Abstract
Background
Langerhans cell histiocytosis (LCH) is a proliferative disorder in which abnormal Langerhans cell (LC)-like cells (LCH cells) intermingle with inflammatory cells. Whether LCH is reactive or neoplastic remains a controversial matter. We recently described Merkel cell polyomavirus (MCPyV) as a possible causative agent of LCH and proposed interleukin-1 loop model: LCH is a reactive disorder with an underlying oncogenic potential and we now propose to test this theory by looking for acute markers of inflammation. We detected MCPyV-DNA in the peripheral blood cells of patients with high-risk organ-type (LCH-risk organ (RO) (+)) but not those with non–high-risk organ-type LCH (LCH-RO (−)); this difference was significant. LCH-RO (−) is further classified by its involvement of either a single organ system (SS-LCH) or multiple organ systems (MS-LCH). In patients with LCH-RO (−), MCPyV-DNA sequences were present in LCH tissues, and significant differences were observed between LCH tissues and control tissues associated with conditions such as dermatopathic lymphadenopathy and reactive lymphoid hyperplasia. Although MCPyV causes subclinical infection in nearly all people and 22 % of healthy adults will harbor MCPyV in their buffy coats, circulating monocytes could serve as MCPyV reservoirs and cause disseminated skin lesions.
Methods
Plasma sample from 12 patients with LCH-RO (−) (5 MS-LCH and 7 SS-LCH) and 5 non-LCH patients were analyzed by peptidomics. Mass spectrometry (MS) spectra were acquired and peptides exhibiting quantitative differences between MS-LCH and SS-LCH patients were targeted.
Results
One new candidate biomarker, m/z 3145 was selected and identified after obtaining a MS/MS fragmentation pattern using liquid chromatography-MS/MS. This peak was identified as a proteolytic fragment derived from inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4, [PDB: Q14624]).
Conclusions
Peptidomics of LCH have revealed that the level of acute-phase ITIH4 distinguishes MS-LCH-RO (−) from SS-LCH-RO (−). Acute-phase proteins serve non-specific, physiological immune functions within the innate immune system. LCH may be a reactive disorder with both underlying neoplastic potential of antigen presenting cells harboring BRAF mutations and hyper-immunity of other inflammatory cells against MCPyV infection. Among LCH-RO (−), MCPyV-DNA sequences were present in both MS-LCH tissues and SS-LCH tissues without significant differences. ITIH4 may show that LCH activity or LCH subtypes correlates with the systemic or localized reactions of MCPyV infection.
Similar content being viewed by others
Background
LCH is a proliferative disorder in which abnormal Langerhans cell (LC)-like cells (LCH cells) intermingle with inflammatory cells [1, 2]. The appearance of LCH lesions under the microscope, which commonly contain only a small number of abnormal immune LCH cells surrounded by many normal immune cells initially led to questions about LCH as a cancer or immune disorder [3]. For decades, it was thought that the disease is a reactive disorder rather than a neoplastic process [4]. The discovery of the B-Rapidly Accelerated Fibrosarcoma gene (BRAF) [GenBank: G_007873] mutation in 2010 [5] gave new insights into LCH pathogenesis. Clonality [6, 7] and BRAF mutation [5] suggest neoplasm [1], whereas granuloma formation [8] with spontaneous regression [1], and hypercytokinemia [1, 9] indicate a reactive process. BRAF mutation does not distinguish LCH subclasses, which vary from self-healing to lethal [5]. Recently, there has been much discussion concerning whether or not LCH is considered a cancer. This discussion started when a recent change to patient information about LCH on the National Cancer Institute’s website [10] stated, “LCH is a type of cancer that can damage tissue or cause lesions to form in one or more places in the body.” On the contrary, the Histiocyte Society Scientific Committee [3] stated “All cancers are considered neoplasias, but not all neoplasias are cancerous.” At present time no clear consensus seem to have been reached regarding whether LCH is reactive or neoplastic [1] and whether LCH is cancerous [10] or not [3].
