Abstract
Background
Excretory-secretory (ES) proteins of E. histolytica are thought to play important roles in the host invasion, metabolism, and defence. Elucidation of the types and functions of E. histolytica ES proteins can further our understanding of the disease pathogenesis. Thus, the aim of this study is to use proteomics approach to better understand the complex ES proteins of the protozoa.
Methods
E. histolytica ES proteins were prepared by culturing the trophozoites in protein-free medium. The ES proteins were identified using two mass spectrometry tools, namely, LC–ESI–MS/MS and LC–MALDI–TOF/TOF. The identified proteins were then classified according to their biological processes, molecular functions, and cellular components using the Panther classification system (PantherDB).
Results
A complementary list of 219 proteins was identified; this comprised 201 proteins detected by LC–ESI–MS/MS and 107 proteins by LC–MALDI–TOF/TOF. Of the 219 proteins, 89 were identified by both mass-spectrometry systems, while 112 and 18 proteins were detected exclusively by LC–ESI–MS/MS and LC–MALDI–TOF/TOF respectively. Biological protein functional analysis using PantherDB showed that 27% of the proteins were involved in metabolic processes. Using molecular functional and cellular component analyses, 35% of the proteins were found to be involved in catalytic activity, and 21% were associated with the cell parts.
Conclusion
This study showed that complementary use of LC–ESI–MS/MS and LC–MALDI–TOF/TOF has improved the identification of ES proteins. The results have increased our understanding of the types of proteins excreted/secreted by the amoeba and provided further evidence of the involvement of ES proteins in intestinal colonisation and evasion of the host immune system, as well as in encystation and excystation of the parasite.
Similar content being viewed by others
Background
Amoebiasis is caused by the protozoan parasite called Entamoeba histolytica. The disease commonly occurs in tropical regions that lack good sanitation. Amoebiasis was ranked second to malaria as the cause of mortality due to protozoan parasites [1]. The infection often begins with the ingestion of the parasite cysts [2]. When the cysts arrive in a conductive environment, such as the intestine, they break out into the invasive trophozoite form.
Up to 90% of amoebiasis patients are mild to asymptomatic cases [3]. In symptomatic patients, amoebiasis primarily manifests as an intestinal infection, and some patients exhibit extraintestinal manifestations, mainly amoebic liver abscess (ALA). The former patients typically experience a gradual onset of abdominal cramps and pain, fever, diarrhoea, and bloody stools, while the latter condition presents with right-upper-quadrant pain, tenderness of the liver, jaundice, and nausea [4]. Diagnosis of amoebiasis includes stool microscopic examination, molecular detection, and antigen and antibody detection-based methods [5–7]. For ALA cases that do not respond to the therapy, invasive procedures such as percutaneous needle aspiration and surgery are performed [8]. Meanwhile, treatment for amoebiasis requires synergy among metronidazole, nitroimidazole, tinidazole and luminal amoebicide [9].
During infection, E. histolytica trophozoites release molecules called excretory-secretory (ES) proteins, which are also known as excretory-secretory antigens (ESA). ES proteins are involved in the invasion of trophozoites into the colonic mucosa by degrading the glycoside substrates and proteins of the host tissues [10–13]. Antibodies to ES proteins have been detected in the sera of both symptomatic and asymptomatic patients who have contracted amoebiasis [14].
The use of ES proteins as potential targets for diagnosis, treatment, and vaccine development for amoebiasis have been reported. The E. histolytica Gal/Gal-NAc lectin antigen is being utilized in commercial antigen detection tests such as the TechLab E. histolytica II ELISA (TechLab Inc). A study on ES proteins showed the diagnostic potential of pyruvate phosphate dikinase (PPDK), and its recombinant form has been used to develop a lateral flow dipstick test [15, 16]. In terms of treatment, auronofin has been identified as an effective drug which targets thioredoxin reductase, an ES protein of E. histolytica [17]. Furthermore, Gal/Gal-NAc lectin also showed potential as a vaccine candidate against E. histolytica [18].
A study on the ES proteins of Trypanosome sp. using proteomic tool has uncovered a range of proteins which include unfolding and degradation classes of proteins, such as serine, cysteine proteases, and metallopeptidases. These proteases play a part in the physiological and pathological functions that favour invasion of the parasite, its growth in hostile host conditions, evasion of the host immune defence, and hydrolysis of host proteins [19].
