Abstract
Background
Calcium oxalate is the most frequent urinary stone component; it exists in three different crystalline forms. In this case, the most common is the calcium oxalate monohydrate (whewellite). However, Morocco is one of the countries that has used the traditional medicine based on natural plants to treat many diseases including urolithiasis. In this respect, the most medicinal plants used for this purpose are Herniaria hirsuta L., Opuntia ficus-indica flowers, Zea mays styles and Ammi visnaga L. seeds. The purpose of this work is to study experimentally the effectiveness of each plant on the dissolution of whewellite stones.
Methods
In 1 L boiled saline solution (9 g/L of NaCl), 5 g of plant extract powder has been introduced. Thereafter, the mixture was left soaked for 15 min and then filtered. Further, a specific installation that resembles the urinary circuit has been conducted in the laboratory. As a starting step, three whewellite stones are placed in contact with extract solutions for 8 weeks. Two other solutions have been used to correct the loss mass: the first one is a solution of potassium citrate of 3 mmol/L served as a positive control, and the second one is a solution of NaCl of 9 g/L without extract used as a reference point.
Results
After 8 weeks, the loss mass is about 54.88 ± 1.32% with Z. mays styles, 49.86 ± 1.69% with H. hirsuta L., 47.10 ± 1019% with A. visnaga L. and 44.97 ± 1.76% with flowers O. ficus-indica, while the loss of mass in the presence of witnesses solutions is 21.95 ± 0.76% for potassium citrate (C Pot) and 21.05 ± 1.07% in the case of saline solutions.
Conclusion
Our experiments show the effectiveness of the extracts of four plants specially for Z. mays styles. These extracts can be effective remedies in the oxalocalcic stones’ dissolution.
Similar content being viewed by others
1 Background
Urolithiasis is a common disease that evolves with the socio-economic and health status of populations. In this regard, it is characterized by crystalline concretion formation in the urinary tract, and it is a widespread illness in the active population. Therefore, it affects nearby 4–20% of the general population of different countries. However, it is readily recurrent and its etiology is often unknown [1]. On the other hand, calcium oxalate is the most abundant element, its prevalence exceeds 70% in most of world's countries [2, 3], and the monohydrate form is oxalo-dependent crystallizing in environments of hyperoxaluria with low or normal calcium. However, to our knowledge there are no effective drugs for the dissolution of calcium oxalate monohydrate stones; in addition, surgical methods are usually invasive with side effects. In recent years, the introduction of expulsive therapy based on drugs able to facilitate the passage of distal ureteral calculi such as nifedipine and tamsulosin, but despite their widespread use, the evidence for the benefit of these agents in the treatment of ureteral calculi remains weak [4,5,6]. In this respect, several medicinal plants have been used traditionally for the treatment of urinary stones such as Herniaria hirsuta L., Opuntia ficus-indica, Zea mays and Ammi visnaga L. These plants have often shown the spectacular therapeutic results [7,8,9]. The aim of this study is to evaluate experimentally the efficiency of some Moroccan medicinal plants for dissolution urinary stones. For this reason, the choice has been focused on calcium oxalate because of both its high prevalence and its extracorporeal lithotripsy resistance.
2 Methods
2.1 The urinary calculi
Calcium oxalate is a calcium salt of oxalic acid with the chemical formula CaC2O4. A major constituent of human kidney stones are formed by calcium oxalate. Various kidney stones of calcium oxalate (Fig. 1) were removed by surgery in an elderly patient of 55 years old suffering from oxalo-dependent lithiasis. These stones whose mass was initially between 125 and 260 mg (Table 1) have a morphology type “Id”, which is characterized by a smooth surface of brown color, and the cross section is compact, with a microcrystalline concentric structure in very thin layers without radial crystallization [10]. Their chemical composition has been analyzed by infrared spectroscopy (Fig. 2).
2.2 Medicinal plants
In this work four medicinal plants are used, the first one is H. hirsuta L., fully used (leaves and stems), it is an annual herb with stems up to 20 inches long and collected from the Taza region of North-Eastern Morocco. The second and third ones are O. ficus-indica flowers and A. visnaga L. seeds. They are collected from the Taounate region of northern Morocco. The fourth one is the very fine filaments that come from the outer shell corn cobs Z. mays styles. It is from the region of Fez in northern Morocco. The taxonomic identification was performed by Prof. A. Bari, Department of Biology, Faculty of Sciences Dhar El-Mahraz, Sidi Mohammed Ben Abdellah University, Fez, Morocco. Voucher specimens were deposited in the herbarium under the following references: RAB 090814; RAB 1264407; RAB 76986 and RAB 1108434, respectively. Then, the four plants were dried at room temperature, ground and stored until the extraction.
