Abstract
Background
Bladder cancer is characterized by high recurrence and progressivity. E-cadherin serves as one of the most important molecules involved in the epithelial cells’ cell-to-cell adherence, suggested to inhibit tumor cell progression. This study aims to investigate the association between the E-cadherin expressions with bladder cancer progressiveness in 3 years.
Methods
This study was a retrospective cohort study involving bladder cancer patients in Cipto Mangunkusumo Hospital, Jakarta. Diagnosis of bladder cancers was confirmed by histopathological and immunohistochemistry examination between 2011 and 2018, with both grading and staging determined by uropathologists and uro-oncologists. E-cadherin was examined through immunohistochemistry examination at the time of diagnosis. Data on demography, muscle invasion, clinical staging, grade, metastasis, multifocality, and recurrence were obtained from medical records and pathology reports. The association of E-cadherin expression to muscle invasion and non-muscle invasion bladder cancer was evaluated and statistically analyzed. Patients’ survival data were followed up by phone.
Results
Forty bladder cancer patients with a mean age of 60.05 ± 10.3 years were included. Most subjects had high E-cadherin expression (85%), muscle invasion (65%), high grade (65%), no metastasis (87.5%), multifocality (65%), and no recurrence (62.5%). Lower expression of E-cadherin was associated with the higher clinical stage (p < 0.02) and metastasis (p < 0.001). Patients with low E-cadherin expression showed worse cumulative survival than the high one (mean 32 months vs. 25 months, p = 0.13).
Conclusions
Low level of E-cadherin was associated with the higher risk of muscle invasion, clinical staging, histological grade, and risk of metastasis. Meanwhile, patients with the high level of E-cadherin showed a better three-year survival rate.
Similar content being viewed by others
1 Background
Bladder cancer ranks as the 10th most commonly occurring cancer and 1.9% of overall cancer mortality are caused by this cancer [1]. Bladder cancer is classified into muscle-invasive bladder cancer (MIBC) and non-muscle invasive bladder cancer (NMIBC). Upon the first diagnosis, usually, about 75–80% of patients were diagnosed as NMIBC (stage Ta/T1/Tis). At this stage, the overall prognosis is good although the recurrence rate is high, showcasing 65–85% of patients achieving five-year survival. About 17% of the patients’ progress into MIBC (stage T2–T4). In this condition, the five-year survival rate drops to 25–50% [2, 3]. Meanwhile, based on its molecular subtypes (Cancer Genome Atlas [TCGA]), bladder cancers are divided into luminal and basal tumors. E-cadherin, a protein, which can be found in luminal tumors, is one of the most important molecules in the process of epithelial cell-to-cell adherence in human tissues [4]. The reduction of E-cadherin expressions causes the barrier made by the cell-to-cell adherence to be disrupted thus increasing the susceptibility of invasion and metastasis due to the uncontrolled proliferation of the tumor cells [2, 3].
The understanding regarding the pathogenesis of bladder cancer invasion should be taken into consideration as the progression of Ta/T1 cases to more advanced cases. To avoid that, many prognostic tools have been developed since 1973, for instances, the world health organization (WHO) grading system. WHO grading system was used to determine bladder cancer prediction of recurrence and progression for NMIBC. The 2004/2016 version outperformed the late grading [5]. Another prognostic scoring system for predicting NMIBC progression is the risk tables published by the European Platform of Cancer Research (EORTC) Genito-Urinary Cancer Group [6]. The first cystoscopy after transurethral resection of bladder tumor (TURBT) has also been used to examine the recurrence and progression in Ta/T1/Tis tumors [7, 8]. Prognostic role of molecular markers has been also investigated, but until now there are insufficient data. E-cadherin is one of the potential molecular markers to determine progressivity since its ability to maintain intercellular connection by adhesion [9]. Ziaran et al. showed E-cadherin was associated with cancer-specific survival and a lower level of E-cadherin gave significantly worse progression-free survival (PFS) in NMIBC [10]. We aim to investigate the association between E-cadherin level expression and to the risk of bladder cancer progressivity, both in NMIBC and MIBC patients.
