Abstract
Background
For a patient presenting with fever, multiple lymphadenopathy and splenomegaly, pathogen infection should be preferentially considered, followed by lymphoid malignancies. When traditional laboratory and pathological detection cannot find the pathogenic microorganism, metagenomic sequencing (MGS) which targets the person’s genome for exceptional genetic disorders may detect a rare pathogen.
Case presentation
Here, we introduced the diagnostic clue of a case of multicentric Castleman disease (MCD) with hemophagocytic syndrome which was elicited from the detection of human herpesvirus-8 in the blood of a HIV-1 infected person by MGS technology during pathogen inspection. This case highlights the need to increase the awareness of MCD among clinicians and pathologists.
Conclusions
MGS technology may play a pivotal role in providing diagnostic clues during pathogen inspection, especially when pathogens are not detectable by conventional methods.
Similar content being viewed by others
Background
Fever, multiple lymphadenopathy and splenomegaly often suggest pathogen infection or lymphoid malignancies. The ability to find the etiology of such symptoms timely is still lacking. Multicentric Castleman disease (MCD) is a rare disease that exhibit lymphadenopathy in > 1 lymph node station as well as a wide spectrum of clinical manifestations, including constitutional symptoms, fluid accumulation, cytopenia, and liver and kidney dysfunction [1]. It also represents a lymphoreticular complication of acquired immunodeficiency syndrome. Human herpes virus-8 (HHV8) was identified as an etiological driver of MCD [2]. Immune deficiency is the primary risk factor for MCD and investigators also noted an association between HIV and MCD [3]. Histopathologic examination of lymph node could help establish the diagnosis. However, if there is no sufficient clinical information provided for pathologist, no right diagnosis could be made. The nonspecific symptoms and conventional clinical practice usually could not make a timely and accurate diagnosis of MCD.
Here, we presented a case of HHV8-positive MCD with hemophagocytic syndrome (HPS) in HIV infection and introduced the diagnostic clue which was elicited from the detection of HHV8 in the plasma of a HIV-1 infected person by metagenomic sequencing (MGS) technology during pathogen detection.
Case presentation
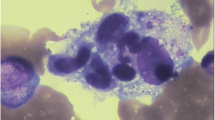
A 49-year-old man with confirmed HIV-1 infection 14 days before treated with lamivudine, tenofovir and efavirenz was admitted with a 3-month history of fatigue, weight loss and fever. He smokes occasionally but denies any alcohol or illicit drug use. He also reported unprotected sex. He has splenomegaly and generalized lymphadenopathy occurred in the neck, axilla, mediastinum and celiac on physical examination and computed tomography scan. A detailed clinical work-up for infection, immune and malignant diseases were performed. His CD4 count was 48 cells/μl and HIV viral load was 8596 copies/ml. Laboratory tests showed pancytopenia, elevated erythrocyte sedimentation rate and C-reactive protein, polyclonal hypergammaglobulinemia and hypoalbuminemia. Serological testing for cryptococcus, Epstein Barr virus, cytomegalovirus was unrevealing (Table 1). Furthermore, peripheral blood cultures incubated for 5 days were also negative. Hemophagocytosis could be seen on bone marrow smears (Fig. 1a), complied with fever, splenomegaly, three-line cytopenia, high level of serum ferritin and soluble CD25, supporting the diagnosis of HPS. A lymph node biopsy with the highest standardized uptake value (SUV = 6.0) provided by a positron emission tomography–computed tomography (PET–CT) scan revealed nonspecific lymphocyte proliferation. Pathological imaging of lymph node biopsy showed nonspecific lymphocyte proliferation and excluded malignancy.
a Hemophagocytosis image in bone marrow. b Mapping of 5621 human herpesvirus-8 (HHV8) reads derived from the patient’s peripheral blood sample. c The distribution of viral sequences identified in the patient’s peripheral blood. d Phylogenetic analysis showed sequences from representative serovars, strains, and species of HHV8. The scale bar denoted the number of nucleotide substitutions per site
To find the cause of HPS, a MGS assay of plasma was performed and the result revealed a HHV8 viremia of 5621 unique reads with coverage of identified viral genes 94.93% (Fig. 1b–d). HHV8-associated diseases were further considered. No Kaposi’s sarcoma evidence was found on the skin, oral and gastrointestinal mucosa by endoscopy. Then the evidence of HHV8 viral load test positive demonstration of in situ hybridization and histopathology evaluation on the lymph node tissue confirmed HHV8-associated multicentric Castleman disease (HHV8-MCD). It was characterized by the presence of sheets of plasma cell in the interfollicular zone. Prominent high endothelial venules could be observed in the interfollicular region. Moreover, the lymphoid follicles were dissolved, with atrophic germinal centers (Fig. 2a, b). Treatment with 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone and combined with ganciclovir for anti-HHV8 treatment in the context of HIV infection, improved the patient’s condition. Nevertheless, the Charlson Comorbidity Index was 8, which suggests poor prognosis.
a Hematoxylin and eosin staining of lymph node showed plentiful plasma cell infiltration in perifollicular and mantle zone. The follicle had an atrophic germinal center. b Positive HHV8 expression in plasma cells localized in perifollicular and mantle zone by immunohistochemical staining (original magnification ×200)
Discussion and conclusions
HHV8 infection is found to correlate with Kaposi's sarcoma (KS) and hematologic diseases, including primary effusion lymphoma and MCD [4]. Here, we have documented a case of HHV8-MCD which was diagnosed based on the detection of HHV8 in the plasma of a HIV-1 infected person by MGS technology and histopathologic findings. It was difficult to diagnosis without HHV8 stains because its histological features can be similar to other diseases such as HIV lymphadenitis.