The main aim of the present study was to better understand the complex ES proteins of E. histolytica. It involved liquid chromatography-mass spectrometry analysis, in which two types of ionisation techniques, namely, electro spray ionization (ESI) and matrix assisted laser desorption ionisation (MALDI), were used in order to obtain the maximum number of protein hits. The combination of both techniques has allowed us to provide improved proteome coverage of the ES proteins.
Methods
Production of E. histolytica ES proteins
Entamoeba histolytica HM1:IMSS trophozoites were axenically cultured in TYI-S-33 medium supplemented with 12.5% bovine serum (GIBCO, New Zealand) and 1× Diamond’s vitamin Tween 80 (Sigma-Aldrich), pH 6.8 at 36 °C. The culture medium was changed every 48 h. The trophozoites were harvested and rinsed three times with protein-free RPMI medium 1640 (ref no.:31800-022) supplemented with 0.1% l-cysteine and 0.02% ascorbic acid (RPMI-C-A medium), by centrifuging at 440×g for 2 min at room temperature (RT). Trophozoites at a density of 0.5 × 106 cells per ml were then seeded into culture tubes containing 80% (8 mL/tube) RPMI-C-A medium and incubated at 36 °C for 6 h. Subsequently, the culture tubes were chilled on ice for 5 min and then centrifuged at 22×g at 4 °C. To protect ES proteins from proteolytic activity, iodoacetamide (IAA) was added to the resultant supernatant at a final concentration of 1 mM. The supernatant was then pooled and centrifuged at 10,000×g for 5 min at 4 °C and filtered through 0.2 µm filter (Sartorius Stedim, Germany). Subsequently, the supernatant was concentrated 1000 times using a spin filter with 5 kDa molecular-weight cut off (Vivapsin, Sartorius), and a cocktail of protease inhibitors (Roche, Germany) was added [20]. Protein samples and RPMI-CA medium (concentrated 100X, as control) were reduced with 0.284 M β-mercaptoethanol by boiling for 5 min. Subsequently the reduced protein was electrophoresed in 10% polyacrylamide resolving gel, pH 8.8 containing 0.4675 M Tris base, 0.1% SDS, 60 µL of 10% ammonium persulphate and 6 µL of TEMED. Before staining, resolving gel was rinsed with dH2O three times (5 min/wash) with gentle agitation. The gel was then stained with RAMA stain which comprised 0.05% Coomasie Briliant Blue (CBB) R250, 10% acetic acid, 15% methanol and 3% ammonium sulphate, for at least 30 min with gentle agitation. The stained gels were then destained with dH2O until the protein bands were clearly seen.
In-solution digestion of ES proteins
One hundred µg of ES proteins were solubilised with 0.05% RapiGest SF surfactant (Waters, USA) at 80 °C for 15 min. The sample was then reduced with 5 µL of 100 mM dithiothreitol (DTT) and incubated at 60 °C for 15 min. The sample was then cooled to RT before alkylating it using 5 µL of 20 mM IAA in the dark at RT for 30 min. Two µg of trypsin (Promega) was then added to the sample and incubated at 37 °C for 16 h. Subsequently, the digestion was stopped by adding trifluoroacetic acid (TFA) at a final concentration of ~1% and incubating at 37 °C for 20 min. The digested proteins were then centrifuged at 14,462×g for 15 min. The supernatant was collected and filtered through a 0.45 µm filter (Sartorius Stedim, Germany) before proceeding to peptide separation by liquid chromatography.
Liquid chromatography and MALDI mass spectrometry
The separation of peptides prior to MALDI–TOF/TOF was performed using eksigent nanoLC ultra 1D plus (Eksigent,Germany) linked to an automated spotter (Eksigent, Germany). To achieve spatial discrimination of the peptide mixtures, 2 µL of the digested proteins was auto-loaded and packed into a C18 column. Mobile phase buffer A consisted of 0.1% TFA in 2% Acetonitrile (ACN) and 97.9% water while mobile phase buffer B consisted of 0.1% TFA in 98% ACN and 1.9% water. The gradient pump was set to elute the peptides with 20–80% acetonitrile for a duration of 165 min and at a flow rate of 0.3 µL/min. The eluted peptides were then automatically spotted between the 30th and 160th min of the gradient phase, with a 5 mg/ml α-cyano-4-hydroxycinnamic acid (CHCA) matrix flow of 1.8 µL/min for a duration of 25 s for each spot. MALDI–MS and MS/MS were performed in an automated LC mode on the AB Sciex TOF/TOF™ 5800 system (AB Sciex, USA). Mass spectra from each spot were obtained in the m/z range from 800 to 4000, whereby up to 500 laser shots were accumulated per spectrum. The signal-to-noise (S/N) ratio was set to a minimum of 10, and the spots with the highest intensity of precursor ion were subjected to MS/MS analysis. A maximum of ten precursors were allowed for the MS/MS analysis; for each spectrum, up to 2000 laser shots were accumulated per spectrum, and the S/N were set to a minimum ratio of 15 S/N. The mass spectrometry data were analysed using ProteinPilot™ Software 4.5 and searched using Paragon against a combined AmoebaDB_4.1 and cRAP (‘protein contaminants database’) which was set to search with the following parameters: false discovery rate of <1%, detected protein threshold of >0.47 (66%), and competitor error margin of 2.00. The cRAP includes the possible contaminant proteins in this study such as BSA and keratin (http://www.thegpm.org/crap/).
