Abstract
Background
Higher age and cognitive impairment are associated with a higher risk of falling. Wearable sensor technology may be useful in objectively assessing motor fall risk factors to improve physical exercise interventions for fall prevention. This systematic review aims at providing an updated overview of the current research on wearable sensors for fall risk assessment in older adults with or without cognitive impairment. Therefore, we addressed two specific research questions: 1) Can wearable sensors provide accurate data on motor performance that may be used to assess risk of falling, e.g., by distinguishing between faller and non-faller in a sample of older adults with or without cognitive impairment?; and 2) Which practical recommendations can be given for the application of sensor-based fall risk assessment in individuals with CI? A systematic literature search (July 2019, update July 2020) was conducted using PubMed, Scopus and Web of Science databases. Community-based studies or studies conducted in a geriatric setting that examine fall risk factors in older adults (aged ≥60 years) with or without cognitive impairment were included. Predefined inclusion criteria yielded 16 cross-sectional, 10 prospective and 2 studies with a mixed design.
Results
Overall, sensor-based data was mainly collected during walking tests in a lab setting. The main sensor location was the lower back to provide wearing comfort and avoid disturbance of participants. The most accurate fall risk classification model included data from sit-to-walk and walk-to-sit transitions collected over three days of daily life (mean accuracy = 88.0%). Nine out of 28 included studies revealed information about sensor use in older adults with possible cognitive impairment, but classification models performed slightly worse than those for older adults without cognitive impairment (mean accuracy = 79.0%).
Conclusion
Fall risk assessment using wearable sensors is feasible in older adults regardless of their cognitive status. Accuracy may vary depending on sensor location, sensor attachment and type of assessment chosen for the recording of sensor data. More research on the use of sensors for objective fall risk assessment in older adults is needed, particularly in older adults with cognitive impairment.
Trial registration
This systematic review is registered in PROSPERO (CRD42020171118).
Similar content being viewed by others
Introduction
With increasing age, cognitive function and motor abilities decline and risk of falling increases [1]. One in three individuals over the age of 65 years experiences one or more falls in any given year, and this prevalence increases to 40% among individuals aged 80 years and older [2]. Falling often leads to severe injuries, hospitalization, loss of autonomy in activities of daily living, reduced quality of life, and an accelerated need for help in older adults [2, 3]. Furthermore, fall-related mortality increases with age [4]. Individuals with cognitive impairment (CI) fall twice as often as their unimpaired peers and have a threefold increased risk of suffering a bone fracture after a fall [5, 6].
A number of motor disabilities are known to be related to a higher risk of falling [7, 8], for example negative changes in gait under single and dual task conditions, balance or lower extremity strength [1, 3, 9]. In individuals with CI, accelerated decline of motor performance is associated with an increased risk of falling as compared to cognitively unimpaired older adults [10, 11].
Physical exercise interventions in fall prevention are promising, as they are associated with improved gait performance, balance and mobility in older adults [12, 13]. Therefore, the identification and quantification of modifiable fall risk factors may be important for the design of effective physical rehabilitation or fall prevention programs that specifically address the needs and burdens of older individuals at high risk of falling [14]. Since falling events in geriatric settings are usually recorded by fall diaries implying a higher risk to recall bias [15], there is a need for the identification and investigation of fall-related factors that may serve as more reliable indicators of a person’s fall risk than recorded total number of prior falls.
To date, such key factors of motor performance are commonly assessed using questionnaires, scales or objective clinician-rated functional performance tests, such as the Short Physical Performance Battery (SPPB) [16] or the Timed- Up and Go Test (TUG) [17], usually evaluated by timekeeping or scoring. Nevertheless, not all of these assessments are feasible, particularly for older individuals with CI [18] and the scales often show a high inter-rater variability [19].
Within the last ten years, wearable technology providing objective data has become more prevalent in clinical settings [20]. Small and lightweight body-worn sensors like accelerometers or gyroscopes hold great promise in the field of fall detection, but also in fall risk assessment [2, 21]. Moreover, these devices are more economic than gold standard methods of motion analysis systems [22] and more applicable in clinical and non-clinical settings as their high level of portability allows the examination of human motion in field instead of laboratory testing [23].