Very recently, we [11] propose a new model for LCH pathogenesis in which the disease is a reactive disorder with underlying neoplastic potential. In other words, LCH is an inflammatory process that is prolonged by BRAF mutation (interleukin (IL)-1 loop model) [11].
The liver, spleen, and bone marrow are considered high-risk organs for LCH [12, 13]. LCH is classified as involving at least one [LCH-risk organ (RO) (+)] or no high-risk organs [LCH-RO (−)] [12]. LCH-RO (−) presents as multisystem (MS-LCH) or single-system disease (SS-LCH) [14]. Nearly all LCH-RO (+) is MS-LCH-RO (+), although SS-LCH-RO (+) has been reported [15, 16]. We [17] reported Merkel cell polyomavirus (MCPyV)-DNA in the peripheral blood mononuclear cells (PBMC) of patients with LCH-RO (+), whereas MCPyV-DNA was significantly restricted to lesional LCH cells in patients with LCH-RO (−) and so we predict that acute phase markers are related to inflammatory activities of LCH (Table 1) [2]. Berres et al. [18] reported that circulating CD11c + and CD14+ cellular fractions in patients with LCH-RO (+) harbored BRAF mutations; the mutation was restricted to LCH cells in patients with LCH-RO (−).
The clinical course of LCH varies quite widely depending on the extent of organ involvement [2] and relationship between LCH subtypes and MCPyV and mutations are shown roughly in Table 1. Treatment of LCH should be planned according to the clinical presentation and the extent of organ involvement [2]. In LCH-RO (+), in which circulating precursor LCH cells as a reservoirs for MCPyV may relate to involving high risk organ ((extramedullary) hematopoietic organ) (Table 1), main aims of treatment are to increase survive and to reduce the incidence of late sequelae [2]. In LCH-RO (−), treatment differs widely from a wait-and-see approach for SS-LCH to systemic chemotherapy for MS-LCH [2]. However, the clinical significance of MCPyV infection and BRAF mutation has not been settled because they occur equally as frequently in MS-LCH-RO (−) and SS-LCH-RO (−) (Table 1) [5].
There are no definite specific laboratory markers for LCH. In many cases, LCH presents with nonspecific inflammatory signs that arise from chronic inflammation [2], although comprehensive analysis of serum levels of cytokines/chemokines and growth factors in pediatric patients with LCH was done [19].
As mentioned above, we predict that acute phase markers are related to inflammatory activities of LCH subtypes (Table 1) and demonstrate, via plasma peptidomics, that acute-phase ITIH4 (inter-alpha-trypsin inhibitor heavy chain 4) levels can distinguish MS-LCH-RO (−) from SS-LCH-RO (−) in this paper.
Methods
Patients and plasma samples
This study was approved by the Institutional Review Boards of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama, Japan; Faculty of Medicine, Tottori University, Tottori, Japan; and Jichi Medical University School of Medicine, Tochigi, Japan. After obtaining written informed consent, plasma samples were obtained from affected patients in the Japan LCH Study Group registry between 2002 and 2009 and Jichi Medical University School of Medicine.
Plasma sample aliquots from 12 patients with LCH-RO (−) (5 MS-LCH and 7 SS-LCH) were stored at −80 °C until further analysis. For confirmative studies, plasma samples were obtained from 5 non-LCH patients at the Division of Pediatrics and Perinatology, Faculty of Medicine, Tottori University.