Liquid chromatography and ESI mass spectrometry
The analyses were performed using an LTQ-Orbitrap Velos Pro (Thermo Scientific, San Jose, CA, USA) mass spectrometer coupled with the Easy-nLC II (Thermo Scientific, San Jose, CA, USA) nano liquid chromatography system. The chromatographic separation of tryptic-digested peptides was performed using Easy-Column C18-A2 (100 × 0.75 mm i.d., 3 µm; Thermo Scientific, San Jose, CA, USA) coupled with pre-column (Easy-Column, 20 × 0.1 mm i.d., 5 µm; Thermo Scientific, San Jose, CA, USA) at a flow rate of 0.3 µL/min and sample injection volume of 10 µL. The pre-column was equilibrated for 15 µL at a flow rate of 3 µL/min whereas the analytical column was equilibrated for 4 µL at a flow rate of 0.3 µL/min. The running buffers used were (A) deionised distilled water with 0.1% formic acid and (B) acetonitrile with 0.1% formic acid. The samples were eluted using a gradient of B from 5 to 100% in 100 min. The fragmentation technique used was collision induced dissociation (CID). De Novo sequencing and database matching against a combined AmoebaDB_4.1 and cRAP (‘protein contaminants database’) was performed using Peaks Studio Version 7 (Bioinformatics Solution, USA). The cRAP includes the possible contaminant proteins in this study such as BSA and keratin (http://www.thegpm.org/crap/). The parameters used in De Novo sequencing were precursor mass tolerance at 0.1 Da and fragment mass error tolerance at 0.8 Da. Subsequently for the database matching, the parameters were carbamidomethylation and methionine oxidation as fixed modifications, 2 maximum missed cleavages, false detection rate (FDR) <0.1% and parent mass and 0.1 Da precursor mass tolerance. Besides, significant score (−10lgP) for protein acceptance were set at >20, whereas minimum unique peptide was set at 1.
Functional group analysis
The list of proteins obtained from the combination of both analyses was then assigned functional categories using the Panther Classification System (pantherdb.org).
Results and discussion
Protein identification
In this study, ES proteins and concentrated RPMI-CA medium were resolved in 10% SDS-PAGE, and stained with RAMA solution. Visual observation of distinct protein bands indicated good protein quality while the concentrated protein free RPMI-CA medium (control) showed no protein band (Fig. 1). Furthermore, no protein smearing was observed, and this also suggested that minimal degradation of the proteins had occurred. Thus, the ES proteins were suitable for downstream mass spectrometry analysis.
We have identified 219 proteins that were excreted-secreted into the extracellular environment, with 18 proteins unique to MALDI and 112 proteins unique to ESI, while 89 proteins were identified by both systems (Fig. 2). Two replicates were analysed with ESI, and three replicates with MALDI TOF–TOF. The proteins there were considered to be significant fell into one of the following categories: a protein that was detected in both replicates using LC–ESI–MS/MS; a protein that was detected in at least two replicates using LC–MALDI–TOF/TOF; and a protein that was detected in only one replicate but detected by both mass-spectrometry systems. The details of the analyses are given in the Additional files 1 and 2. Tables 1 and 2 show representatives of the ten highest protein scores from ESI and MALDI respectively. The results of all protein identifications, peptides summaries, De novo and contaminant proteins analyses are provided in the Additional files 1, 2, 3, 4 and 5.