Fall detection using wearable sensors can reduce fall-related injuries and healthcare costs, and is often used as an alarm system in case of an emergency, i.e. accidental fall. The recognition of fall events can be used to trigger helping systems (e.g. alarming signals to caregivers) and may help to understand the mechanism underlying the fall incident [24, 25]. Thereby, fall detection systems may prevent an individual from remaining in a helpless position on the floor for an extended period of time [25]. A recent review on single and multiple sensor-based fall detection concluded wearable sensor-based solutions to be of accuracy to detect fall-events in older adults [25]. Nevertheless, fall detection systems using multiple input sources may lead to high costs and their use is often restricted to indoor locations [25, 26]. Furthermore, fall detection systems may help to identify external fall risk (e.g., uneven ground) but they are limited in providing information about internal fall risk factors, e.g., dysfunctional patterns of gait or required motor tasks that are of interest to conceptualize fall prevention strategies. To this end, using wearable sensors for fall risk assessment may comprehensively capture characteristics of different motor tasks allowing an estimation of human motion (e.g. spatio-temporal characteristics of balance or gait or transfer performance from sitting to standing) [20, 27].
Current reviews on body-worn sensors for the assessment of fall risk focus either on methodological aspects such as applied classification methods and model assessment outcomes, or on practical aspects such as type, number and location of sensors and are often limited to older people without CI [2, 20, 27,28,29]. Moreover, most of published reviews are limited to either a supervised or a unsupervised setting or included studies with other quantitative measures like instrumented walkways or motion capturing systems [30].
Therefore, the overarching aim of the present systematic review was to provide an overview and update of the existing body of literature that examined the feasibility of body-worn sensors for the assessment of motor fall risk among older adults. Furthermore, we deliberately aimed at including studies that focused on older adults with CI to give practical advice on the use of wearable sensors in individuals with CI. To this end, we addressed two specific research questions: 1) Can wearable sensors provide accurate data on motor performance that may be used to assess risk of falling, e.g., by distinguishing between faller and non-faller in a sample of older adults with or without cognitive impairment?; and 2) Which practical recommendations can be given for the application of sensor-based fall risk assessment in individuals with CI?
The following paragraphs contain a detailed description of the methodological procedure of this systematic review, i.e. search strategy, study selection and data synthesis. In the results section we present study design, detection of fall status, use of sensors to assess fall risk, and classification models of the included studies. Furthermore, results of studies including individuals with CI are presented separately. Finally, we summarize our findings in accordance with the objectives with this systematic review and discuss the strengths and limitations as well as practical implications.
Methods
Protocol
We followed the Preferred Reporting Item for Systematic review and Meta-Analysis (PRISMA) guidelines in preparing this systematic review [31]. Furthermore, we registered this review in PROSPERO (CRD42020171118).
Search strategy and eligibility criteria
We performed a literature search using PubMed, Web of Science and Scopus databases with no time filter set. Articles were searched using the following combination of key words: (fall risk OR fall risk factor*) AND (sensor* OR objectively measured OR objective measurement OR acceleromet*). Population or cognitive status were not included in the search term because we did not want to restrict our results, for example by potentially excluding articles that had mixed study populations. Rather, we deliberately kept our literature search as inclusive as possible. No filter was applied at this stage. The complete literature search can be found in supplementary material (Additional file 1). We screened the reference lists of included articles for relevant secondary literature. The initial database search was conducted in July 2019 with an updated search in August 2020. The following inclusion criteria for the studies were defined:
-
a)
Original research articles in peer reviewed journals in English language;
-
b)
Studies including individuals with a mean age of 60 years or older, with or without presence of CI;
-
c)
Studies assessing fall-related motor performance using body-worn, sensor-based tools in a clinical or community-based setting or in nursing homes, and;
-
d)
Studies sub-dividing their sample into fallers and non-fallers, or into individuals at high and low fall risk based on prospective or retrospective falls, clinical assessments or the combination of these methods.
Studies were excluded if a) the mean age of the reported sample was younger than 60 years, b) the individuals showed concomitant severe chronical conditions (e. g., stroke, Parkinson’s Disease), and c) only environmental sensor-based systems (e.g. 2D video analysis) were applied. As the focus on fall risk assessment may provide more pertinent information that enables the design of new preventive approaches, i.e., physical exercise interventions, we also excluded studies with the purpose of fall detection.
Study selection
After detection and removal of duplicates, two authors (JB and JKR) independently screened all titles and abstracts of the literature search. Both authors repeated this process by screening the abstracts (or full texts if more information was needed) of the remaining articles based on the above defined inclusion criteria. In case of any discrepancy, a third author (TE) was consulted. If there was disagreement about the final inclusion of an article, the third author read the full text and made a final decision. Literature management was performed using Citavi Software (Version 6.3.0.0, Swiss Academic Software GmbH).