2.3 Extraction process
In 1L of physiological saline solution (9 g/L NaCl) boiled, 5 g of the plant powder has been introduced, and the mixture was left soaked for 15 min and then filtered in three steps (Fig. 3). The first via a sieve with port diameter of 125 µm, followed by filtration through filter paper with port diameter of 20 to 25 µm and then through filter paper with port diameter from 7 to 10 µm. The outcome of the plant extracts was compared to an aqueous solution of potassium citrate to 3 mmol/L [8], which corresponds to the average concentration of urinary citrate obtained during the treatment of whewellite, and an aqueous NaCl solution at 9 g/L was used as a witnesses solution.
2.4 Experimental device
A specific installation that resembles the urinary circuit has been done in the laboratory (Fig. 4). Indeed, the solution of the plant extract is placed in a large tank and whewellite stones are placed in an enclosure using the sample holder, attached at both ends by two tubulars. The first one is to pump the solution from the large tank, and the second one is attached to a second collecting vessel. In this respect, the stones undergo regular flow from the tank through two valves placed upstream and downstream of the chamber for controlling the flow rate of the solution at 1.5 mL/min (2L/day): average urine flow is recommended for the lithiasis patients. Meanwhile, the pH value of the recovered solution is monitored daily using a pH meter. Thereafter, the solution is filtered using a sieve with port diameter of 125 μm and given to the initial tank. Further, the operation lasted for 8 weeks, period recommended in traditional medicine. Hence, every 2 weeks, the stones are removed, dried for 16 h at a temperature of 40 °C and weighed with a precision balance to measure the mass loss and then delivered to the infrared spectroscopy to determine their composition. The same procedure was carried out for two other witnesses’ solutions to correct the mass loss (Dissolution Rate): The first is a solution of potassium citrate 3 mmol/L, and the second is a saline solution of NaCl 9 g/L [8, 11].
3 Results
3.1 The effect of plant extracts on stones
The results provided, expressed as the dissolution rate (DR), were calculated by the following formula and expressed as a mean ± standard deviation
with Wi: initial stones weight, Wf: final stones weight.
The initial stones weights "Wi" are given in Table 1.
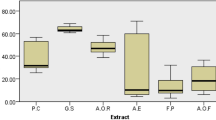
The analysis of the histogram (Fig. 5) shows a continuous increasing kinetic evolution of DR for the different solutions over the 8 weeks. For this reason, this trend is more important for the all extract solutions than the control solutions. The comparison of DR for the four plants displays that Zea mays styles have a slightly greater impact than the others. Indeed, after 2 weeks, this plant has given a DR of about 14.43 ± 2.22%, compared to 9.94 ± 1.63% for seeds of Ammi visnaga L., 8.76 ± 1.23% for the H. hirsuta L. plant, and 8.69 ± 0.87% for flower extracts of O. ficus-indica. Similarly citrate and physiological saline solutions have provided a DR of 7.02 ± 0.65 and 7.93 ± 1.01%, respectively. The same result has been observed after contact of 8 weeks with the plant extracts. Thereby, the DR of 54.88 ± 1.32% was obtained with Z. mays styles extracts and the DR values of 49.86 ± 1.69, 47.10 ± 1019 and 44.97 ± 1.76% were found with plant Herniaria hirsute L, seeds of Ammi visnaga L. and flowers of O. ficus-indica, respectively, while with citrate and physiological solutions, the dissolution rates after 8 weeks were 21.95 ± 0.76 and 21.05 ± 1.07%, respectively.
3.2 Kinetic evolution of pH for the four plants
Figure 6 exhibits that the initial pH of the solution is slightly acidic or basic. Thus, it ranges from 6 for the extract of ficus-indica flowers to 7.8 for the citrate solution. However, the pH values have slightly increased linearly during treatment time to the values of 7.5 and 9 for Hirnia Hirustat, potassium citrate and physiological solutions.
3.3 Proposed mechanisms of action.
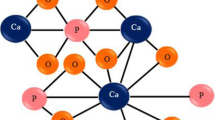
Figure 7 shows the mechanisms of action proposed to explain the interactions between the calcium oxalate molecule and the active ingredient of medicinal plants.