Excretory-secretory (ES) proteins detected by LC–ESI MS/MS and LC–MALDI TOF/TOF. Venn diagram depicting 219 ES proteins detected through the complementary use of LC–ESI MS/MS and LC–MALDI TOF/TOF. The list of proteins found in LC–ESI MS/MS and LC–MALDI TOF/TOF can be found in Additional files 1 and 2
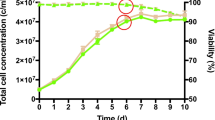
The protein-free media (RPMI-C-A) used in this experiment has previously been shown to support 95% viability of E. histolytica trophozoites for up to 8 h [21]. Nevertheless, there may still be a small number of dead E. histolytica trophozoites that were lysed and released proteins into the medium. Therefore it was possible that among the 219 proteins identified, a small proportion may be non-ES proteins of the amoeba.
The results of this study highlighted the important contributions of each mass-spectrometry system and showed that the use of only a single method does not provide good protein identification of a complex sample. This observation is consistent with the report by Bodnar [22] in which they observed that 16 and 22% proteins were identified uniquely by ESI and MALDI respectively.
This study showed that the ESI system identified more ES proteins compared to MALDI. The nature of the peptides and the ionization processes may have contributed to this unequal results [23, 24]. As described by Stapels and Barofsky [25], the ESI system tends to ionize hydrophobic peptides while MALDI tends to identify basic and aromatic peptides. MALDI is able to detect higher proportions of arginine (R) terminating peptides while ESI favours lysine (K) terminating peptides [26]. In addition, compared to MALDI, ESI tends to detect lower mass peptides [27, 28].
We have identified 25% (52) well-annotated proteins, as well as 65% (136) putative, and 10% (21) hypothetical proteins. Pawlowski [29] discussed the importance of presenting uncharacterized proteins, as these may hold novel and promising discoveries. Therefore, we have included putative and hypothetical proteins, as they embody unexplored information and clues for new source of biological markers.
Functional protein classification
When categorised according to biological functions (Fig. 3), a total of 78 (27%) proteins were associated with the metabolic process, 62 (21%) were involved with the cellular process, 6 (2%) were biological regulation proteins, 20 (7%) were proteins involved in localization, 22 (7%) were cellular component proteins, and 9 (3%) proteins were associated with response to stimulus. The largest category by molecular function (Fig. 4) was associated with catalytic activity whereby there was a total of 95 (35%) proteins, followed by 34 (13%) binding proteins, 29 (10%) proteins involved in structural molecule activity, 10 (4%) proteins involved in antioxidant activity, 5 (2%) proteins with transporter activity, and 3 (1%) translation regulator proteins.
The top two categories in the biological and molecular functions were metabolic process and catalytic activity. The major protein representatives in these two categories were peroxiredoxins (PRXs) (EHI_139570, EHI_145840, EHI_121620, EHI_122310, EHI_123390, EHI_061980, EHI_001420, EHI_201250, and EHI_114010). PRXs are involved in redox metabolism, oxidoreductase activity, and peroxidase activity. The presence of these antioxidant enzymes in the extracellular environment aids in the defence mechanism of the parasite against reactive oxygen species (ROS) that is imposed by the host immune system [30]. Wassmann et al. [31] reported an increased expression of PRXs in metronidazole resistant E. histolytica. They also showed that there is an increased expression of iron-containing superoxide dismutase (SOD), and hypothesised that PRXs and SOD play a role in the resistance of metronidazole in E. histolytica. In the present study, we have also identified superoxide dismutase [Fe];SODB (EHI_159160), which is involved in the metabolic process.
In another finding, PRX showed interaction with N-acetyl-galactosamine inhibitable (GalNAc) lectin in protecting the parasite [32]. It is also believed that the recruitment of PRX by lectin leads to a signal transduction that protects the parasite from oxidative stress [33]. The Gal/GalNAc lectin is an adhesin involved in the attachment of the parasite to the host. This was observed when the parasite failed to engage adhesion and contact-dependant cytotoxicity with cells that lacked terminal Gal/GalNAc residues [34, 35]. We have identified twelve GalNAc lectin-related proteins, i.e., EHI_006980, EHI_065330, EHI_148790, EHI_035690, EHI_058330, EHI_049690, EHI_042370, EHI_133900, EHI_012270 and EHI_077500, although this group of proteins does not fall into any of the classified functional annotations by PantherDB. Nonetheless, GalNAc lectin proteins are well studied, and their potential has already been explored and exploited. They contribute to the virulence of the parasite by their adherence to the host tissue, and cause contact-dependent cytolysis of target cells; they are also involved in phagocytosis and contribute to resistance to cell lysis inflicted by the host [36]. The GalNAc lectin protein was also reported by Wong et al. [15] as one of the components of excretory-secretory antigens of E. histolytica. In addition, the abundance of the Gal/GalNAc lectin protein on the surface of the parasite and its antigenic property have allowed it to be used for antigen detection in the TechLab Entamoeba histolytica II ELISA kit (TechLAB Inc.) [37].