2 Methods
2.1 Study design
The design of this study was a retrospective cohort, with a total sampling of all patients with bladder cancer that had treatment in Cipto Mangunkusumo National Central Referral Hospital, Jakarta, Indonesia (a tertiary referral hospital). The subjects included in this study were treated bladder cancer patients who had undergone histopathological and immunohistochemistry evaluation in Cipto Mangunkusumo National Referral Hospital between January 2011 and December 2018. Staging of bladder cancers was evaluated by experienced uropathologists and uro-oncologists accounting signs, symptoms, and results from supportive modalities using the standard staging system by American Joint Committee for Cancer (AJCC). Demographics and cancer characteristics (muscle invasion, clinical staging, grade, metastasis, multifocality, and recurrence) data were obtained through medical records and pathology reports. All patients who underwent both histopathological and E-cadherin immunohistochemistry examinations, with complete medical record data, were included in the study. All subjects were followed up by phone 36 months after treatment to evaluate all-cause mortality. All data were recorded prior to this study. This study has been approved by the Ethics Committee of Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital (Protocol number: 18-03-0213). All methods were performed in accordance with the relevant guidelines and regulations. Requirement of informed consent was waived by Ethics Committee of Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital.
2.2 Immunohistochemistry
Immunohistochemistry examination was done from bladder cancer formalin-fixed paraffin-embedded (FFPE) specimens, which were cut into 4 µm tissues. Antigen retrieval was done through heat-induced epitope retrieval (HIER) in pH6 using a pressure boiler 125 °C and cooled to 90 °C. Endogenous peroxidase enzymes were blocked using H2O2 0.3% and ethanol 95%. E-cadherin antibody used was produced by Sigma Aldrich (St Louis, Missouri). Incubation was done in labelled polymer HRP followed by chromogenization in DAB (substrate DAB:chromogen DAB was 1:20) using DAKO stainer KIT. The specimens were then counterstained using hematoxylin. The examination was performed visually by two examiners and then confirmed by an experienced pathologist.
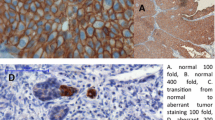
The expression of the protein was considered positive if cells were positively stained with brownish color on the cell membrane of the epithelial cells (Fig. 1.). A 3+ mark was given to cells with full-thickness circumferential expression of the E-cadherin, a 2+ mark was given to cells with full-thickness non-circumferential expression of the E-cadherin, a 1+ mark was given to cells with a faint expression of the E-cadherin, and a 0 mark was given to cells without any visible expression of E-cadherin. Each cell assigned to different mark groups was counted and processed into a specific formula: [1 \(\times\) (%cells + 1) + 2 \(\times\) (%cells + 2) + 3 \(\times\) (%cells + 3)]. The H-score ranged from 0 to 300, depending on the expression of E-cadherin. Further grouping for E-cadherin levels was done by defining low E-cadherin levels as a score below 100, as published by Horne et al., Liu et al., and Rakha et al. [11,12,13].
Immunohistochemistry of antibody-stained FFPE bladder cancer preparation for E-cadherin was done per specimen counting minimum of 1000 cells using Aperio Imagescope (Leica Biosystems, Buffalo Grove, IL, USA); a. field dominant with 0 expression mark (× 400 magnification); b. field dominant with + 1 expression mark (× 400); c. field dominant with + 2 expression mark (× 400); d. field dominant with + 3 expression mark (× 400)
2.3 Statistical analysis
All collected data were analyzed using IBM SPSS version 25 (IBM statistics, New York). For 2 \(\times\) 2 bivariate analysis, Fisher’s exact test was done with a 95% confidence interval (p-value < 0.05). For more than 2 \(\times\) 2 table, Kruskal–Wallis test was done. Survival analysis based on E-cadherin category was done by Kaplan–Meier test with log-rank.
3 Results
After evaluating the eligibility of the patients, 40 subjects were included in the study. The mean age of subjects was 10.3 ± 60.05 years and 31 of the subjects were male. Mean H-score of bladder cancers was 177.15 (95% CI 157.88–196.42). After 36 months, 17 subjects were lost to follow-up, so the data of all-cause mortality were incomplete.
Data of bivariate statistical analysis of E-cadherin expression and each outcome were shown in Table 1. None of NMIBC patients was found in low E-cadherin levels and the majority of MIBC patients had high E-cadherin levels. Only stage and metastasis had statistically significant difference with E-cadherin. The relative risk (RR) for metastasis based on E-cadherin expression was 22.67 (95% CI 3.03–169.53).
