9], cattle [10, 11] and other animals worldwide due to excessive use of antibiotics in livestock and aquaculture. Similarly, studies have shown that ARGs were detected in all types of environments, such as the atmosphere [12], water [13], and soil [14, 15]. In particular, the occurrence and spread of ARGs in lakes and reservoirs will affect the bacterial community structures and functions in the water body, which would affect the biochemical cycle of the lakes/reservoirs and the migration, transformation and natural degradation of pollutants, and the aquatic ecosystems have been destroyed ultimately [16, 17]. Studies have proved that ARGs can persist in the environment and can be spread therein, relying on the mobile genetic elements (MGEs) (e.g., insertion sequence, integrons, and plasmids) by the horizontal gene transfer (HGT) among different bacterial communities, which may have more detrimental influence on the ecological environment and human health than the antibiotics themselves [15, 16]. In addition, the environmental factors such as temperature, nutrients and heavy metals can impact the HGT of sediment and bacterial communities in water. In Karst River (Guizhou), the environmental factors such as bicarbonate ions (HCO- 3) and nitrate–nitrogen (NO- 3–N) concentration are obviously correlated with ARGs and MGEs [16]. Heavy metals such as Cd and Cr are significantly correlated with ARGs in solid waste leachates in Shanghai [18]. In recent years, antibiotics and ARGs as new environmental pollutants have been extensively studied, because they pose a threat to human and environment [19,20,21,22].
The aquatic environment is an important source and sink of ARGs, which is highly susceptible to interference by human activities. Although the wastewater (including the antibiotics and ARGs) produced from human activities such as clinical, pharmaceutical, breeding, industrial and agricultural activities has been treated by the sewage treatment plants (STPs) before discharge, many studies also have shown that the STPs cannot remove them altogether, which introduce a large amount of antibiotics and ARGs into the lakes/rivers ultimately [23, 24]. Therefore, a large number of drinking water source reservoirs at domestic and abroad are widely contaminated by antibiotics and ARGs due to the inadequate treatment of the upstream wastewater [25, 26]. For instance, in the Taipu River and Jinze Reservoir (Shanghai), it was reported that 283 ARGs were detected in all the samples, with an absolute abundance of 1.59 × 109–5.69 × 1010 copies/L [27].
With the continuous advancement of urbanization, high-density material flow and population have greatly accelerated the migration, and spread of antibiotics and their resistance genes in the environment. Therefore, reservoirs and the rivers between them can provide an ideal environment for the physical transmission, acquisition and dissemination of ARGs. These environments are closely related to the comprehensive influence between natural processes and human activities. In fact, there is no practical significance to only discuss the changes in the diversity and abundance of ARGs (increase or decrease). Differences in water and sediment environmental factors caused by seasonal hydrological gradients, such as total nitrogen (TN), total phosphorus (TP), dissolved oxygen (DO), and electrical conductivity (EC), may also affect the distribution characteristics of ARGs. Exploring the underlying causes behind variations of changes in ARGs is very important to control the occurrence and spread of ARGs to ensure the safety of the aquatic environment. However, there are few studies on the fate and related transmission mechanism of ARGs. Therefore, this study conducted a comprehensive investigation of ARGs in the water and sediment of the Douhe Reservoir and cascade reservoir group, Tangshan, northern China in summer (flood period) and winter (dry period) in 2019 using the quantitative polymerase chain reaction (qPCR). Technically, the high-throughput quantitative PCR (HT-qPCR) used in this study includes 381 primer sets (including 1 for 16S rRNA, 325 for ARGs and 55 for MGEs), covering almost all common major types of ARGs and MGEs to ensure obtain more comprehensive and reliable results for the ARGs in the Douhe Reservoir. Overall, this study investigated the occurrence and distribution characteristics of ARGs and MGEs in water and sediments in different seasons of the Douhe Reservoir, and analyzed the correlations among the occurrence of ARGs and bacterial communities, MGEs and environmental factors to explore the factors influencing the occurrence of ARGs. This work will help to comprehensively understand the mechanism of occurrence and transmission of ARGs, which will benefit for the ecological sustainability and human health of drinking water sources of the Douhe Reservoir.
Materials and methods
Location selection and sample collection
The Douhe Reservoir belongs to drinking water sources of lake reservoirs, which is the only surface drinking water sources in Tangshan City (38°55’ to 40°28’, 117°31’ to 119°19’), northern China, and the supply of water to Tangshan City started since 1988. Especially, the Douhe Reservoir is not only the single surface water source of drinking water in Tangshan City, but also the terminal regulating reservoir and water supply source of the "Luanhe Water to the Tangshan Diversion Project". The annual water consumption is about 235 million m3, and the annual water supply to Luanhe River is 300 million m3. The average precipitation of the Douhe Reservoir in the past 5 years was close to 600 mm. There are Panjiakou Reservoir, Daheiting Reservoir and Qiuzhuang Reservoir on the upstream water delivery channel of Douhe Reservoir. Among them, the Panjiakou Reservoir and Daheiting Reservoir are the main sources of the water supply project of the Luanhe River.
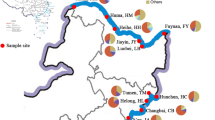
In this study, eighteen sampling locations were selected in the Douhe Reservoir and cascade reservoir group on the basis of the geographic and residential conditions of the Douhe Reservoir and its surroundings (Fig. 1). The GPS information of the sampling locations was summarized in Supplementary Information (Additional file 1: Table S1). The survey for the Douhe Reservoir was performed in two seasons: summer (flood period, July 2019) and winter (dry period, October 2019), respectively. Eighteen water samples were collected from sampling locations 1 to 18 in the Douhe Reservoir and cascade reservoir group, and ten sediment samples were collected from the sampling locations 9 to 18 in the Douhe Reservoir area in each sampling campaign, respectively. Eighteen water samples of surface water (3 L, 0.5 m depth) were taken from all sampling locations and were placed in a pre-prepared clean brown glass bottles, and were stored in a refrigerator at 4 ℃ as soon as possible for processing within 24 h. For sediment samples, all collected samples (0 − 0.3 m depth) were kept in self-sealing plastic bag and stored at – 80 ℃ as soon as possible until analysis.
Map of the sampling locations of the Douhe Reservoir and cascade reservoir group. Sampling location 1 is located in the Panjiakou Reservoir, sampling location 2 is located in the river channel between the Panjiakou Reservoir and Daheiting Reservoir, sampling locations 3–5 are located in the Daheiting Reservoir, sampling locations 6–7 are located in the river channel between the Daheiting Reservoir and Douhe Reservoir, sampling locations 8–18 are located in the Douhe Reservoir
Environmental factors of water and sediment
Water quality parameters including DO, pH, temperature (T), Oxidation–Reduction Potential (ORP), EC were determined in the field using an analysis instrument (YSI ProDss, USA), and the details of the analysis methods for other physicochemical factors (including ammonium nitrogen (NH + 4–N), NO − 3–N, Pb, Cu and As, etc.) of sediment and water were listed in the previous study [15]. Analysis results are shown in Additional file 1: Tables S2 and S3, respectively.