Whole-plasma peptidome analyses (Peptidomics)
The whole-plasma peptidome was directly analyzed via a lithium dodecyl sulfate (LDS)-based 1-D polyacrylamide gel electrophoresis (PAGE)/matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS)-based rapid quantitative method using BLOTCHIP® (Protosera, Amagasaki, Japan) [20]: plasma samples were treated with NuPAGE LDS sample buffer (Life Technologies, Carlsbad, CA, USA), heated for 10 min at 70 °C, and applied to NuPAGE Novex Bis-Tris Mini Gels 4–12 % (Life Technologies, No gel is shown). After 1-D PAGE, slab gels were sliced into gel strips; two strips were placed on each chip. Peptides contained in the gel strips were electroblotted onto the chip using an XCell IITM Blot Module (Life Technologies). MALDI matrix-cyano-4-hydroxycinnamic acid (CHCA) (Sigma-Aldrich, MO, USA) was applied to each BLOTCHIP® using an automatic matrix-dispensing machine (Protosera).
MS spectra were acquired on an UltraFlex II MALDI-time of flight (TOF)/TOF (Bruker Daltonics, Billerica, MA, USA) interfaced with flexAnalysis version 2.4 software (Bruker Daltonics) as previously described [20] under the following conditions: laser intensity, 28–37 %; detector voltage, 1685 V; suppression, 500, fuzzy mode; and molecular mass range, m/z 1000–30,000.
All plasma sample measurements were repeated four times. The resulting 29 MS spectra per each measurement were combined using flexAnalysis version 2.4 to generate an integrated MS spectrum in the molecular mass range of m/z 1000–20,000. The mean relative peak intensities, normalized to a total ion current of m/z between 1000 and 6000, were expressed as arbitrary units (a.u.) [21].
Measurement of monoisotopic masses on BLOTCHIP®
Monoisotopic masses of statistically significant peaks were recorded in reflector mode on an Ultraflex II instrument (Bruker Daltonics).
Purification and MS/MS identification of peptides
Plasma samples were selected for peptide purification as follows: peptides exhibiting quantitative differences between MS-LCH and SS-LCH patients were targeted based on the statistical analysis of MS data. Blood samples containing the highest target peptide concentrations were used by Protosera as sources for further identification studies.
MS/MS spectra of the target peptides were obtained via liquid chromatography (LC)-MS/MS (Q Exactive, Thermo Fisher Scientific K. K., Yokohama, Japan); fragmentation data were applied to a “nonredundant” human database search (both NCBInr and Swiss-Prot) via the MASCOT MS/MS ions search program version 2.1.0 (Matrix Science, Boston, MA, USA) in Biotools software (Bruker Daltonics).
Statistical analysis
All statistical MS data analyses were conducted using ClinPro Tools version 2.2 (Bruker Daltonics) [22]. Peak heights exhibiting significant statistical differences between two groups (MS-LCH versus SS-LCH) were selected. MS data intensity comparisons between patients with MS-LCH and SS-LCH were performed using Student’s t-test. Differences between values were considered statistically significant at P < 0.05.
Results and discussion
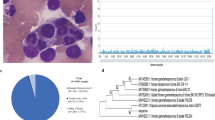
Clinical profiles of patients in this study with LCH and non-LCH are summarized in Table 2. Plasma samples from LCH (n = 12) and non-LCH patients (n = 5) were subjected to BLOTCHIP® followed by MS analysis. Subsequent differential profiling analyses of the two sample sets were performed based on data obtained from the MS analysis. Quantitative differences in peptide peaks between the groups were noticeable primarily in the 2000–6500 m/z range. Statistical analysis indicated that 32 peptide peaks differed significantly between MS-LCH and SS-LCH patients at an average m/z of <6000 (Table 3 and Fig. 1a).