Another family/sub family group of proteins that was included in the metabolic process is 60 s acidic ribosomal proteins (EHI_138770, EHI_052610). To the best of our knowledge, these are putative proteins with only two other reports on E. histolytica [38, 39]. They are strongly acidic proteins that are commonly found on the surface of the ribosome in all organisms [40]. Eukaryotic 60 s acidic ribosomal proteins are called ribosomal P proteins because they can be phosphorylated [41]. P protein is a structural constituent of the ribosome and is involved in nucleic acid binding and translation. Furthermore, the presence of P proteins has been reported to be related to infections caused by protozoa since anti-P-antibodies were detected against Trypanosoma cruzi and Leishmania species [42, 43].
Categorised under metabolic process and catalytic activity, ES proteins of E. histolytica with proteolytic properties were also identified. Among them, we have identified a protein family called cysteine proteinase (EHI_168240, and EHI_074180). The roles of cysteine proteinase include the degradation of fibrinogen, collagen, and the basement membrane matrix [10, 12, 13]. When proteinase or proteases of parasites are expressed on the cell surfaces and/or released into the extracellular environments, they often damage the host. An example is the action of E. histolytica cysteine proteinase on the disruption of the cysteine-rich MUC2 polymer of the host tissue [44].
Interestingly, we have detected an F-actin capping protein beta subunit (EHI_005020) that is involved in a variety of biological functions including metabolic process, biological regulation, cellular component organization/biogenesis, cellular process, and developmental process. In addition, it is also involved in binding activity by molecular function. The capping protein is involved in the motility of E. histolytica and is known to inhibit the elongation of the actin filament for the motility of the parasite [45]. The capping proteins are also part of a larger protein complex called Wiskott–Aldrich Scar Homology (WASH) whereby the complex is associated with the endosomes [46], thus explaining the presence of the capping protein in the extracellular environment.
We have also detected Calmodulin (EHI_023500) in the ES proteins of E. histolytica. Calmodulin (CaM) is involved in many categories under biological and molecular functions, including metabolic process, cellular process and localization, catalytic activity, and binding. CaM is associated with the secretion of electron-dense granules containing collagenase, as well as the growth and encystation of Entamoeba spp. [47, 48]. Makioka et al. [49] demonstrated the function of CaM in the excystation and metacystic development of E. invadens as a model for E. histolytica.
Within the biological function category of proteins related to response to stimulus, several heat shock proteins that respond to stress were identified. The 70 kDa heat shock protein (EHI_199590), also known as BiP, has been suggested to be involved in encystation in an experiment using E. invadens which showed 88% sequence homology to E. histolytica [50]. In addition, heat shock protein (HSP) 70 of E. histolytica was reported to stimulate immune responses in patients with amoebiasis [51]. Furthermore, amoebic HSPs have been suggested to be involved in the degradation of cytoskeletal proteins for the purpose of encystation [52, 53]. On the other hand, the presence of HSP may not indicate stress response, as a previous study demonstrated that HSP was present in both heated and unheated cells of Amoeba proteus [54].
By molecular functional analysis, a notable number of proteins that were classified under the binding category were actin binding proteins. Previously, Váazquez et al. [55] described four actin binding proteins (vinculin, α-actinin, tropomyosin and myosin I) of amoebic adhesion plaques/plates. In the present study, we identified actin-binding proteins (EHI_168340), cofilin/tropomyosin family (EHI_186840), and myosin heavy chain (EHI_110180). These proteins do not only exist on the surface of E. histolytica as a component in adhesion plaques, but also aid the parasite in locomotion and invasion of the host tissue [55].
Analysis by cellular component (Fig. 5) characterizes proteins according to their subcellular location and macromolecule complex level. A large number of proteins detected (54 proteins) belonged to the category of ‘cell part’, which includes intracellular and plasma membranes, followed by 31 proteins that are associated with the organelle (cytoskeleton), 4 extracellular region proteins, 2 proteins that are related to the membrane, and 3 proteins that are affiliated with the macromolecular complex.