DNA extractions and quantitative PCR amplification
Microorganisms in each water sample were enriched by filtering 1 L of water through a 0.22 μm membrane (Millipore, USA), and then the filter membrane was cut into pieces (2 mm × 2 mm) to extract DNA in water, and 1 g of fresh sediment was used to extract DNA in each sediment sample. EZNA™ Soil DNA kit (OMEGA, USA) was used for DNA extraction. The quality and concentration of the DNA extract of the samples were verified by spectrophotometry (Thermo Fisher Scientific, USA) and agarose gel electrophoresis (1%), and then the qualified DNA was adjusted to 50 ng/mL. Three DNA extracts were obtained for each sample, and all the sample DNA extracts were stored at – 80 ℃ until PCR analysis. All the profiles of ARGs and MGEs were determined by HT-qPCR using a Quantus™ Fluorometer (Promega, USA). There are 381 primer sets used in this research (including 325 for ARGs, 55 for MGEs, and 1 for 16S rRNA gene) [28,29,30] and the detailed information about each ARG and MGE is listed in Additional file 1: Table S4.
High-throughput illumina sequencing
In this study, high-throughput Illumina sequencing (Majorbio, Shanghai, China) was used to characterize the composition of bacterial communities in the water and sediment samples collected from the Douhe Reservoir, which was described in the previously study [28, 31]. Briefly, the PCR reaction was used to characterize the bacterial communities on the V4 − V5 regions of 16S ribosomal RNA gene, and each reaction was performed in a 20 μL mixture, and the details of the PCR amplification conditions were consistent with the previous research [32]. To minimize the potential PCR bias of all the samples, the triple PCR reactions were implemented and purified, and then all purified products were adjusted to the same concentration and then mixed uniformly. Finally, the mixtures were sequenced on the MiSeq platform (Illumina, San Diego, USA). The chimeric sequences were removed after identification, and the operational taxonomic units (OTUs) with 97% similarity were clustered using UPARSE (usearch8.0.1616) [33]. The NCBI Sequence Read Archive (SRA) database was utilized for depositing the raw reads (Accession Number: SRP291227).
Statistical analysis
The ArcMap10.3 was used for visualization of the information for sampling locations. For the HT-qPCR data, all statistical analyses were performed using Excel 2017, and the difference in the occurrence and distribution of ARGs and MGEs were compared by software SPSS 20, and the results were considered significant at the 5% level (p = 0.05). Origin 2017 was used to produce the bar chart and the boxplot. Meanwhile, the heatmap of the composition of the bacterial community was analyzed with R (version 3.5.2). The nonmetric multidimensional scaling (NMDS) analysis and the Adonis test were used to reveal the difference of the composition of bacterial communities between water and sediment samples collected form the Douhe Reservoir. The redundancy analysis (RDA) was applied to reveal the relationship between the ARGs, MGEs and bacterial community using Canoco 5.0 software. The ordinary least squares (OLS) was applied to analyze ARGs and MGEs through ‘basic Trendline’ package. Finally, the visualization of the network analysis was performed using the Gephi (version 0.9.1, https://gephi.org/) software.
Results and discussion
Occurrence of ARGs and MGEs in the water and sediment samples
Diversity of ARGs
In all samples of the Douhe Reservoir and cascade reservoir group, there are 9 major types of ARGs detected (details data are given in Additional file 1: Table S5), which included trimethoprim, vancomycin, sulfonamide, tetracycline, MLSB (macrolides–lincosamides–streptomycin B), aminoglycoside, multidrug, FCA (fluoroquinolone, quinolone, florfenicol, chloramphenicol, and amphenicol), Beta-lactam resistance genes (Fig. 2). A total of 263 out of the 325 ARG subtypes were detected in the current study (261 were detected in water samples, including 250 and 197 subtypes in samples of summer and winter, respectively, Fig. 2a, b, 200 were detected in sediment samples, including 179 and 163 subtypes in samples of summer and winter, respectively, Fig. 2c, d), which indicated that the water and sediments of the Douhe Reservoir and cascade reservoir group have been extensively polluted by ARGs. In fact, it is not surprising that ARGs are ubiquitous in fresh surface drinking water sources, since intrinsic antibiotic resistance also occurs in natural ecosystems, such as forest biomes with less human disturbance [34], Siberian permafrost and Beringian stage permafrost deposits 30,000 years ago [35]. Although ARGs are generally believed to be ancient and widespread, each ecosystem contains different bacterial communities and different dominant ARGs. Meanwhile, drinking water sources are inevitably disturbed by human activities, and the influencing factors of ARGs may be more complex and diverse. This result about the number and types of detected ARGs in this study was similar with that in many previous studies for rivers, lakes and STPs [16, 28, 32, 36, 37]. The total number of ARGs detected in water and sediment samples of summer and winter of the Douhe Reservoir and cascade reservoir group are shown in Fig. 2, and the detailed data are given in Additional file 1: Table S6.
Among all the detected ARGs, the number of ARG of fw (fw1–fw18, water samples in flood period) were highest, ranging from 62.75 to 108.75, with a mean of 80.13, and followed by the number of fs samples (fs1–fs10, sediment samples in flood period) (32.50–86.50, mean of 50.90) and ds samples (ds1–ds10, sediment samples in dry period) (28.25–74.25, mean of 48.35). The lowest number of ARGs were in dw (dw1–dw18, water samples in dry period), ranging from 10.50 to 80.75, with a mean of 45.97. The results indicated that the total number of ARGs in water samples of the two seasons were significantly higher (One-way ANOVA, F = 4.85, p < 0.05) than those in sediment samples of the two seasons. At the same time, seasonally, the total number of detected ARGs in the water of summer was significantly higher (One-way ANOVA, F = 35.34, p < 0.01) than in the water of winter. This result was consistent with the results of the Karst River and Ebinur Lake (Xinjiang) [16, 38]. There are many types of resistance genes associated with antibiotics. Therefore, the selective pressure of antibiotics on microbial may be responsible for the production of ARGs [16]. As anthropogenic pollutant, evidence suggests that the movement of people and substances enhances the dispersal and proliferation of resistant organisms (including bacteria) between environments, so the type of antibiotic use and regional transport may have an impact on the distribution of resistance genes, and may lead to differences in the abundance and diversity of resistance genes [39]. In addition, higher water temperature in summer is favorable for bacterial activities, which promotes the occurrence of ARGs, resulting in significantly higher numbers of resistance genes detected in summer than in winter. In contrast, there was no significant difference in the total number of detected ARGs of water and sediment samples from the two seasons (Welch t test, p > 0.05). Basing on the number of detected ARGs, the aminoglycoside (20.32–36.94%, average of 25.81%), multidrug (4.76–26.87%, 19.31%), MLSB (9.91–24.46%, 17.74%), Beta-lactam (4.64–16.51%, 10.23%) and tetracycline (1.49–12.55%, 8.62%) resistance genes were the five main types of ARGs in water and sediment samples of the Douhe Reservoir (Additional file 1: Table S6). The surveys of dominant ARG classes in the river-reservoir system of Guangdong and Jiangxi Provinces (including Beiling River, Xunwu River and Fengshuba Reservoir) also showed similar results with this current research [32]. All the detected ARGs in this study were divided into 3 classes according to the resistance mechanism, in which antibiotic deactivation (average of 50.15%) were the most, followed by cellular protection (26.15%) and efflux pump (18.77%), respectively (Additional file 1: Table S4).