Plasma peptidomics of patients with LCH-RO (−). (a) Blue and red lines indicate integrated MS spectra of each MS-LCH and SS-LCH sample, respectively. All plasma sample measurements were repeated four times. The resulting 29 MS spectra per each measurement were combined using flexAnalysis version 2.4 to generate an integrated MS spectrum. Statistical analysis indicated that 32 peptide peaks differed significantly between MS-LCH and SS-LCH patients at an average m/z of <6000. Peaks (arbitrary unit [a.u.]) in the range of m/z 2000–5000, which contains m/z 3145 (arrow), are shown. (b) Blue and red lines indicate each MS spectrum of MS-LCH and SS-LCH samples, respectively. Peaks in the range of m/z 3127–3162, which contains m/z 3145 (arrow), are shown. (c) Blood samples containing the highest concentrations of the target peptide m/z 3145 were used for further identification studies. MS/MS spectra of the target peptides were obtained via LC-MS/MS (Q Exactive). The MS/MS fragmentation data of m/z 3145 were applied to a “nonredundant” human database search (both NCBInr and Swiss-Prot) using MASCOT MS/MS ions search program version 2.1.0 in Biotools software. MS/MS fragmentations of NFRPGVLSSRQLGLPGPPDVPDHAAYHPF, a peptide found in inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4, [PDB: Q14624]), were detected (ion score: 89; expect: 0.00062). Y-axis: relative intensity. (d) A profile summary of peptide fragment m/z 3145 (ITIH4). The amino acid sequence is presented according to the Paris Convention guidelines for peptidomics data presentation. (e) Peptidomics data indicating the intensities of m/z 3145 (ITIH4 fragment) in MS-LCH, SS-LCH, and non-LCH plasma samples were plotted as box-whisker plots. For the MS-LCH, SS-LCH, and non-LCH samples, the median intensities are 15710.537, 2412.950, and 14157.62538, lower quartiles are 4081.623, 1583.592, and 12148.52916, and upper quartiles are 17688.457, 3635.496, and 20523.77099, respectively. P values (Student’s t-test) are also indicated (An asterisk means P > 0.05. A double asterisk means P < 0.05. A triple asterisk means P < 0.01). LCH-RO (−), LCH with no involvement of high-risk organ (spleen, liver, and bone marrow)
Three new candidate biomarkers, m/z 2555, m/z 2962 and m/z 3145 (Fig. 1b), differed quantitatively between MS-LCH and SS-LCH patients and were selected. Among three peptides, only m/z 3145 was identified after obtaining a MS/MS fragmentation pattern using LC-MS/MS (Fig. 1c). This peak was identified as a proteolytic fragment derived from ITIH4, ([PDB: Q14624]; Integrated into UniProtKB/Swiss-Prot: July 15, 1998) (Fig. 1d).
ITIH4 is an acute-phase-related protein [23] and potential new biomarker for distinguishing MS-LCH and SS-LCH (Table 3 and Fig. 1e). Acute-phase proteins serve non-specific, physiological immune functions within the innate immune system [24]. ITIH4 has been detected in animals during experimental bacterial and viral infections [25].
MCPyV causes subclinical infection in nearly all people [26]. Although 22 % of healthy adults will harbor MCPyV in their buffy coats [27], circulating monocytes could serve as MCPyV reservoirs and cause disseminated skin lesions [28]. Martel-Jantin et al. [29] reported seroprevalence rate of MCPyV antibodies of children 12 months or younger (49/105) in Cameroon and pointed out the presence of maternal antibodies in very young children. Their data indicated that MCPyV infections mostly occurred during early childhood, after the disappearance of specific maternal antibodies [29]. On the contrary Tolstov et al. [30] reported seroprevalence rate of MCPyV antibodies of children of 1 year or younger (0/6) in patients with LCH. We [17] identified a relationship between LCH and MCPyV. MCPyV-DNA in PBMC correlated with LCH-RO (+) [17]. Among patients with LCH-RO (−) (MS-LCH and SS-LCH), MCPyV-DNA was restricted to lesional LCH cells [17], and we predict that primary MCPyV infection may influence the LCH subtype involving cells in an early-activated state [4].