A log-rank test was run to determine if there were differences in the survival distribution for the E-cadherin expression. The survival distributions for the high and low E-cadherin expression were different at cumulative survival analysis, even though it was not statistically significantly different (mean 32 months vs 25 months, p = 0.13). Subgroup analysis of survival based on muscle invasion and E-cadherin expression was done. Mean survival time of subjects with MIBC with low E-cadherin, MIBC with high E-cadherin, and NMIBC with high E-cadherin were 25.2 months (95% CI 16.3–34.1 months), 32.2 months (95% CI 26.1–38.2 months), and 32 months (95% CI 24.7–39.2 months), respectively (p = 0.24) (Table 2 and Fig. 2).
4 Discussion
Our study showed that lower expression of E-cadherin was associated with the higher clinical staging as well as higher number of metastases. E-cadherin, one of the members of cadherin family, plays a vital role in cell-to-cell adhesion. It is a basic part of the adherence junction. Its role as tumor suppressor has been recognized. Without E-cadherin, cells are able to grow on top of each other, leading to initial formation of cancer [2]. This pathophysiology justified the role of low E-cadherin in cancer progression.
Earlier studies showed that lower E-cadherin expression contributed to higher number of MIBC and high histological cancer grades [14, 15]. Those findings showed the same result as the study conducted by Ziaran et al. All NMIBC cases in our study had high E-cadherin expression, corresponding to other studies [14, 15]. Interestingly, some of the high-grade and MIBC cases retained their E-cadherin expression. This finding suggested the phenomenon of E-cadherin mutation as a factor of cancer progression, other than loss of expression. Mutated E-cadherin is detected in an immunohistochemistry assay, but no longer had its original function in cell adhesion and tumor suppression [2]. Some cancers may also exhibit the ability to downregulate E-cadherin adhesive activity through an unknown mechanism [2]. Both mutated and downregulated E-cadherin retain their expression, which may explain why some cancers with high E-cadherin expression progress to MIBC.
E-cadherin was not statistically associated with multifocality, but data showed high E-cadherin resulted in the existence of multifocality (70.6% vs. 29.4%) than low E-cadherin which the majority found in no multifocality cases (66.7% vs. 33.3%). Current literatures showed that multifocal bladder cancer may arise from two sources: divisions of single cell (clonogenic) and simultaneous multiple transformations (field changes). In theory, clonogenic multifocality may need loss of cell adhesion (represented by E-cadherin), while field changes do not. Both hypotheses were still the subject for extensive studies [16,17,18]. The other explanation is the mutated or downregulated expressed E-cadherin, as discussed above [2]. However, we found no research that specifically studied the impact of the “non-active” expressed E-cadherin to multifocality of bladder cancer.
There was no statistically significant difference between E-cadherin expression with mortality and recurrence (p = 0.29 and p = 0.6, respectively) in our study. Loss to follow-up data was maybe one of the reasons for this result. In contrast, Shi et al. concluded the expression of E-cadherin was very closely associated with the biological behavior of bladder cancer, which may suggest a potential role of E-cadherin in the recurrence and progression of bladder cancer, perhaps with more samples [19,20,21,22,23,24]. Meanwhile, multivariate analysis by Ziaran et al. showed low E-cadherin expression can be a predictor for worse cancer-specific survival. [10].
Mean of three-year survival was higher in the high E-cadherin expression group. Cumulative survival of low E-cadherin group showed only 40% probability of surviving beyond 36 months, while the high E-cadherin group showed around 70% subjects survived to 36 months. Furthermore, in subgroup analysis, subjects with MIBC had lower probability of survival (64% in high E-cadherin and 40% in low E-cadherin) compared to NMIBC (86%) within three years. The lack of statistical association was perhaps due to loss to follow-up in 17 (42.5%) of subjects. As discussed earlier, E-cadherin holds a significant role in cell-to-cell adhesion [2]. With low E-cadherin, there is the higher risk for cancers to progress and metastasize [10]. This result would be comparable to the differences of expression found in the different staging and grading of bladder cancer which also translate into the tumor’s aggressiveness, and subsequently, the patients’ prognosis. Therefore, E-cadherin expression had the potential to become a prognostic factor in MIBC and NMIBC.