Abundance of ARGs
The total absolute abundance of 16S rRNA in each sample ranged from 2.50 × 108 to 2.48 × 1010 copies/L (water) and 9.14 × 108 to 5.34 × 109 copies/g (sediment); the total absolute abundance of ARGs ranged from 4.70 × 106 to 5.16 × 108 copies/L (water) and 4.95 × 106 to 2.39 × 108 copies/g (sediment). The absolute abundance of the nine major types of ARGs are shown in Fig. 3, and the detailed data are given in Additional file 1: Table S5. It can be observed that the absolute abundances of ARGs and 16S rRNA in summer were significantly higher than that in winter (p < 0.01 for ARGs, p < 0.05 for 16S rRNA). The absolute abundance of 16S rRNA in this study was lower than those in the Qingcaosha Reservoir (Shanghai) (1.4 × 105–1.1 × 108 copies/mL for water, 1.8 × 108–1.8 × 1011 copies/g for sediment) and higher than that in drinking water source of Yuqiao Reservoir (Tianjin) (109 copies/L) [40, 41]. The absolute abundance of ARGs was also lower than that in the Qingcaosha Reservoir (3.7 × 103 to 1.4 × 106 copies/mL for water, and 2.1 × 107 to 4.4 × 109 copies/g for sediment) and comparable to the abundance of ARGs in drinking water sources in China in previous study (1.68 × 105 to 2.31 × 107 copies/100 mL) [40, 42]. Meanwhile, it was observed that the absolute abundance of ARGs in water samples were significantly higher than that in sediment samples (p < 0.05), but the absolute abundance of 16S rRNA in water and sediment samples was not significant difference (p > 0.05). The dominant ARGs in water and sediment samples were multidrug, aminoglycoside and tetracycline resistance genes, with total average absolute abundance of 1.03 × 109, 6.28 × 108 and 1.47 × 108 copies/L for water samples, followed by 2.76 × 108, 1.00 × 108 and 2.87 × 107 copies/g for sediment samples, respectively. The result was consistent with that of the Karst River [16]. Previous studies on ARG pollution in large Chinese lakes (such as Dongting, Taihu, Chaohu, Honghu and Bosten Lake) showed that the sulfonamide, tetracycline, multidrug and aminoglycoside resistance genes were the most commonly observed in the aquatic environment [28, 32, 43, 44]. The different types and abundance of detected ARGs in the aquatic environment worldwide may be depend on antibiotic usage patterns and bacterial species in different regions [44,45,46].
Characterization of MGEs
In the current study, a total of 51 MGEs were detected in the 55 target MGE subtypes (Additional file 1: Table S5). The total number of detected MGEs in the fw, fs, dw and ds samples were 50, 38, 39 and 34, respectively, and in water and sediment samples were 51 and 39, respectively. The number of MGEs was higher in summer than in winter, which was also consistent with the results of the ARGs (Fig. 2), and the corresponding absolute abundances of MGEs are shown in Fig. 3. The highest MGE abundance (total 2.39 × 108 copies/g) was detected in the fs6 sample location. The absolute abundance of MGEs was significantly higher in the flood season water samples than in the dry season water samples (p < 0.01), but the absolute abundance of MGEs had no significant difference between sediment and water samples (p > 0.05).
Specifically, the 51 MGE subtypes detected include integrase genes intI1 (class I integrase), intI2 (class II integrase) and intI3 (class III integrase), transposase genes (e.g., tnpA-7, tnpA-1, tnpA-4, tnpA-5, tnpA-6, tnpA-3, IS26, IS91, TP614, IS256, IS200-2, IS3, IS6100, IS613, and IS1247), plasmids (e.g., IncN_korA, IncP_oriT, IncN_oriT, IncN_rep, IncHI2-smr0018, IncI1_repI1, IncW_trwAB, lncF_FIC, IncQ_oriT).
Bacterial communities in the Douhe Reservoir samples
Diversity and richness of bacterial communities
Among all the samples collected from the Douhe Reservoir, the average Shannon index was 3.63 in the fw1–fw18 samples, 3.98 in the dw1–dw18 samples, 6.18 in the fs1–fs10 samples and 6.49 in the ds1–ds10 samples, respectively, see Additional file 1: Table S7. In addition, the richness and diversity of the bacterial communities in the sediment samples were significantly higher than those in the water samples of two seasons, which were indicated by the Chao1 (Welch t test, p < 0.01) and Shannon (Welch t test, p < 0.01) indices, respectively. Meanwhile, the richness and diversity had significant difference between the sediment samples of summer and winter, based on the Shannon and Chao1 indices (p < 0.01). However, there was no comparable significant difference in the water samples of the two seasons (p > 0.05).
Composition of bacterial communities in samples of the Douhe Reservoir
There were about 53 phyla, 163 classes, 402 orders, 640 families and 1,220 genera identified from the total of 36 water samples (including fw1–fw18 in summer and dw1–dw18 in winter), respectively, and there were about 64 phyla, 188 classes, 444 orders, 698 families and 1,229 genera identified from the total of 20 sediment samples (including fs1–fs10 in summer and ds1–ds10 in winter), respectively. The compositions of the bacterial communities of the water and sediment samples at the phylum and genus levels are shown in Additional file 1: Fig. S1 and Fig. 4, and the detailed data of percents of community abundance at the phylum and genus levels see Additional file 1: Table S8. At the phylum level of water samples (Additional file 1: Fig. S1), Firmicutes, Proteobacteria, Actinobacteriota, Bacteroidota, Cyanobacteria and Chloroflexiwere, the six major phyla, and the Firmicutes was overwhelmingly dominant for the largest contribution (with the average of 30.87%), followed by Proteobacteria (18.90%), Actinobacteriota (18.39%) and Bacteroidota (9.83%). Less abundant phyla were Cyanobacteria (9.61%) and Chloroflexi (4.17%). In the Karst River (Guizhou), Proteobacteria, Bacteroidetes and Cyanobacteria were the most abundant bacterial phyla [16]. Most studies have shown that the Firmicutes, Proteobacteria, Bacteroidota, and Actinobacteriota were the dominant composition of the bacterial communities at the phylum level of rivers, reservoirs and domestic waste water in many regions of the world [21, 28, 47,48,49]. At the genus level of the water samples of the Douhe Reservoir (Fig. 4), Exiguobacterium, Acinetobacter, CL500-29_marine_group, norank_f__Caldilineaceae, hgcI_clade, and Bacillus were the six dominant genera, and the Exiguobacterium was overwhelmingly dominant for the largest contribution (average of 24.14%), followed by Acinetobacter (6.46%), CL500-29_marine_group (5.02%), and norank_f__Caldilineaceae (3.64%). Less abundant genus was hgcI_clade (3.30%) and norank_f__norank_o__Chloroplast (3.10%). At the phylum level of sediment samples (Additional file 1: Fig. S1), Proteobacteria, Chloroflexi, Firmicutes, Acidobacteriota, Actinobacteriota and Bacteroidota were the six dominant phyla, and the Proteobacteria was the greatest contribution (average of 25.55%) to the composition of the bacterial community, followed by Chloroflexi (16.62%), Firmicutes (17.77%) and Acidobacteriota (9.51%). Less abundant phyla were Actinobacteriota (9.10%) and Bacteroidota (5.92%). At the genus level of sediment samples (Fig. 5), Thiobacillus, norank_f__Anaerolineaceae, norank_f__norank_o__norank_c__KD4-96, norank_f__Steroidobacteraceae, norank_f__norank_o__Vicinamibacterales and Clostridium_sensu_stricto_13 were the six major genera, and the Thiobacillus was the most abundant genus (average of 3.97%) of the bacterial community, followed by norank_f__Anaerolineaceae (3.79%), norank_o__norank_c__KD4-96 (3.77%) and norank_f__Steroidobacteraceae (3.52%). Less abundant phyla were norank_f__norank_o__Vicinamibacterales (2.85%) and Clostridium_sensu_stricto_13 (2.61%).