Data extraction and data synthesis
First, relevant data of the included studies were independently extracted and systematically recorded by two authors (JB and JKR) using a standardized data extraction form. Second, the collected data was cross-checked to ensure complete and correct data extraction. We extracted first author’s name, publication date, study design, sample size and population characteristics (i.e., sex, age, cognitive status). We also collected information on fall classification methods that was used to differentiate between fallers and non-fallers or individuals at high and low fall risk. Additionally, the following specific characteristics about the use of body-worn sensors were collected: type of sensor(s), location of sensor(s), activities while sensor data were collected (e.g., during clinical assessment of the TUG) and the parameters of sensor data collected. Furthermore, results of prediction models were extracted and accuracy, sensitivity and specificity were extracted. Accuracy is defined as the ability to discriminate between fallers and non-fallers or between people at high and people at low fall risk. Sensitivity describes the true-positive proportion and specificity describes the true-negative proportion. An accuracy of 50% means that no discrimination exists and that this performance can be achieved by chance [32]. After data extraction, one author (JB) synthesized the data.
Assessment of methodological quality
Two authors (JB and JKR) independently assessed the methodological quality of each study included in this systematic review using the Newcastle-Ottawa Scale (NOS) for cross-sectional and for prospective or cohort studies [33, 34]. The scale uses an evaluation system with stars across three categories, i.e. selection (cross-sectional: 0–5 stars; prospective: 0–4 stars), comparability (cross-sectional: 0–2 stars; prospective: 0–2 stars) and outcome (cross-sectional: 0–3 stars; prospective: 0–3 stars). A higher number of total stars (cross-sectional: range 0–10; prospective: 0–9) reflects a higher study quality with regard to the respective categories.
Results
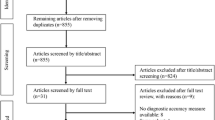
After the identification of 527 studies and the screening of 307 abstracts, 82 full-text articles were checked for the inclusion criteria. Finally, a total of 27 studies were included in this systematic review (Fig. 1). An updated search in July 2020 resulted in one additional article. Screening the reference lists resulted in no additional articles.
Study design and sample characteristics
The included studies were published between 2009 and 2020. Sixteen of the included studies had a cross-sectional design, ten studies had a prospective / longitudinal design and two studies combined cross-sectional with prospective design. The follow-up period of included prospective studies differed between 2 and 24 months. Seventeen studies were conducted in a supervised setting (e. g., clinical setting), whereas six studies collected unsupervised sensor data during daily life. Five studies combined the two settings.
A total of 2896 participants (range = 35–303; 65% females) were included in the studies. Twenty studies included community-dwelling participants, eight studies were conducted in patients who were hospitalized or residing in a geriatric care facility. The mean age of the older participant groups ranged between 68 and 86 years. Two studies [19, 35] included younger control groups with a mean age between 21 and 35 years, however, we did not consider these groups for the purpose of this review. CI was an exclusion criterion in most of the studies (n = 14) and only one study deliberately focused on older people with dementia [36]. To determine cognitive status, the Mini-Mental-State Examination (score 0–30) [37], the MiniCog (score 0–5) [38] or the Short Orientation-Memory-Concentration Test (score 0–26) [39] were used. The remaining studies did not explicitly state CI as an exclusion criterion but required to be able to understand the test instructions. Further information about study characteristics and the main findings of the studies is presented in Table 1.
Detection of fall status
Classification into fallers and non-fallers or in older adults at high risk or low risk of falling was conducted using three different methods: retrospective assessment (e.g., fall history questionnaire), prospective assessment (e.g., fall diaries) or clinical assessment of fall risk (e.g., Tinetti Score, TUG, SPPB). Moreover, five of the studies combined two of these methods (Table 1). The majority of studies compared fallers and non-fallers. A faller was defined as a person having at least one fall over a certain period of time, usually the past or prospective 12 months. Eight studies compared older adults at high and low risk of falling [19, 40,41,42] or non-fallers and multiple fallers [43,44,45,46]. Multiple fallers were defined as participants that had fallen at minimum twice during the investigation period.
Use of sensors to assess fall risk
To obtain the data, the included studies used between one and five inertial sensors. That were mainly located close to the centre of the body at the lower back [40, 42,43,44,45,46,47,48,49,50,51,52,53,54] or legs [43, 50, 55,56,57,58] of the participants. Less frequently used sensor locations were chest [19, 58,59,60], pelvis [41, 56, 57], waist [61, 62], foot [19, 45, 46], head [56, 57] and wrist [63]. The majority of the studies used sensor-derived data to distinguish between the different fall status groups or for fall classification during clinical testing (e. g. gait analysis under single or dual task conditions. Nine studies assessed walking and other related tasks during daily life, i.e. in homes of participants with a duration of three to eight days (Table 2).