This study has revealed proteins considered as surface membrane proteins. Slightly more than half of the proteins that we identified were also E. histolytica membrane surface proteome reported by Biller [39], such as disulphide isomerase, enolase, heat shock proteins, malate dehydrogenase, triosephosphate isomerase, thioredoxin, and superoxide dismutase. The identification of membrane-related proteins in the ES products may be due to membrane recycling. An example in Acanthamoeba demonstrated by Hohman and Bowers [56] showed that proteins secreted when trapped in the shuttle vesicles were transported from secondary lysosomes to the surface membrane.
Conclusion
The ES proteome of E. histolytica was studied using two methods of mass spectrometry analyses. The results showed that ES proteins are involved in the colonisation and evasion of the host immune system as well as in encystation and excystation. The results of this study can further our understanding of the pathogenesis of E. histolytica. In future research, ES proteins from parasites isolated from an infected animal can be analysed in order to provide deeper insights into the host-parasite interactions. Furthermore, real patient samples could be analysed to detect the presence of some of the identified and unknown proteins found in this study.
References
Ackers J, Clark CG, Diamond LS, Duchêne M, Cantellano ME, Jackson TF, Martínez-Palomo A, Mirelman D, Hernández OMWHO. PAHO, UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28–29 January, 1977. Epidemiol Bull PAHO. 1997;1997(18):13–4.
Petri WA, Singh U. Diagnosis and management of amebiasis. Clin Infect Dis. 1999;29:1117–25.
Gatti S, Swierczynski G, Robinson F, Anselmi M, Corrales J, Moreira J, Scaglia M. Amebic infections due to the Entamoeba histolytica–Entamoeba dispar complex: a study of the incidence in a remote rural area of Ecuador. Am J Trop Med Hyg. 2002;67(1):123–7.
Stanley SL. Amoebiasis. The Lancet. 2003;361:1025–34.
Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511–32.
Paul J, Srivastava S, Bhattacharya S. Molecular methods for diagnosis of Entamoeba histolytica in a clinical setting: an overview. Exp Parasitol. 2007;116:35–43.
Tanyuksel M, Petri WA. Laboratory diagnosis of amebiasis. Clin Microbiol Rev. 2003;16(4):713–29.
Akgun Y, Tacyildiz IH, Çelik Y. Amebic liver abscess: changing trends over 20 years. World J Surg. 1999;23:102–6.
Farthing MJ. Treatment options for the eradication of intestinal protozoa. Nat Clin Pract Gastroenterol Hepatol. 2006;3:436–45.
Keene WE. The major neutral proteinase of Entamoeba histolytica. J Exp Med. 1986;163:536–49.
Moncada D, Keller K, Chadee K. Entamoeba histolytica-secreted products degrade colonic mucin oligosaccharides. Infect Immun. 2005;73:3790–3.
Reed S, Bouvier J, Pollack AS, Engel JC, Brown M, Hirata K, et al. Cloning of a virulence factor of Entamoeba histolytica. Pathogenic strains possess a unique cysteine proteinase gene. J Clin Invest. 1993;91:1532–40.
Scholze H, Werries E. Cysteine proteinase of Entamoeba histolytica. Partial purification and action on different enzymes. Mol Biochem Parasitol. 1986;18:103–12.
Pal S, Sengupta K, Manna B, Sarkar S, Bhattacharya S, Das P. Comparative evaluation of somatic & excretory-secretory antigens of Entamoeba histolytica in serodiagnosis of human amoebiasis by ELISA. Indian J Med Res. 1996;104:152–6.
Wong WK, Tan ZN, Othman N, Lim BH, Mohamed Z, Garcia AO, et al. Analysis of Entamoeba histolytica excretory-secretory antigen and identification of a new potential diagnostic marker. Clin Vaccine Immunol. 2011;18:1913–7.
Saidin S, Yunus M, Zakaria N, Razak K, Huat L, Othman N, et al. Production of recombinant Entamoeba histolytica pyruvate phosphate dikinase and its application in a lateral flow dipstick test for amoebic liver abscess. BMC Infect Dis. 2014;14:182.
Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, García-Rivera G, Orozco E, Martínez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, Mckerrow JH, Reed SL. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18:956–60.
Quach J, St-Pierre J, Chadee K. The future for vaccine development against Entamoeba histolytica. Human Vaccines Immunother. 2014;10:1514–21.
Nten CMA, Sommerer N, Rofidal V, Hirtz C, Rossignol M, Cuny G, et al. Excreted/secreted proteins from Trypanosome Procyclic strains. J Biomed Biotechnol. 2009;2010:1–8.