Differences in abundances of the top 15 on Phylum level in different samples. Note: a fw: water samples in summer, dw: water samples in winter, b fs: sediment samples in summer, ds: sediment samples in winter, c w: water samples, s: sediment samples, the significance of the difference in abundance in all different samples is marked by Student’s t test, * means p < 0.05, ** means p < 0.01, *** means p < 0.001. d difference of the composition of bacterial communities among all samples in summer and winter used the NMDS analysis
Difference of bacterial community of all the samples in the Douhe Reservoir
At the OTU level, the NMDS was performed to analyze the differences of the bacterial community distribution (Fig. 5d). It can be seen from Fig. 5d that the similarity of the bacterial communities composition of the fw1–fw18 and dw1–dw18. It also can be seen that the similarity of the bacterial communities composition of the fs1–fs10 and ds1–ds10, despite difference in their water period. By contrast, the Bray–Curtis distance of the water samples cluster was significantly different from that of the sediment samples cluster (Adonis, p = 0.001, Fig. 5d), which indicated that the clustering mode of the bacterial community was mainly affected by the sample type (water or sediment), little affected by the water period.
The differences between the composition of bacterial community on the phylum level identified in water and sediment samples in summer and winter were analyzed (Fig. 5). The proportions of Firmicutes, Planctomycetes, Deinococcus-Thermus, and Chlamydiae in the water samples of summer were significantly higher than those in winter samples, while the proportions of Actinobacteria and Bacteroidetes in water samples of winter were significantly higher than that in summer samples (p < 0.01, Fig. 5a). For the sediment samples, the proportions of Firmicutes and Actinobacteria in the summer samples were significantly higher than those in winter samples, while the proportions of Bacteroidetes, Patescibacteria, Gemmatimonadetes and Planctomycetes in the winter samples were significantly higher than those in summer samples (p < 0.01, Fig. 5b). It can be seen the differences between water and sediment samples from Fig. 5c that the proportions of Firmicutes, Actinobacteria, Cyanobacteria, Verrucomicrobia and Planctomycetes in water samples were significantly higher than those in sediment samples, in contrast, the proportions of Proteobacteria, Chloroflexi, Acidobacteria, Nitrospirae, Nitrospinae, Gemmatimonadetes and Latescibacteria in sediment samples were significantly higher than those in water samples (p < 0.01).
Factors that shape the distribution of ARGs and MGEs
Correlation between bacterial and ARGs vs MGEs
Most previous studies have indicated that the composition of bacterial community might influence the occurrence and abundance of ARGs and MGEs in aquatic and soil environments [50,51,52,53]. Due to the complexity of the correlation between bacterial community and ARGs in the Douhe Reservoir, the redundancy analysis (RDA) was used to reveal the correlation between bacterial community and ARGs (Fig. 6). Among the nine major bacterial genera in the Douhe Reservoir, norank_f__Caldilineaceae (Monte carlo permutation tests, p = 0.002, phylum of the Chloroflexi), Cylindrospermopsis_CRJ1 (p = 0.122, phylum of the Cyanobacteria), Bacillus (p = 0.284, phylum of the Firmicutes), hgcI_clade (p = 0.184, Actinobacteriota) and CL500-29_marine_group (p = 0.314, both the phylum of the Actinobacteriota) were the five significant microbial genera that affected the abundance of ARGs in the water of summer (flood period) (Fig. 6a). In water of winter (dry period), Flavobacterium (p = 0.161, phylum of the Bacteroidota), norank_f__Chloroplast (p = 0.159, phylum of the Cyanobacteria), norank_f__Rhizobiales_Incertae_Sedis (p = 0.158, phylum of the Proteobacteria) were the three significant bacteria at the genus level that impact the abundance of ARGs (Fig. 6b). In the sediment samples of summer and winter, norank_f__SC-I-84 (p = 0.012, phylum of the Proteobacteria), Thiobacillus (P = 0.058, phylum of the Proteobacteria) and Acinetobacter (p = 0.454, phylum of the Proteobacteria) and norank_f__SC-I-84 (p = 0.008, both the phylum of the Proteobacteria), norank_f__Vicinamibacterales (p = 0.096, phylum of the Acidobacteriota), fnorank_f__Thermodesulfovibrionia (p = 0.184, phylum of the Nitrospirota) were the three major bacteria at the genus level that influenced the abundance of ARGs in the sediment of flood and dry periods, respectively (Fig. 6c, d). These correlations between ARGs and bacterial communities at genus level indicated that genus was the major factor that influence the occurrence, distribution and abundance of ARGs.
There are 19 transposase genes (including tapA-7, tnpA-1, tapA-4, tapA-6, IS26, IS91, TP614, etc.) and 3 integrase genes (including intI1, intI2 and intI3) were detected among 51 MGEs, which indicated that the widespread of MGEs in antibiotic-polluted the Douhe Reservoir. Furthermore, the prevalence of tnpA gene could accelerate the abundance of ARGs through HGT. OLS was applied to reveal the correlation between ARGs and MGEs (Fig. 6e, f), it can be seen from Fig. 6e, f that there exist a positive liner correlation between ARGs and MGEs in the water (R = 0.462, p = 0.0045) and sediment samples (R = 0.957, p = 3.9 × 10–11). It indicates that the HGT as a main mechanism is likely to have occurred in the water and sediment for the widespread distribution of ARGs of the Douhe Reservoir and the MGEs may play a role in the persistence and proliferation of related resistance phenotypes [54, 55].
Network analysis among physicochemical factors, microbial communities, MGEs and ARGs
The co-occurrence network analysis was used to investigate the relationship between the major bacterial genera, environmental physicochemical factors, MGEs and major ARG subtypes based on the ARGs’ relative abundance (Fig. 7), and in each network analysis, the Spearman’s correlation coefficient was greater than 0.6 (p < 0.05). Furthermore, we hypothesized that if there was a significantly positive correlation among ARG subtypes and coexisting bacterial genera, MGEs and the environmental physicochemical factors of the samples, then the co-occurrence pattern between ARGs and different types of bacterial genera could be used to provide potential host information for ARGs, and obtain the main drivers of ARGs based on their correlation with bacterial genera and environmental factors.