There were several limitations of our study. First, the sample size of our study was limited because our study was a single-center study. Next, our follow-up data were incomplete. Because our hospital was a national referral hospital with patients originated across the country, we encountered difficulties in tracking the subjects after three years. Third, type of treatments received by the subjects were unaccounted in our study, which could lead to outcome bias. Other limitation of this study was unaccounted co-founding factors, such as obesity, diabetes mellitus, chronic lung disease, and congestive heart failure, which may aggravate the recurrence and survival rate. Those co-founding factors were difficult to adjust because of the rarity of the disease and single-centered nature of our study. Nevertheless, we conducted this study because E-cadherin is an important prognostic marker and scarcely studied in our country.
5 Conclusions
In our study, a low level of E-cadherin was associated with higher clinical staging and risk of metastasis. It also has the tendency to be associated with muscle invasion and histological grade. Low E-cadherin expression also has the tendencies to worsen survival and maybe potential to predict patients’ prognosis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MIBC:
-
Muscle invasive bladder cancer
- NMIBC:
-
Non-muscle invasive bladder cancer
References
International Agency for Research on Cancer (2019) Bladder Globocan 2018. http://globocan.iarc.fr. Accessed 10 Aug 2020
Mendonsa AM, Na TY, Gumbiner BM (2018) E-cadherin in contact inhibition and cancer. Oncogene 37:4769–4780. https://doi.org/10.1038/s41388-018-0304-2
Bryan RT, Tselepis C (2010) Cadherin switching and bladder cancer. J Urol 184:423–431. https://doi.org/10.1016/j.juro.2010.04.016
Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J et al (2014) Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25:152–165. https://doi.org/10.1016/j.ccr.2014.01.009
Soukup V, Čapoun O, Cohen D, Hernández V, Babjuk M, Burger M et al (2017) Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non–muscle-invasive bladder cancer: a European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel Syst. Eur Urol 72:801–813. https://doi.org/10.1016/j.eururo.2017.04.015
Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L et al (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466–477. https://doi.org/10.1016/j.eururo.2005.12.031
Horwich A, Babjuk M, Bellmunt J, Bruins HM, De Reijke TM, De Santis M et al (2019) EAU–ESMO consensus statements on the management of advanced and variant bladder cancer—an international collaborative multi-stakeholder effort: under the auspices of the EAU and ESMO Guidelines Committees. Ann Oncol 30:1697–1727. https://doi.org/10.1093/annonc/mdz296
Witjes JA, Babjuk M, Bellmunt J, Bruins HM, De Reijke TM, De Santis M et al (2020) EAU-ESMO consensus statements on the management of advanced and variant bladder cancer—an international collaborative multistakeholder effort. Eur Urol 77:223–250. https://doi.org/10.1016/j.eururo.2019.09.035
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G et al (2020) European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol S 0302–2838:30230. https://doi.org/10.18632/aging.104115
Ziaran S, Harsanyi S, Bevizova K, VarchulovaNovakova Z, Trebaticky B, Bujdak P et al (2020) Expression of E-cadherin, Ki-67, and p53 in urinary bladder cancer in relation to progression, survival, and recurrence. Eur J Histochem 64(2):3098. https://doi.org/10.4081/ejh.2020.3098
Horne HN, Oh H, Sherman ME, Palakal M, Hewitt SM, Schmidt MK et al (2018) E-cadherin breast tumor expression, risk factors and survival: pooled analysis of 5,933 cases from 12 studies in the Breast Cancer Association Consortium. Sci Rep 8(1):6574. https://doi.org/10.1038/s41598-018-23733-4
Liu JB, Feng CY, Deng M, Ge DF, Liu DC, Mi JQ et al (2017) E-cadherin expression phenotypes associated with molecular subtypes in invasive non-lobular breast cancer: evidence from a retrospective study and meta-analysis. World J Surg Oncol 15(1):139. https://doi.org/10.1186/s12957-017-1210-8