Generally, no response is observed after secondary viral infection [25]. Primary respiratory syncytial virus infection at 6 months or younger often induces severe disease [31], although nearly all children are infected by 2–3 years of age [32]. Primary Epstein-Barr virus and cytomegalovirus infections in elderly individuals cause a severe condition called infectious mononucleosis; again, nearly all children are infected with these viruses [33]. Although no response is observed after MCPyV infection [26, 27], Kumar et al. [34], however, found that MCPyV-specific T helper cells (in vitro model of a secondary infection) secrete several cytokines, including IL-10. IL-10 is an anti-inflammatory cytokine and is one of cytokines produced in LCH [2, 35]. ITIH4 production is up-regulated by IL-6 [23], which is known produced in LCH [2], one of the mediators coordinating the interface between adaptive and innate immunity [36], and might be a target for the therapy [37]. Innate immune function between newborns and elderly is extremely different and large quantities of IL-6 after stimulation of receptors, such as Toll like receptor, by term newborns are indicated [38]. In LCH, MCPyV infection may induce hyper-immunity in both LCH cells [11] and other inflammatory cells [1, 2]. Increased mRNA expression of BRAF, which was proven in MCPyV-positive non-small cell lung cancer [39], may also influence BRAF mutated precursor LCH cells.
Conclusions
LCH is a proliferative disorder in which LCH cells intermingle with inflammatory cells [1, 2]. Very recently, we [11] proposed a new model for LCH pathogenesis in which the disease is a reactive disorder with both underlying neoplastic potential of LCH cells and other inflammatory cells such as T cells, macrophages, and eosinophils. In other words, LCH is an inflammatory process that is prolonged by mutations of antigen presenting cells (IL-1 loop model). The other inflammatory cells may be informed and instigated by the mutated antigen presenting cells. We tested this theory by examining the abundance of a well-established acute phase marker ITIH4. The presence of the acute phase markers observed is compatible with our proposed theory. Cytokine storm is one of characteristics of LCH and the fact that serum levels of some cytokines but not LCH histology, which is so uniform that pathologists cannot determine whether a given biopsy originates from a patient with LCH-RO (+) or LCH-RO (−) [40], deeply correlate to LCH activity and LCH subtypes [19, 41–44] explicitly reach this inflammatory reactive model in LCH.
Peptidomics suggests that the systemic or localized reaction against MCPyV may influence LCH activity or subtypes. Primary infection without maternal immunoglobulins against MCPyV [29, 30] may influence LCH activity or subtypes. In the aspect of data validation, an orthogonal quantitation method has to be conducted, e.g., an ELISA for absolute quantification of ITIH4 in the future.
References
Weitzman S, Egeler RM. Histiocytic disorders of children and adults: introduction to the problem, overview, historical perspective and epidemiology. In: Weitzman S, Egeler RM, editors. Histiocytic disorders of children and adults. Cambridge: Cambridge University Press; 2005. p. 1–13.
Morimoto A, Oh Y, Shioda Y, Kudo K, Imamura T. Recent advances in Langerhans cell histiocytosis. Pediatr Int. 2014;56:451–61.
Is Langerhans cell histiocytosis a cancer? In: Histiocytosis Association’s web site. https://histio.org/feb-2015/news/is-lch-a-cancer?srctid=1&erid=1082042&trid=03b181ee-42d5-4703-a572-cba81b5dc45e. Accessed 26 March 2015.
da Costa CE, Annels NE, Egeler RM. The immunological basis of Langerhans cell histiocytosis. In: Weitzman S, Egeler RM, editors. Histiocytic disorders of children and adults. Cambridge: Cambridge University Press; 2005. p. 66–82.
Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–23.
Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, et al. Langerhans’-cell histiocytosis (histiocytosis X)–a clonal proliferative disease. N Engl J Med. 1994;331:154–60.
Yu RC, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;343:767–8.
Jaffe R, Weiss LM, Facchetti F. Tumours derived from Langerhans cells. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 358–60.
Egeler RM, Favara BE, van Meurs M, Laman JD, Claassen E. Differential in situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999;94:4195–201.
General Information About Langerhans Cell Histiocytosis (LCH). In: Langerhans cell histiocytosis treatment (PDQ®) (National Cancer Institute’s web site). http://www.cancer.gov/cancertopics/pdq/treatment/lchistio/Patient. Accessed 26 March 2015.
Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, Nagata K, et al. Interleukin-1 loop model for pathogenesis of Langerhans cell histiocytosis. Cell Commun Signal. 2015;13:13.
Donadieu J, Egeler RM, Pritchard J. Langerhans cell histiocytosis: a clinical update. In: Weitzman S, Egeler RM, editors. Histiocytic disorders of children and adults. Cambridge: Cambridge University Press; 2005. p. 95–129.
Ronceray L, Potschger U, Janka G, Gadner H, Minkov M, German Society for Pediatric Hematology and Oncology, Langerhans Cell Histiocytosis Study Group. Pulmonary involvement in pediatric-onset multisystem Langerhans cell histiocytosis: effect on course and outcome. J Pediatr. 2012;161:129–33.
Chu T, D’Angio GJ, Favara B, Ladisch S, Nesbit M, Pritchard J. Histiocytosis syndromes in children. Lancet. 1987;1:208–9.
Kaplan KJ, Goodman ZD, Ishak KG. Liver involvement in Langerhans’ cell histiocytosis: a study of nine cases. Mod Pathol. 1999;12:370–8.
Finn LS, Jaffe R. Langerhans’ cell granuloma confined to the bile duct. Pediatr Pathol Lab Med. 1997;17:461–8.
Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, Horie Y, et al. Merkel cell polyomavirus DNA sequences in peripheral blood and tissues from patients with Langerhans cell histiocytosis. Hum Pathol. 2014;45:119–26.
Berres ML, Lim KP, Peters T, Price J, Takizawa H, Salmon H, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J Exp Med. 2014;211:669–83.
Morimoto A, Nakamura S, Shioda Y, Imamura T, Oh Y, Imashuku S, et al. Comprehensive analysis of serum levels of cytokines/chemokines and growth factors in pediatric patients with Langerhans cell histiocytosis [abstract]. Pediatr Blood Cancer. 2011;56:696.
Tanaka K, Tsugawa N, Kim YO, Sanuki N, Takeda U, Lee LJ. A new rapid and comprehensive peptidome analysis by one-step direct transfer technology for 1-D electrophoresis/MALDI mass spectrometry. Biochem Biophys Res Commun. 2009;379:110–4.
Sogawa K, Satoh M, Kodera Y, Tomonaga T, Iyo M, Nomura F. A search for novel markers of alcohol abuse using magnetic beads and MALDI-TOF/TOF mass spectrometry. Proteomics Clin Appl. 2009;3:821–8.
Araki Y, Nonaka D, Tajima A, Maruyama M, Nitto T, Ishikawa H, et al. Quantitative peptidomic analysis by a newly developed one-step direct transfer technology without depletion of major blood proteins: its potential utility for monitoring of pathophysiological status in pregnancy-induced hypertension. Proteomics. 2011;11:2727–37.
Pineiro M, Alava MA, Gonzalez-Ramon N, Osada J, Lasierra P, Larrad L, et al. ITIH4 serum concentration increases during acute-phase processes in human patients and is up-regulated by interleukin-6 in hepatocarcinoma HepG2 cells. Biochem Biophys Res Commun. 1999;263:224–9.
Braciale TJ, Hahn YS, Burton DR. Adaptive immune response to viral infections. In: Knipe DM, Howley PM, editors. Fields virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, a Wolter Kluwer business; 2013. p. 214–53.
Pineiro M, Andres M, Iturralde M, Carmona S, Hirvonen J, Pyorala S, et al. ITIH4 (inter-alpha-trypsin inhibitor heavy chain 4) is a new acute-phase protein isolated from cattle during experimental infection. Infect Immun. 2004;72:3777–82.
Foulongne V, Kluger N, Dereure O, Mercier G, Moles JP, Guillot B, et al. Merkel cell polyomavirus in cutaneous swabs. Emerg Infect Dis. 2010;16:685–7.