Many studies have shown that the bacterial communities can significantly affect the distribution of ARGs and may act as hosts of ARGs, some bacteria can even synthesize antibiotics [56, 57]. Hence, the composition of the bacterial community was studied based on the heatmap of community and NMDS analysis (Figs. 4 and 5 ), and it can be concluded that the bacterial community has a significant difference in temporal (two seasons) and spatial (water and sediment) distribution. Especially, the opportunistic pathogens in tap water mostly come from drinking water sources and may pose risk to human health and even cause diseases. In this study, it can be seen from Fig. 7 that the bacteria (genus-level) not only have a significant correlation with ARGs subtypes in water (Fig. 7a) and sediment (Fig. 7b), but also have a significant correlation with physicochemical factors. However, there showed more significantly positive correlations between the bacteria genera and ARG subtypes in water samples than in sediment samples. For instance, in water samples (Fig. 7a), Acinetobacter (phylum of the Proteobacteria) was the possible host of some major ARGs such as multidrug-resistance genes (e.g., floR), tetracycline (tet(39)), Caldilineaceae (phylum of the Chloroflexi) was the possible host of Beta-lactam-resistance genes (bla-ACT), MLSB-resistance genes (lnu(F)) in the Douhe Reservoir. Likewise, the Bacillus (phylum of Firmicutes) may be the potential host of FCA (catQ), Beta-lactam (beta_ccra and blaOXY-1), MLSB-resistance genes (mefA) and multidrug-resistance (czcA). Furthermore, a total of 25, 21, 11, 7, and 5 genera affiliated with Proteobacteria, Firmicutes, Bacteroidota, Actinobacteriota, and Cyanobacteria, respectively, were positively correlated with some ARGs and MGEs. Han [58] have also demonstrated that Bacillus was significantly correlated with some ARGs, and Proteobacteria, Actinobacteria, Chloroflexi were significantly correlated with some ARGs and MGEs in many drinking water sources of cities of China. Furthermore, Acinetobacter and Bacillus belong to opportunistic pathogens which have been observed in many drinking water sources. The results indicating that opportunistic pathogens might play role of host of ARGs. It is worth noting that Acinetobacter can cause pneumonia and has potential to antimicrobial resistance [15]. Hence, opportunistic pathogens carry ARGs may pose risks to human health.
In sediment samples (Fig. 7b), both Thermodesulfovibrionia (phylum of Nitrospirota) and MB-A2-108 (phylum of Actinobacteriota) were the possible hosts of MLSB resistance genes (mdtA), Rhodobacteraceae (phylum of Proteobacteria) was also found to be the potential host of tetracycline resistance genes (tetB(P)). Acinetobacter and Rhizobiales_Incertae_Sedis (both phylum of Proteobacteria) were the potential host of MGE (traN). Especially, the Nitrospirota is widely present in polluted aquatic environment, as a kind of nitrite oxidizing bacteria, it plays an essential role in the nitrogen cycle of the microbial communities [59]. These correlations between bacterial genera and ARG types indicated a result that bacterial genera were the main driver of ARG distribution pattern in water and sediment of reservoirs, which has been demonstrated in previous studies of water of the Karst River and Poyang Lake, and sediment of the Ebinur Lake [16, 38, 60]. These results also indicate that the host bacterial communities of ARGs are different in the environmental gradients. In addition, it should be noted that network co-occurrence is only a mathematical statistical analysis based on correlation testing, and its reliability in predicting potential host bacteria for ARGs will be affected. To ensure the safety of drinking water sources, there is an urgent need for more substantial investigations on opportunistic pathogen of bacteria that may carry ARGs, such as molecular detection of ARG-host.
Many studies have demonstrated that the HGT between bacterial communities mediated by MGEs can accelerate the spread and increase the abundance of ARGs in the environment [61,62,63]. Hence, MGEs were strongly correlated with ARGs and bacterial communities because of their significant effect on driving ARGs among various microorganisms in environment [64]. For instance, integrase intI1 as a good represent for the anthropogenic pollution has been demonstrated that have key influence in the occurrence of ARGs, and is widely used in environmental investigation [15, 65]. In this study, it can be seen from Fig. 7 that some relative abundance of MGEs (IS26, ISEcp1 and mobA) and MGEs (IS1247, intI1 and ISEcp1) showed the strong correlations (Spearman's r = 0.65–0.96 and 0.61–0.92, p < 0.05) with ARGs in the water and sediment samples, respectively, which indicate that the occurrence and prevalence of MGEs play an important role in the abundance of ARGs in the water and sediment environment. Specifically, 22 detected ARG subtypes belong to almost all types of ARGs (e.g., aminoglycoside, MLSB, FCA, multidrug, and tetracycline resistance genes) were significantly related to intI1 (p < 0.05), which indicates that intI1 played a key role in the proliferation and dissemination of many types of ARGs. Wang et al. [38] also found a similar phenomenon that sul1 and sul2 were significantly positively correlated with intI1 (p < 0.01) in samples. Nevertheless, we also found that the co-occurrence patterns between ARGs and MGEs were obviously different between water and sediment samples (Fig. 7a, b), this indicated that the proliferation and spread of ARGs by the MGEs may be influenced by the different environment medium. In particular, the influence on ARGs by MGEs in the water was much stronger than that of in sediment, resulting the same spatial distribution characteristic of ARGs and MGEs (details described in Figs. 2 and 3 ).
In addition to bacterial communities, environmental factors may also influence the occurrence and fate of ARGs, studies have demonstrated the environmental factors, such as T, pH, EC, NH + 4–N and NO- 3–N present positive correlation with ARGs [15, 16]. In current study, the environmental physicochemical factors (including T, NO–3–N, TDN and TP) mostly present positive correlation with ARGs and bacterial genera in water samples, and merely a few physicochemical factors had correlations with the ARGs and bacterial genera in sediment samples, which means that the physicochemical factors had a certain impact on the composition of bacterial communities and the occurrence of ARGs in the Douhe Reservoir. Besides, it also can be seen from Fig. 7 that some environmental factors, such as TDN, NO- 3–N, T and pH were positively correlated with some bacterial genera (Bacillus, Cylindrospermopsis_CRJ1, Saprospiraceae, Nodosilinea_PCC-7104), indicating that these bacteria may use TDN and NO- 3–N as the major nutrients and energy for their own growth and metabolism or may be affected by temperature and pH. Xiao [66] confirmed that Bacillus is resistant to heavy metals and participates in nutrient cycling. This indicates that environmental factors may affect the occurrence of ARGs by affecting bacterial community. In addition, studies also have shown that there is a significant correlation between the content of heavy metals in sediments and the abundance of ARGs, indicating that heavy metal pollution may be a major factor affecting the abundance of ARGs. Zhao [67] have confirmed that the collaborative screening of nutrients and heavy metals played an important role in the dynamic changes of ARGs in sediments by analyzing the relationship between ARGs and heavy metals and nutrients in the Yellow River Delta sediments. Overall, the network analysis results about the correlations among physicochemical factors, microbial communities, MGEs and ARGs further confirmed that the fate of ARGs is closely related to their potential host bacteria [68], and the physicochemical factors may impact the composition of bacterial communities.
Conclusions
This study revealed the characteristics of ARGs, MGEs, and bacterial communities of water and sediment in the drinking water sources of the Douhe Reservoir in summer and winter. There were significant differences in the structure of bacterial communities and profiles of ARGs and MGEs between the water and sediment samples of the two seasons, but there were no differences of the major types of ARGs between the water and sediment samples in winter and summer. Six classes of MGE subtypes (IS26, ISEcp1 and mobA for water, IS1247, intI1 and ISEcp1 for sediment) played an important role in the spread of ARGs. The evolution of bacterial communities has the most direct contribution to changes in ARGs. Furthermore, the variation of bacterial communities, ARGs and MGEs in drinking water sources of the Douhe Reservoir appeared to be related to the environmental factors, particularly of the values of T, NO– 3–N, TDN and TP (p < 0.05), and the further research is necessary. Overall, the results of this research will help us understand the ARGs in the drinking water sources and provide us essential information to improve the management strategies for reservoir.
Availability of data and materials
The complete data set of this study is included within the article and the Additional file.