Rakha EA, Abd El Rehim D, Pinder SE, Lewis SA, Ellis IO (2005) E-cadherin expression in invasive non-lobular carcinoma of the breast and its prognostic significance. Histopathology 46:685–693. https://doi.org/10.1111/j.1365-2559.2005.02156.x
Xie Y, Li P, Gao Y, Gu L, Chen L, Fan Y et al (2017) Reduced E-cadherin expression is correlated with poor prognosis in patients with bladder cancer: a systematic review and meta-analysis. Oncotarget 8:62489–62499. https://doi.org/10.18632/oncotarget.19934
Erdemir F, Ozcan F, Kılıcaslan I, Parlaktas BS, Uluocak N, Gokce O (2007) The relationship between the expression of E-cadherin and tumor recurrence and progression in high-grade stage T1 bladder urothelial carcinoma. Int Urol Nephrol 39:1031–1037. https://doi.org/10.1007/s11255-006-9159-5
Hafner C, Knuechel R, Stoehr R, Hartmann A (2002) Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies. Int J Cancer 101:1–6. https://doi.org/10.1002/ijc.10544
Habuchi T (2005) Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol 12:709–716. https://doi.org/10.1111/j.1442-2042.2005.01155.x
Duggan BJ, Gray SB, McKnight JJ, Watson CJ, Johnston SR, Williamson KE (2004) Oligoclonality in bladder cancer: The implication for molecular therapies. J Urol 171:419–425. https://doi.org/10.1097/01.ju.0000100105.27708.6c
Lin HH, Ke HL, Wu WJ, Lee YH, Chang LL (2012) Hypermethylation of E-cadherin, p16, p14, and RASSF1A genes in pathologically normal urothelium predict bladder recurrence of bladder cancer after transurethral resection. Urol Oncol Semin Orig Investig 30:177–181. https://doi.org/10.1016/j.urolonc.2010.01.002
Jäger T, Becker M, Eisenhardt A, Tilki D, Tötsch M, Schmid KW et al (2010) The prognostic value of cadherin switch in bladder cancer. Oncol Rep 23(4):1125–1132. https://doi.org/10.3892/or_00000741
Muramaki M, Miyake H, Terakawa T, Kumano M, Sakai I, Fujisawa M (2012) Expression profile of E-cadherin and N-cadherin in non-muscle-invasive bladder cancer as a novel predictor of intravesical recurrence following transurethral resection. Urol Oncol Semin Orig Investig 30:161–166. https://doi.org/10.1016/j.urolonc.2010.01.005
Otto W, Breyer J, Herdegen S, Eder F, Bertz S, May M et al (2017) WHO 1973 grade 3 and infiltrative growth pattern proved, aberrant E-cadherin expression tends to be of predictive value for progression in a series of stage T1 high-grade bladder cancer after organ-sparing approach. Int Urol Nephrol 49:431–437. https://doi.org/10.1007/s11255-016-1491-9
Breyer J, Gierth M, Shalekenov S, Aziz A, Schäfer J, Burger M et al (2016) Epithelial–mesenchymal transformation markers E-cadherin and survivin predict progression of stage pTa urothelial bladder carcinoma. World J Urol 34:709–716. https://doi.org/10.1007/s00345-015-1690-5
Hussein S, Mosaad H, Rashed HE, Ahmed S, Ragab A, Ismail EI (2017) Molecular factors regulating E-cadherin expression in urothelial bladder cancer and their correlations with the clinicopathological features. Mol Biol Rep 44:365–377. https://doi.org/10.1007/s11033-017-4118-z
Acknowledgements
Not applicable.
Funding
No funding is to be declared.
Author information
Authors and Affiliations
Contributions
ARAHH designed, supervised, and provided funding for the study. MPT collected the data from medical records, contacted the participants, and wrote the draft of the manuscript. MS provided histopathological data and supervised the histopathological examination aspect of the study. RU and CAM supervised and provided guidance to other authors on the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of the Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital (Protocol number: 18–03-0213). All methods were performed in accordance with the relevant guidelines and regulations. Requirement of informed consent was waived by the Ethics Committee of Faculty of Medicine Universitas Indonesia-Cipto Mangunkusumo Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tambunan, M.P., Saraswati, M., Umbas, R. et al. E-cadherin expressions on bladder and its association with cancer progressivity: a retrospective cohort study. Afr J Urol 28, 14 (2022). https://doi.org/10.1186/s12301-022-00280-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-022-00280-z