For a case of fever unknown origin, pathogenic microorganism inspection commonly does not include HHV8 detection. But specific HHV8 detection is suggested for a HIV-1 infected patient with fever and lymphatic hyperplasia, because immunodeficiency increases the risk of HHV8 infection. MGS assay may provide a valuable diagnostic support for HHV8 infection [5]. Further, for a HIV/AIDS patient with HHV8 co-infection, KS and Castleman disease (CD) should be considered [6]. HPS is a reactive disorder of the reticuloendothelial system, and occasionally be found in HIV/AIDS patients with malignancies, severe opportunistic infections or autoimmune diseases [7]. CD with HPS is extremely rare and it is difficult to differentiate HPS and CD due to their similar presentations [8]. Thus, HHV8 finding may provide a diagnostic clue for CD with HPS.
To our knowledge, previously documented case of HHV8-associated MCD was mainly diagnosed by the histologic confirmation of lymph node [9, 10]. However, the diagnostic clues and process remain unclear. Here, we presented the diagnostic procedure of the infectious disease, which may provide reference for the diagnosis of unusual pathogens.
In conclusion, physicians should be expanding the knowledge for diseases presenting with fever and lymphadenopathy and be aware about the possibility of MCD in patients with immunodeficiency. The diagnosis might be difficult, but the etiology should be repeatedly sought. MGS may provide a diagnostic clue for infection-associated diseases.
Abbreviations
- MGS:
-
Metagenomic sequencing
- MCD:
-
Multicentric Castleman disease
- HHV8:
-
Human herpes virus-8
- HPS:
-
Hemophagocytic syndrome
- PET–CT:
-
Positron emission tomography–computed tomography
- HHV8-MCD:
-
HHV8-associated multicentric Castleman disease
- KS:
-
Kaposi's sarcoma
- CD:
-
Castleman disease
References
Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135(16):1353–64.
Fajgenbaum DC, Shilling D. Castleman disease pathogenesis. Hematol Oncol Clin North Am. 2018;32(1):11–21.
Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–80.
Campogiani L, Cerva C, Maffongelli G, Teti E, Pupo L, Vaccarini S, et al. Remission of an HHV8-related extracavitary primary effusion lymphoma in an HIV-positive patient during antiretroviral treatment containing dolutegravir. AIDS Res Ther. 2019;16(1):15.
The Human Microbiome Project Consortium. A framework for human microbiome research. Nature 2012; 486(7402):215–221.
Suda T, Katano H, Delsol G, Kakiuchi C, Nakamura T, Shiota M, et al. HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman’s disease. Pathol Int. 2001;51(9):671–9.
Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814–22.
Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet (London, England). 2014;383(9927):1503–16.
Osakwe N, Johnson D, Klein N, Azim DA. A rare case of HHV-8 associated hemophagocytic lymphohistiocytosis in a stable HIV Patient. Case Rep Infect Dis. 2019;2019:3297463.
Zondag TC, Rokx C, van Lom K, van den Berg AR, Sonneveld P, Dik WA, et al. Cytokine and viral load kinetics in human herpesvirus 8-associated multicentric Castleman’s disease complicated by hemophagocytic lymphohistiocytosis. Int J Hematol. 2016;103(4):469–72.
Acknowledgements
We are thankful to the patient for the support given in providing the data.
Funding
This work was funded by the National Natural Science Foundation of China (Grant Numbers 81873761 and 81672026); Youan Foundation of Liver Disease and AIDS (Grant Number YNKTTS20180123); Beijing Fengtai Health System Research Project (Grant Number 2018–63) and National Science and Technology Major Special Program of the 13th Five-Year Plan of China (Grant Numbers 2018ZX10302104 and 2018ZX10302205-002–002). The funders play a role in study design, collection, management, analysis and interpretation of data, the study supervision and providing important intellectual content.
Author information
Authors and Affiliations
Contributions
XC and YW collected clinical data and drafted the manuscript; LJ interpreted the data and critical revised the manuscript; JC performed the pathological diagnosis; CL performed the lymph node biopsy; CG, TZ and YM revised the manuscript for important intellectual content; YZ obtained funding and performed study supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with regulations issued by the National Health Commission of China and was approved by the local Commission of Ethics (reference number LL 2019-053-K). Informed consent was obtained from the participant included in the study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, X., Wu, Y., Jia, L. et al. A case of Castleman disease with hemophagocytic syndrome derived from HHV8 infection. Eur J Med Res 26, 119 (2021). https://doi.org/10.1186/s40001-021-00589-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-021-00589-5