Abbreviations
- ARGs:
-
Antibiotic resistance genes
- MGEs:
-
Mobile genetic elements
- NO– 3–N:
-
Nitrate–nitrogen
- STPs:
-
Sewage treatment plants
- OTUs:
-
Operational taxonomic units
- TDN:
-
Total dissolved nitrogen
- MLSB:
-
Macrolides–lincosamides–streptomycin B
- TP:
-
Total phosphorus
- NH + 4–N:
-
Ammonia nitrogen
- T:
-
Temperature
- HT-qPCR:
-
High-throughput quantitative PCR
- EC:
-
Electrical conductivity
- HGT:
-
Horizontal gene transfer
- DO:
-
Dissolved oxygen
- FCA:
-
Fluoroquinolone, quinolone, florfenicol, chloramphenicol, and amphenicol
- NMDS:
-
Nonmetric multidimensional scaling
- OLS:
-
Ordinary least squares
- RDA:
-
Redundancy analysis
- HCO- 3:
-
Bicarbonate ions
- qPCR:
-
Quantitative polymerase chain reaction
References
Wang GG, Zhou SH, Han XK, Zhang LL, Ding SY, Li Y, Zhang DJ, Zarin K (2020) Occurrence, distribution, and source track of antibiotics and antibiotic resistance genes in the main rivers of Chongqing city, southwest China. J Hazard Mater 389:122110. https://doi.org/10.1016/j.jhazmat.2020.122110
Aminov RI (2009) The role of antibiotics and antibiotic resistance in nature. Environ Microbiol 11:2970–2988. https://doi.org/10.1111/j.1462-2920.2009.01972.x
An LX, Su JQ, Li B, Ouyang WY, ZhaoY CLQ, Cui L, Chen H, Gillings MR, Zhang T, Zhu YG (2018) Tracking antibiotic resistome during wastewater treatment using high throughput quantitative PCR. Environ Int 117:146–153. https://doi.org/10.1016/j.envint.2018.05.011
Martínez JL (2008) Antibiotics and antibiotic resistance genes in natural environments. Science 321:365–367. https://doi.org/10.1126/science.1159483
Hyejun J, Shahbaz R, Adeel F, Jungman K, Tatsuya U (2021) Fish farm effluents as a source of antibiotic resistance gene dissemination on Jeju Island, South Korea. Environ Pollut 276:116764. https://doi.org/10.1016/j.envpol.2021.116764
Ye LT, Liu GF, Yao T, Lu J (2021) Monitoring of antimicrobial resistance genes in the spotted sea bass (Lateolabrax maculatus): association with the microbiome and its environment in aquaculture ponds. Environ Pollut 276:116714. https://doi.org/10.1016/j.envpol.2021.116714
Su HC, Liu S, Hu XJ, Xu XR, Xu WJ, Xu Y, Li ZJ, Wen GL, Liu YS, Cao YC (2017) Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci Total Environ 607:357–366. https://doi.org/10.1016/j.scitotenv.2017.07.040
Zhou RJ, Zeng SZ, Hou DW, Liu J, Weng SP, He JG, Huang ZJ (2020) Temporal variation of antibiotic resistance genes carried by culturable bacteria in the shrimp hepatopancreas and shrimp culture pond water. Ecotoxicol Environ Saf 199:110738. https://doi.org/10.1016/j.ecoenv.2020.110738
Cao RK, Wang J, Ben WW, Qiang ZM (2020) The profile of antibiotic resistance genes in pig manure composting shaped by composting stage: mesophilic-thermophilic and cooling-maturation stages. Chemosphere 250:126181. https://doi.org/10.1016/j.chemosphere.2020.126181
Zhang YJ, Hu HW, Gou M, Wang JT, Chen DL, He JZ (2017) Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ Pollut 231:1621–1632. https://doi.org/10.1016/j.envpol.2017.09.074
Sardar MF, Zhu CX, Geng B, Ahmad HR, Song TT, Li HN (2021) The fate of antibiotic resistance genes in cow manure composting: shaped by temperature-controlled composting stages. Bioresour Technol 320:124403. https://doi.org/10.1016/j.biortech.2020.124403
Zhang Y, Zheng YH, Zhu ZP, Chen YX, Dong HM (2021) Dispersion of Antibiotic Resistance Genes (ARGs) from stored swine manure biogas digestate to the atmosphere. Sci Total Environ 761:144108. https://doi.org/10.1016/j.scitotenv.2020.144108
Zainab MS, Junaid M, Xu N, Malik RN (2020) Antibiotics and antibiotic resistant genes (ARGs) in groundwater: a global review on dissemination, sources, interactions, environmental and human health risks. Water Res 187:116455. https://doi.org/10.1016/j.watres.2020.116455
Cheng WX, Li JN, Wu Y, Xu LK, Su C, Qian YY, Zhu YG, Chen H (2016) Behavior of antibiotics and antibiotic resistance genes in eco-agricultural system: a case study. J Hazard Mater 304:18–25. https://doi.org/10.1016/j.jhazmat.2015.10.037
Feng TS, Su WH, Zhu JX, Yang JW, Wang YJ, Zhou R, Yu QL, Li H (2021) Corpse decomposition increases the diversity and abundance of antibiotic resistance genes in different soil types in a fish model. Environ Pollut 286:117560. https://doi.org/10.1016/j.envpol.2021.117560
Zhang B, Qin S, Guan XY, Jiang KD, Jiang MH, Liu F (2021) Distribution of antibiotic resistance genes in Karst River and its ecological risk. Water Res 203:117507. https://doi.org/10.1016/j.watres.2021.117507
Corcoll N, Acuña V, Barceló D, Casellas M, Guasch H, Huerta B, Petrovic M, Ponsatí L, Rodríguez-Mozaz S, Sabater S (2014) Pollution-induced community tolerance to non-steroidal anti-inflammatory drugs (NSAIDs) in fluvial biofilm communities affected by WWTP effluents. Chemosphere 112:185–193. https://doi.org/10.1016/j.chemosphere.2014.03.128
Wu D, Huang ZT, Yang K, Graham D, Xie B (2015) Relationships between antibiotics and antibiotic resistance gene levels in municipal solid waste leachates in Shanghai, China. Environ Sci Technol 49:4122–4128. https://doi.org/10.1021/es506081z
Namrata R, Sruthi AA, Chandrasekaran N, Mukherjee A, Kannabiran K (2021) A comprehensive update on antibiotics as an emerging water pollutant and their removal using nano-structured photocatalysts. J Environ Chem Eng 9:104796. https://doi.org/10.1016/j.jece.2020.104796
Yang Y, Li B, Zou SC, Fang-Herbert HP, Zhang T (2014) Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Res 62:97–106. https://doi.org/10.1016/j.watres.2014.05.019
Adhikari NP, Liu YQ, Liu KS, Zhang F, Adhikari S, Chen YY, Liu XB (2019) Bacterial community composition and diversity in Koshi River, the largest river of Nepal. Ecol Indic 104:501–511. https://doi.org/10.1016/j.ecolind.2019.05.009
Li LX, Guo CS, Fan SS, Lv JP, Zhang Y, Xu Y, Xu J (2018) Dynamic transport of antibiotics, antibiotic resistance genes under different treatment processes in a typical pharmaceutical wastewater treatment plant. Environ Sci Pollut Res 25:30191–30198. https://doi.org/10.1007/s11356-018-2913-2
Cho S, Jackson CR, Frye JG (2020) The prevalence and antimicrobial resistance phenotypes of Salmonella, Escherichia coli and Enterococcus sp. in surface water. Lett Appl Microbiol 71:3–25. https://doi.org/10.1111/lam.13301
Gao PP, Mao DQ, Luo Y, Wang LM, Xu BJ, Xu L (2012) Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res 46:2355–2364. https://doi.org/10.1016/j.watres.2012.02.004