Wong WK, Tan ZN, Lim BH, Mohamed Z, Olivos-Garcia A, Noordin R. Comparison of protein-free defined media, and effect of l-cysteine and ascorbic acid supplementation on viability of axenic Entamoeba histolytica. Parasitol Res. 2011;108:425–30.
Wong WK, Tan ZN, Boon Lim BH, Mohamed Z, Olivos-Garcia A, Noordin R. Comparison of protein-free defined media, and effect of l-cysteine and ascorbic acid supplementation on viability of axenic Entamoeba histolytica. Parasitol Res. 2011;108:425–30.
Bodnar WM, Blackburn RK, Krise JM, Moseley MA. Exploiting the complementary nature of LC/MALDI/MS/MS and LC/ESI/MS/MS for increased proteome coverage. J Am Soc Mass Spectrom. 2003;14:971–9.
Gonnet F, Lemaître G, Waksman G, Tortajada J. MALDI/MS peptide mass fingerprinting for proteome analysis: identification of hydrophobic proteins attached to eucaryote keratinocyte cytoplasmic membrane using different matrices in concert. Proteome Sci. 2003;1(1):1.
Tholey A, Heinzle E. Ionic (liquid) matrices for matrix-assisted laser desorption/ionization mass spectrometry—applications and perspectives. Anal Bioanal Chem. 2006;386:24–37.
Stapels MD, Barofsky DF. Complementary use of MALDI and ESI for the HPLC-MS/MS analysis of DNA-binding proteins. Anal Chem. 2004;76:5423–30.
Seymour S, Booy A, Gundry R, Eyk JV, Hunter C. Assessing the complementarities of MALDI and ESI for protein identification in complex mixtures. In: AB SCIEX; 2010.
Hansen KC, Gerold S, Robert JC, Hirsh J, Baldwin MA, Burlingame AL. Mass spectrometric analysis of protein mixtures at low levels using cleavable 13C-ICAT and multi-dimensional chromatography. Mol Cell Proteom. 2003. doi:10.1074/mcp#0021-MCP200.
Lubec G, Afjehi-Sadat L. Limitations and pitfalls in protein identification by mass spectrometry. ChemInform. 2007;107(8): 3568–84.
Pawlowski K. Uncharacterized/hypothetical proteins in biomedical ‘omics’ experiments: is novelty being swept under the carpet? Brief Funct Genom Proteom. 2008;7:283–90.
Choi M-H, Sajed D, Poole L, Hirata K, Herdman S, Torian BE, et al. An unusual surface peroxiredoxin protects invasive Entamoeba histolytica from oxidant attack. Mol Biochem Parasitol. 2005;143:80–9.
Wassmann C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem. 1999;274:26051–6.
Hughes MA, Lee CW, Holm CF, Ghosh S, Mills A, Lockhart LA, Reed SL, Mann BJ. Identification of Entamoeba histolytica thiol-specific antioxidant as a GalNAc lectin-associated protein. Mol Biochem Parasitol. 2003;127:113–20.
Sen A, Chatterjee NS, Akbar MA, Nandi N, Das P. The 29-kilodalton thiol-dependent peroxidase of Entamoeba histolytica is a factor involved in pathogenesis and survival of the parasite during oxidative stress. Eukaryot Cell. 2007;6:664–73.
Li E. Use of Chinese hamster ovary cells with altered glycosylation patterns to define the carbohydrate specificity of Entamoeba histolytica adhesion. J Exp Med. 1988;167:1725–30.
Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. J Clin Invest. 1981;68:1305–13.
Petri WA, Haque R, Mann BJ. The bittersweet interface of parasite and host: lectin–carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu Rev Microbiol. 2002;56:39–64.
Haque R, Mollah NU, Ali IKM, Alam K, Eubanks A, Lyerly D, Petri WA. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J Clin Microbiol. 2000;38(9):3235–9.
Tchórzewski M. The acidic ribosomal P proteins. Int J Biochem Cell Biol. 2002;34(8):911–5.
Biller L, Matthiesen J, Kuhne V, Lotter H, Handal G, Nozaki T, et al. The cell surface proteome of Entamoeba histolytica. Mol Cell Proteomics. 2014;13:132–44.
Crisóstomo-Vázquez MDP, Marevelez-Acosta VA, Flores-Luna A, Jiménez-Cardoso E. The MAK16 gene of Entamoeba histolytica and its identification in isolates from patients. Korean J Parasitol. 2014;52:429–33.