Pancaldi C, Corazzari V, Maniero S, Mazzoni E, Comar M, Martini F, et al. Merkel cell polyomavirus DNA sequences in the buffy coats of healthy blood donors. Blood. 2011;117:7099–101.
Mertz KD, Junt T, Schmid M, Pfaltz M, Kempf W. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2010;130:1146–51.
Martel-Jantin C, Pedergnana V, Nicol JT, Leblond V, Tregouet DA, Tortevoye P, et al. Merkel cell polyomavirus infection occurs during early childhood and is transmitted between siblings. J Clin Virol. 2013;58:288–91.
Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250–6.
Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98.
Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6.
Bravender T. Epstein-Barr virus, cytomegalovirus, and infectious mononucleosis. Adolesc Med State Art Rev. 2010;21:251–64.
Kumar A, Chen T, Pakkanen S, Kantele A, Soderlund-Venermo M, Hedman K, et al. T-helper cell-mediated proliferation and cytokine responses against recombinant Merkel cell polyomavirus-like particles. PLoS One. 2011;6:e25751.
Senechal B, Elain G, Jeziorski E, Grondin V, de Serre Patey-Mariaud N, Jaubert F, et al. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Med. 2007;4:e253.
Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–19.
Kolde G, Schulze P, Sterry W. Mixed response to thalidomide therapy in adults: two cases of multisystem Langerhans’ cell histiocytosis. Acta Derm Venereol. 2002;82:384–6.
Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–83.
Lasithiotaki I, Antoniou KM, Derdas SP, Sarchianaki E, Symvoulakis EK, Psaraki A, et al. The presence of Merkel cell polyomavirus is associated with deregulated expression of BRAF and Bcl-2 genes in non-small cell lung cancer. Int J Cancer. 2013;133:604–11.
Delprat C, Arico M. Blood spotlight on Langerhans cell histiocytosis. Blood. 2014;124:867–72.
Murakami I, Morimoto A, Oka T, Kuwamoto S, Kato M, Horie Y, et al. IL-17A receptor expression differs between subclasses of Langerhans cell histiocytosis, which might settle the IL-17A controversy. Virchows Arch. 2013;462:219–28.
Ishii R, Morimoto A, Ikushima S, Sugimoto T, Asami K, Bessho F, et al. High serum values of soluble CD154, IL-2 receptor, RANKL and osteoprotegerin in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2006;47:194–9.
Coury F, Annels N, Rivollier A, Olsson S, Santoro A, Speziani C, et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med. 2008;14:81–7.
Lourda M, Olsson-Akefeldt S, Gavhed D, Bjornfot S, Clausen N, Hjalmars U, et al. Detection of IL-17A-producing peripheral blood monocytes in Langerhans cell histiocytosis patients. Clin Immunol. 2014;153:112–22.
Chilosi M, Facchetti F, Calio A, Zamo A, Brunelli M, Martignoni G, et al. Oncogene-induced senescence distinguishes indolent from aggressive forms of pulmonary and non-pulmonary Langerhans cell histiocytosis. Leuk Lymphoma. 2014;55:2620–6.
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31.
Acknowledgments
The authors thank the physicians who participated in this study.
Funding disclosure
This work was partly supported by JSPS KAKENHI (Grants-in-Aid for Scientific Research (C)) Numbers 23590426 and 26460451 and research grants 2011, 2012, 2013, and 2014 from the Japan LCH Study Group.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IM and KH conceived and wrote the initial study proposal; IM, MM, TI, SK, MK, and KN conducted the research; HS, SK, YO, AM, and SI conducted the clinical study; KH, SI, and JG contributed to report writing; KH, JG, FJ, TO, and TY supervised the project; and all authors read and approved the manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Murakami, I., Oh, Y., Morimoto, A. et al. Acute-phase ITIH4 levels distinguish multi-system from single-system Langerhans cell histiocytosis via plasma peptidomics. Clin Proteom 12, 16 (2015). https://doi.org/10.1186/s12014-015-9089-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12014-015-9089-2