Liu XH, Zhang GD, Liu Y, Lu SY, Qin P, Guo XC, Bi B, Wang L, Xi BD, Wu FC, Wang WL, Zhang TT (2019) Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ Pollut 246:163–173. https://doi.org/10.1016/j.envpol.2018.12.005
Su HC, Liu YS, Pan CG, Chen J, He LY, Ying GG (2018) Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment system: from drinking water source to tap water. Sci Total Environ 616:453–461. https://doi.org/10.1016/j.scitotenv.2017.10.318
Li P, Wu YF, He YL, Zhang B, Huang YS, Yuan QY, Chen YH (2020) Occurrence and fate of antibiotic residues and antibiotic resistance genes in a reservoir with ecological purification facilities for drinking water sources. Sci Total Environ 707:135276. https://doi.org/10.1016/j.scitotenv.2019.135276
Ding HJ, Qiao M, Zhong JY, Zhu YG, Guo CJ, Zhang QQ, Ynag P, Han L, Zhang WH, Wu YX, Liu JT, Zhang LT, Sun JH (2020) Characterization of antibiotic resistance genes and bacterial community in selected municipal and industrial sewage treatment plants beside Poyang Lake. Water Res 174:115603. https://doi.org/10.1016/j.watres.2020.115603
Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Natl Acad Sci USA 110:3435–3440. https://doi.org/10.1073/pnas.1222743110
Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, Sul WJ, Stedtfeld TM, Chai BL, Cole JR, Hashsham SA, Tiedje JM, Stanton TB (2012) In-feed antibiotic effects on the swine intestinal microbiome. Natl Acad Sci USA 109:1691–1696. https://doi.org/10.1073/pnas.1120238109
Ma Y, Zhao HZ, Shan QJ, Xu YQ, Yu MD, Cui J, Liu T, Qiao LK, He XS (2021) K-strategy species plays a pivotal role in the natural attenuation of petroleum hydrocarbon pollution in aquifers. J Hazard Mater 420:126559. https://doi.org/10.1016/j.jhazmat.2021.126559
Chen YH, Su JQ, Zhang JY, Li P, Chen HJ, Zhang B, Gin K-H, He YL (2019) High-throughput profiling of antibiotic resistance gene dynamic in a drinking water river-reservoir system. Water Res 149:179–189. https://doi.org/10.1016/j.watres.2018.11.007
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Hu HW, Wang JT, Singh B, Liu YR, Chen YL, Zhang YJ, He JZ (2017) Diversity of herbaceous plants and bacterial communities regulates soil resistome across forest biomes. Environ Microbiol 20:3186–3200. https://doi.org/10.1111/1462-2920.14248
D’Costa VM, King CE, Kalan L, Mariya M, Sung Wilson WL, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding GB, Poinar HN, Wright GD (2011) Antibiotic resistance is ancient. Nature 477:457–461. https://doi.org/10.1038/nature10388
Zhao RX, Feng J, Liu J, Fu WJ, Li XY, Li B (2019) Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res 151:388–402. https://doi.org/10.1016/j.watres.2018.12.034
Zheng J, Gao RX, Wei YY, Chen T, Fan JQ, Zhou ZC, Makimilua TB, Jiao YN, Chen H (2017) High-throughput profiling and analysis of antibiotic resistance genes in East Tiaoxi River, China. Environ Pollut 230:648–654. https://doi.org/10.1016/j.envpol.2017.07.025
Wang YQ, Lu SY, Liu XH, Chen J, Han MZ, Wang Z, Guo W (2021) Profiles of antibiotic resistance genes in an inland salt-lake Ebinur Lake, Xinjiang, China: the relationship with antibiotics, environmental factors, and microbial communities. Ecotoxicol Environ Saf 221:112427. https://doi.org/10.1016/j.ecoenv.2021.112427
Zhu YG, Gillings M, Simonet P, Stekel D, Banwart S, Penuelas J (2017) Microbial mass movements. Science 357:1099–1100. https://doi.org/10.1126/science.aao3007
Huang ZF, Zhao WT, Xu T, Zheng BH, Yin DQ (2019) Occurrence and distribution of antibiotic resistance genes in the water and sediments of Qingcaosha Reservoir, Shanghai China. Environ Sci Eur 31:81. https://doi.org/10.1186/s12302-019-0265-2
Zhang K, Niu ZG, Lv ZW, Zhang Y (2017) Occurrence and distribution of antibiotic resistance genes in water supply reservoirs in Jingjinji area, China. Ecotoxicol 26:1284–1292. https://doi.org/10.1007/s10646-017-1853-9
Zhang K, Xin R, Zhao Z, Ma YZ, Zhang Y, Niu ZG (2020) Antibiotic Resistance Genes in drinking water of China: occurrence, distribution and influencing factors. Ecotoxicol Environ Saf 188:109837. https://doi.org/10.1016/j.ecoenv.2019.109837
Storteboom H, Arabi M, Davis JG, Crimi B, Pruden A (2010) Identification of antibiotic resistance gene molecular signatures suitable as tracers of pristine river, urban, and agricultural sources. Environ Sci Technol 44:1947–1953. https://doi.org/10.1021/es902893f
Jiang L, Hu XL, Xu T, Zhang HC, Sheng D, Yin DQ (2013) Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu River and the drinking water sources, Shanghai, China. Sci Total Environ 458:267–272. https://doi.org/10.1016/j.scitotenv.2013.04.038
Xu S, Qasim MZ, Zhang T, Wang RY, Li C, Ge SJ (2021) Diversity, abundance and expression of the antibiotic resistance genes in a Chinese landfill: effect of deposit age. J Hazard Mater 417:126027. https://doi.org/10.1016/j.jhazmat.2021.126027
Chen BW, Liang XM, Huang XP, Zhang T, Li XD (2013) Differentiating anthropogenic impacts on ARGs in the Pearl River Estuary by using suitable gene indicators. Water Res 47:2811–2820. https://doi.org/10.1016/j.watres.2013.02.042
Campos M, Rilling JI, Acuña JJ, Valenzuela T, Larama G, Peña-Cortés F, Ogram A, Jaisi DP, Jorquera MA (2021) Spatiotemporal variations and relationships of phosphorus, phosphomonoesterases, and bacterial communities in sediments from two Chilean rivers. Sci Total Environ 776:145782. https://doi.org/10.1016/j.scitotenv.2021.145782
Zhang H, He HY, Chen SG, Huang TL, Lu KY, Zhang ZH, Wang R, Zhang XY, Li HL (2019) Abundance of antibiotic resistance genes and their association with bacterial communities in activated sludge of wastewater treatment plants: geographical distribution and network analysis. J Environ Sci 82:24–38. https://doi.org/10.1016/j.jes.2019.02.023
Carles L, Artigas J (2020) Interaction between glyphosate and dissolved phosphorus on bacterial and eukaryotic communities from river biofilms. Sci Total Environ 719:137463. https://doi.org/10.1016/j.scitotenv.2020.137463
Yang YY, Liu GH, Chen Y, Liu WZ (2019) Bacterial community and climate change implication affected the diversity and abundance of antibiotic resistance genes in wetlands on the Qinghai-Tibetan Plateau. J Hazard Mater 361:283–293. https://doi.org/10.1016/j.jhazmat.2018.09.002
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Huerta-Cepas J (2018) Structure and function of the global topsoil microbiome. Nature 560:233–237. https://doi.org/10.1038/s41586-018-0386-6