4 Discussion
The crystallization of calcium oxalate is related to the molar calcium oxalate product; however, the crystalline form which is noticed in the urine is strongly dependent on the molar ratio of calcium/oxalate. In this regard, whewellite is oxalo-dependent and formed in urine when the oxalate concentration is above 0.3 mmol/L and the molar calcium/oxalate ration is low [12, 13]. The use of medicinal plants to dissolve whewellite stones remains an interesting alternative.
The tests performed in vitro in the presence of different extracts exhibit a very higher solubilization kinetics than those given by both saline and potassium citrate solutions. In this case, the dissolving power of Whewellite stones shown by extracts of Z. mays styles, Opuntia Ficus-Indica flowers, H. hirsuta L. and seeds of Ammi visnaga could be related to the interaction between the calcium oxalate and the molecules present in the plant extracts tested. On the other hand, the results obtained from the four plants are largely exceeding those obtained by Khouchla et al. [14] who worked on the Zizyphus lotus L aqueous extract and kachkoul et al. [15] on the infusion and ethanolic extract of A. unedo plant.
However, the studies relating to the chemical composition of the four plants show that the H. hirsuta L plant contains saponosides such as medicagenic acid, bidesmosidic saponosides and flavonoids derivatives. Z. mays styles are rich in polyphenols, tannins, and potassium, while Ammi visnaga L. seeds are mostly composed of furanochromes like khellin, visnagin. As for the flowers of O. ficus-indica, we deduce that they are mostly rich in flavonoids, pendulum, rutin, quercetin and luteolin [16,17,18,19]. In fact, catechin (flavonoid) shows a preventive effect of the calcium oxalate crystallization induced by ethylene glycol [20]. Also, quercetin and hyperoside have been shown a significant effect in inhibiting the deposition of calcium oxalate crystals in the rats kidneys [21].
The examination of all chemical constituents present in the various plants used suggests that a mechanism of action independent to the pH maybe responsible for the dissolution of whewellite stones. Therefore, this effect could be attributed to the formation of complex oxalate-molecules, such as oxalate-saponin, oxalate-tannins or oxalate-quercetin (Fig. 7) of which the stability would be both ensured by hydrogen and hydrophilic links between the functional groups of active molecules and calcium oxalate. Consequently, the complexes formed are very soluble than calcium oxalate which causes the dissolution of the stones while maintaining in solution the high amounts of dissolved calcium oxalate. Indeed, Meiouet et al. [8] have already proposed almost the same mechanism to explain the plant extracts effect on cystine stones.
5 Conclusion
The outcomes of our experiments display the efficiency of four plants extracts in the dissolution urinary stones. In this respect, these extracts may have interesting use as curative and/or prophylactic agents for patients with urinary tract stones. However, an optimization study is necessary to determine the optimum concentration of the plant used.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- HHL:
-
Herniaria hirsuta L.
- OFI:
-
Opuntia ficus-indica
- ZMS:
-
Zea mays styles
- AVL:
-
Ammi visnaga L.
- W:
-
weight
- DR:
-
dissolution rate
- C pot:
-
potassium citrate
- S phy:
-
physiological saline solution
References
Johnson CM, Wilson DM, O’fallon WM, Malek RS, Kurland (1979) Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int 16:624
Daudon M, Bounxouei B, Santa Cruz F, Da Silva SL, Diouf B, Angwafoo FF III, Talati J, Desrez G (2004) Composition des calculs observés aujourd’hui dans les pays non industrialisés. Prog Urol 14:1151–1161
El Habbani R, Chaqroune A, Sqalli Houssaini T, Arrayhani M, El Ammari J, Dami F, Chouhani BA, Lahrichi A (2016) Étude épidémiologique sur les calculs urinaires dans la région de Fès et sur le risque de récidive. Prog Urol 26:287–294
Arumuham V, Bycroft J (2016) The management of urolithiasis. Surgery (United Kingdom) 34(7):352–360. https://doi.org/10.1016/j.mpsur.2016.04.007
De Coninck V, Antonelli J, Chew B, Patterson JM, Skolarikos A, Bultitude M (2019) Medical expulsive therapy for urinary stones: future trends and knowledge gaps. Eur Urol 76(5):658–666. https://doi.org/10.1016/j.eururo.2019.07.053