Shahi P, Trebicz-Geffen M, Nagaraja S, Alterzon-Baumel S, Hertz R, Methling K, et al. Proteomic identification of oxidized proteins in Entamoeba histolytica by resin-assisted capture: insights into the role of arginase in resistance to oxidative stress. PLOS Negl Trop Dis. 2016;10(1): e0004340. doi:10.137/journal.pntd.0004340.
Zinker S, Warner JR. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J Biol Chem. 1976;251(6):1799–807.
Mesri EA, Levitus G, Hontebeyrie-Joskowicz M, Dighiero G, Van Regenmortel MH, Levin MJ. Major Trypanosoma cruzi antigenic determinant in Chagas’ heart disease shares homology with the systemic lupus erythematosus ribosomal P protein epitope. J Clin Microbiol. 1990;28(6):1219–24.
Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci. 2006;103:9298–303.
Isenberg G, Aebi U, Pollard TD. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980;288:455–9.
Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–23.
Makioka A, Kumagai M, Ohtomo H, Kobayashi S, Takeuchi T. Effect of calcium antagonists, calcium channel blockers and calmodulin inhibitors on the growth and encystation of Entamoeba histolytica and E. invadens. Parasitol Res. 2001;87:833–7.
Muñoz MDL, Moreno MA, Pérez-Garcia JN, Tovar GR, Hernandez VI. Possible role of calmodulin in the secretion of Entamoeba histolytica electron-dense granules containing collagenase. Mol Microbiol. 1991;5:1707–14.
Makioka A, Kumagai M, Kobayashi S, Takeuchi T. Possible role of calcium ions, calcium channels and calmodulin in excystation and metacystic development of Entamoeba invadens. Parasitol Res. 2002;88:837–43.
Ali IKM, Haque R, Siddique A, Kabir M, Sherman NE, Gray SA, et al. Proteomic analysis of the cyst stage of Entamoeba histolytica. PLoS Negl Trop Dis. 2012;6(5):e1643. doi:10.1371/journal.pntd.0001643.
Ortner S, Plaimauer B, Binder M, Wiedermann G, Scheiner O, Duchene M. Humoral immune response against a 70-kilodalton heat shock protein of Entamoeba histolytica in a group of patients with invasive amoebiasis. Mol Biochem Parasitol. 1992;54:175–83.
Field J, Dellen K, Ghosh SK, Samuelson J. Responses of Entamoeba invadens to heat shock and encystation are related. J Eukaryot Microbiol. 2000;47:511–4.
Sherman MYS, Goldberg AL. Involvement of molecular chaperones in intracellular protein breakdown. In: Feige U, Yahara I, Morimoto RI, Polla BS, editors. Stress-inducible cellular responses, vol. 77. Basel: Birkhäuser; 1996. p. 57–78.
Podlipaeva YI. Heat shock protein of 70 kDa in Amoeba proteus. Protistology. 2001;2(2):123–29.
Váazquez J, Franco E, Reyes G, Meza I. Characterization of adhesion plates induced by the interaction of Entamoeba histolytica trophozoites with fibronectin. Cell Motil Cytoskelet. 1995;32:37–45.
Hohman TC, Bowers B. Hydrolase secretion is a consequence of membrane recycling. J Cell Biol. 1984;98(1):246–52.
Authors’ contributions
JU: Performed majority of the experiments, analysed the data and wrote the first draft of the manuscript. KSH: Performed the LC–ESI–MS/MS. MNI: Participated in the experimental design of LC–ESI–MS/MS, analysed the data and edited the manuscript. LBH: Provided the E. histolytica culture, established the protocol to isolate ES proteins and edited the manuscript. RN: Participated in the experimental design and data analysis; and edited the manuscript. NO: Principle investigator of the project, designed the experiment, analysed the whole data, wrote and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was funded by a short term grant from Universiti Sains Malaysia (304/CIPPM/6312134). We would like to thank Mr. Muhammad Hafiznur Yunus (Institute for Research in Molecular Medicine, Universiti Sains Malaysia) for his technical assistance in the MALDI-TOF/TOF analysis. In addition, this study is partly supported by HiCOE program, Malaysian Ministry of Higher Education.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data and material can be obtained from the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ujang, J.A., Kwan, S.H., Ismail, M.N. et al. Proteome analysis of excretory-secretory proteins of Entamoeba histolytica HM1:IMSS via LC–ESI–MS/MS and LC–MALDI–TOF/TOF. Clin Proteom 13, 33 (2016). https://doi.org/10.1186/s12014-016-9135-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12014-016-9135-8