Andrea DC, Ester ME, Michela R, Gianluca C (2017) Rainfall increases the abundance of antibiotic resistance genes within a riverine microbial community. Environ Pollut 226:473–478. https://doi.org/10.1016/j.envpol.2017.04.036
Su YL, Wang JX, Huang ZT, Xie B (2017) On-site removal of antibiotics and antibiotic resistance genes from leachate by aged refuse bioreactor: effects of microbial community and operational parameters. Chemosphere 178:486–495. https://doi.org/10.1016/j.chemosphere.2017.03.063
Dobrovol’skaya TG, Khusnetdinova KA, Manucharova NA, Golovchenko AV (2017) Structure of epiphytic bacterial communities of weeds. Microbiology 86:247–254. https://doi.org/10.1134/S0026261717020072
Szekeres E, Chiriac CM, Baricz A, Szőke-Nagy T, Lung I, Soran ML, Rudi K, Dragos N, Coman C (2018) Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas. Environ Pollut 236:734–744. https://doi.org/10.1016/j.envpol.2018.01.107
Jang JY, Kim M, Baek S, Shin J, Shin J, Shin SG, Kim YM, Cho KH (2021) Hydrometeorological Influence on Antibiotic-Resistance Genes (ARGs) and bacterial community at a recreational Beach in Korea. J Hazard Mater 403:123599. https://doi.org/10.1016/j.jhazmat.2020.123599
Colomer-Lluch M, Calero-Cáceres W, Jebri M, Hmaied F, Muniesa M, Jofre J (2014) Antibiotic resistance genes in bacterial and bacteriophage fractions of Tunisian and Spanish wastewaters as markers to compare the antibiotic resistance patterns in each population. Environ Int 73:167–175. https://doi.org/10.1016/j.envint.2014.07.003
Han ZM, Zhang Y, An W, Lu JY, Hu J, Yang M (2020) Antibiotic resistomes in drinking water sources across a large geographical scale: multiple drivers and co-occurrence with opportunistic bacterial pathogens. Water Res 183:116088. https://doi.org/10.1016/j.watres.2020.116088
Nielsen PH, Mielczarek AT, Kragelund C, Nielsen JL, Saunders AM, Kong YH, Hansen AA, Vollertsen J (2010) A conceptual ecosystem model of microbial communities in enhanced biological phosphorus removal plants. Water Res 44:5070–5088. https://doi.org/10.1016/j.watres.2010.07.036
Liang XM, Guan FL, Chen BW, Luo PY, Guo CF, Wu GQ, Ye Y, Zhou QB, Fang HS (2020) Spatial and seasonal variations of antibiotic resistance genes and antibiotics in the surface waters of Poyang Lake in China. Ecotoxicol Environ Saf 196:110543. https://doi.org/10.1016/j.ecoenv.2020.110543
Zhang SH, Yang GL, Hou SG, Zhang TJ, Li ZG, Liang F (2018) Distribution of ARGs and MGEs among glacial soil, permafrost, and sediment using metagenomic analysis. Environ Pollut 234:339–346. https://doi.org/10.1186/s13100-017-0102-3
Sachin KG, Hanseob S, Dukki H, Hor-Gil H, Tatsuya U (2018) Metagenomic analysis reveals the prevalence and persistence of antibiotic-and heavy metal-resistance genes in wastewater treatment plant. J Microbiol 56:408–415. https://doi.org/10.1007/s12275-018-8195-z
Zhu LJ, Zhao Y, Yang KJ, Chen J, Zhou HX, Chen XM, Liu Q, Wei ZM (2019) Host bacterial community of MGEs determines the risk of horizontal gene transfer during composting of different animal manures. Environ Pollut 250:166–174. https://doi.org/10.1016/j.envpol.2019.04.037
Ma LP, Li AD, Yin XL, Zhang T (2017) The prevalence of integrons as the carrier of antibiotic resistance genes in natural and man-made environments. Environ Sci Technol 51:5721–5728. https://doi.org/10.1021/acs.est.6b05887
Hu HW, Wang JT, Singh BK, Liu YR, Chen YL, Zhang YJ, He JZ (2018) Diversity of herbaceous plants and bacterial communities regulates soil resistome across forest biomes. Environ Microbiol 20:3186–3200. https://doi.org/10.1111/1462-2920.14248
Xiao EZ, Ning ZP, Xiao TF, Sun WM, Qiu YQ, Zhang Y, Chen JY, Gou ZL, Chen YX (2019) Variation in rhizosphere microbiota correlates with edaphic factor in an abandoned antimony tailing dump. Environ Pollut 253:141–151. https://doi.org/10.1016/j.envpol.2019.06.097
Zhao ZL, Wang J, Han Y, Chen JW, Liu GF, Lu H, Yan B, Chen S (2017) Nutrients, heavy metals and microbial communities co-driven distribution of antibiotic resistance genes in adjacent environment of mariculture. Environ Pollut 220:909–918. https://doi.org/10.1016/j.envpol.2016.10.075
Chen J, Wang TT, Zhang K, Luo HB, Chen W, Mo Y, Wei ZL (2021) The fate of antibiotic resistance genes (ARGs) and mobile genetic elements (MGEs) from livestock wastewater (dominated by quinolone antibiotics) treated by microbial fuel cell (MFC). Ecotoxicol Environ Saf 218:112267. https://doi.org/10.1016/j.ecoenv.2021.112267
Acknowledgements
Not applicable.
Funding
The research was supported by the Basic Research Project of the Chinese Research Academy of Environmental Sciences (No. 2020YSKY-009), Drinking Water Environmental Supervision Project of Ministry of Ecology and Environment of China, and the National Natural Science Youth Foundation of China (No. 51508539).
Author information
Authors and Affiliations
Contributions
KZ: writing—original draft; YF: supervision, methodology; SC: resources, funding acquisition; QF: visualization, data curation; QZ: project administration; GY: software, data curation; XS: reviewed drafts of the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
GPS location of sampling locations. Table S2. Water quality of the selected samples. Table S3. Sediment quality of the selected samples. Table S4. Primer sets used in this study. MLSB (Macrolide–Lincosamide–Streptogramin B), MGEs (mobile genetic elements) and FCA (fluoroquinolone, quinolone, florfenicol, chloramphenicol, and amphenicol). Table S5. Relative mean abundance of each ARG and MGE subtypes in sample (copies/16S rRNA gene). Table S6. Average number of detected ARGs and MGEs of the each sample. Table S7. Diversity indices of bacterial communities in each sample. Table S8. Bacterial phyla percent in each water samples. Bacterial genus percent in each water samples. Bacterial phyla percent in each sediment samples. Bacterial genus percent in each sediment samples. Figure S1. Relative abundance of different bacterial phyla in water and sediment samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, K., Fan, Y., Chang, S. et al. Characterization of antibiotic resistance genes in drinking water sources of the Douhe Reservoir, Tangshan, northern China: the correlation with bacterial communities and environmental factors. Environ Sci Eur 34, 56 (2022). https://doi.org/10.1186/s12302-022-00635-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-022-00635-x