El Khebir M, Fougeras O, Le Gall C, Santin A, Perrier C, Sureau C, Miranda J, Ecollan P, Bagou G, Trinh-Duc A, Traxer O (2009) Updating 2008 of 8th Conference of consensus of the French Society of medical emergencies of 1999 The treatment of adult renal colic by the emergency services and in ER. Progres Urol 19(7):462–473
Kachkoul R, Sqalli Houssaini T, Miyah Y, Mohim M, El Habbani R, Lahrichi A (2018) The study of the inhibitory effect of calcium oxalate monohydrate’s crystallization by two medicinal and aromatic plants: Ammi visnaga and Punica granatum. Prog Urol 28(3):156–165. https://doi.org/10.1016/j.purol.2017.12.003
Meiouet F, El Kabbaj S, Daudon M (2011) Étude in vitro de l’activité litholytique de quatre plantes médicinales vis-a-vis des calculs urinaires de cystine. Prog Urol 21:40–47
Hannache B, Bazin D, Boutefnouchet A, Daudon M (2012) Effet des extraits de plantes médicinales sur la dissolution des calculs rénaux de cystine in vitro: étude à l’échelle mésoscopique. Prog Urol 22:577–582
Daudon M, Dessombz A, Frochot V, Letavernier E, Haymann J-P, Jungers P, Bazin D (2016) Comprehensive morpho-constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. C R Chim 19(11–12):1470–1491. https://doi.org/10.1016/j.crci.2016.05.008
Roberston WG, Peacock M (1972) Calcium oxalate crystalluria and inhibitors of crystallization in recurrent renal stone-formers. Clin Sci 43:499–506
Caudarella R, Rizzoli E, Malavolta N, Severi B, Vasi V, Biagini G (1986) Cristallurie urinaire. Un problème à débattre. Act Urol Belg 54:49–56
Daudon M (1989) Modèles de cristallisation. Jungers P, Daudon M, Le Duc A (1989) Lithiase urinaire. Flammarion Médecine-Sciences, Paris, pp 158–195
Khouchla A, Talbaoui A, El Idrissi AEY, Bouyahya A, Ait Lahsen S, Kahouadji A, Tijane M (2017) Détermination des composés phénoliques et évaluation de l’activité litholytique in vitro sur la lithiase urinaire d’extrait de Zizyphus lotus L d’origine marocaine. Phytothérapie. https://doi.org/10.1007/s10298-017-1106-3
Kachkoul R, Squalli Housseini T, Mohim M, El Habbani R, Miyah Y, Lahrichi A (2019) Chemical compounds as well as antioxidant and litholytic activities of Arbutus unedo L. leaves against calcium oxalate stones. J Integr Med 17(6):430–437. https://doi.org/10.1016/j.joim.2019.08.001
Freiler M, Reznicek G, Jurenistsch J, Al Et (1996) New triterpene saponins from Herniaria glabra. Helv Chim Acta 79:385–390
Solihah MA, Wan Rosli WI, Nurhanan AR (2012) Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int Food Res J 19(4):1533–1538
Gu Naydın K, Erim FB (2002) Determination of khellin and visnagin in Ammi visnaga fruits by capillary electrophoresis. J Chromatogr A 954:291–294
Moussa-Ayoub TE, El-Hady EAA, Omran HT, El-Samahy SK, Kroh LW, Rohn S (2014) Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res Int 64:864–872
Zhai W, Xu Y-F, Feng Y, Peng B, Che J-P, Liu M, Zheng J-H (2013) Catechin prevents the calcium oxalate monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complement Altern Med 13(1):228. https://doi.org/10.1186/1472-6882-13-228
Zhu W, Zheng J, Yao X, Peng B, Liu M, Huang J, Wang G, Xu Y (2014) Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis 42(6):519–526. https://doi.org/10.1007/s00240-014-0695-7
Acknowledgements
This work has been supported by Nephrology Department, University Hospital Hassan II – Fez (Morocco). The authors express their thanks for this support. The authors thank Professor Amina BARI (Department of Biology, Faculty of Sciences Dhar El-Mahraz, Sidi Mohammed Ben Abdellah University, Fez, Morocco) for the plants identification.
Funding
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Contributions
RH conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; and wrote the paper. TSH, AL, and AC conceived and designed the experiments; analyzed and interpreted the data. MM, RK, and BAC performed the experiments and analyzed and interpreted the data. All authors read and approved the manuscript."
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We have not working on the animal model or on the patients.
Consent for publication
Not applicable.
Competing interests
We wish to confirm that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
El Habbani, R., Lahrichi, A., Sqalli Houssaini, T. et al. In vitro mass reduction of calcium oxalate urinary calculi by some medicinal plants. Afr J Urol 27, 28 (2021). https://doi.org/10.1186/s12301-021-00132-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-021-00132-2