Classification models
Nineteen studies applied different types of machine learning models (e.g. receiver operating curves, Naïve Bayes, decision tree) and logistic regression analysis in order to correctly assign the study participants to the right category (e.g. faller and non-faller) using the sensor data. Besides sensor-derived variables, four studies also included height, body mass index, age [19, 54, 58], fall efficacy and information processing speed [50]. Prediction models achieved sensitivities between 48.1 and 91.3%, specificities between 66.3 and 100.0% and accuracies between 68.0 and 90.0% (Table 3). When comparing the analysed classification models of the different assessment conditions, the best model was found for daily-life data of three consecutive days with accuracy of 90.6%, sensitivity of 91.7% and specificity of 89.2% [35]. The models with sensor-derived data of laboratory assessment were on average not as precise, but accuracies, sensitivities and specificities were still acceptable (best in-lab data model [50]: accuracy = 89.4%, sensitivity = 92.7%, specificity = 84.9%).
Results for individuals with CI
Since most of the included studies were conducted in a community setting, participants with severe CI are less likely to have participated. In addition to the only study that included individuals with severe dementia [36], five studies were conducted in a geriatric or hospital setting but provided no information concerning the cognitive status of their participants [19, 42, 48, 61, 62] and three more studies did not explicitly exclude participants with CI [44, 52, 64]. Overall, these nine studies may reveal information about the use and ability of sensors and sensor-derived data to distinguish between groups of fall status or to predict fall risk in a sample of older individuals with CI.
Six of the nine studies [36, 44, 48, 52, 62, 64] had a prospective design with between six- and 24-months follow-up. Three studies had a cross-sectional design [19, 42, 61] and collected sensor-derived data during clinical assessments. Sensors were placed at the lower back [44, 48, 52, 64], the shank [36], the waist [61] and the chest [19, 42] and sensor data were collected within seven [36, 44] or eight [52] days of daily-life, a 20-m gait analysis [48, 61], the TUG [42, 62], the Tinetti Test [19] or a walking test [64]. Only two studies gave information on how the sensor was applied to the participant’s body. In the study of Gietzelt et al. [36], sensors were applied by instructed nursing staff while in the study of van Schooten et al. [52] study participants had to attach the sensor by themselves.
For daily-life data of gait quality (e.g. gait velocity, step frequency) classification models of those studies including older adults with CI revealed accuracies between 68.0–76.0%, sensitivities of 67.0–78.2% and specificities of 66.3–80.0% [36, 44, 52] and therefore performed worse than the best model found for individuals without CI [35]. For sensor data collected during clinical assessments accuracies of 70.0–90.0%, sensitivities of 50.0–86.0% and specificities of 73.9–100.0% were achieved [19, 61, 62, 64].
Quality assessment
All studies included in this systematic review used reasonable methodology (Table 4) measured with NOS. Most studies did not apply randomized stratified sampling. Furthermore, not all included studies controlled for age and sex differences or other important factors resulting in a lower evaluation of the category “comparability”. Overall, cross-sectional and prospective studies achieved a mean score of six stars out of ten and nine total stars.
Discussion
As a consequence of the aging process, falls are a major issue in geriatric populations and require special consideration in the design and conduct of effective physical exercise interventions. Therefore, a comprehensive understanding of motor performance is required to detect underlying fall risk factors more precisely. Assessment of motor performance in geriatric settings is usually based on scales, questionnaires and time-keeping, and wearable sensors may present a more objective and reliable approach. This systematic review provides an update of the existing body of literature concerning the assessment of fall risk factors in motor performance using wearable sensors with a special consideration of older adults with CI.
All studies included in this systematic review, except for one prospective study [48], found that sensor-derived data are successful in distinguishing between groups of faller status, or are useful in fall classification models. When classification ability of sensor data was compared to conventional clinical assessment, sensor-derived variables outperformed data of clinical assessment [56]. Wearable sensors may thus be considered a good alternative to conventional clinical assessment methods for fall risk assessment.
With regard to the setting of data collection, our review shows that data derived from both daily-life and clinical assessments was used to predict, classify or distinguish between groups of fall status. For in-lab sensor-based gait analysis, using the mean of at least two walks for more reliable data was recommended [40]. Furthermore, gait features may differ depending on walking distance [40] and longer walking distance in clinical assessment may better reflect everyday walking [57]. Nevertheless, sensor data of in-lab assessments might be biased because participants might be affected by the awareness of direct observation or cameras and therefore might not behave naturally (e. g. adjustment of gait) [35, 41, 45, 46]. Hence, daily-life data might better represent everyday functioning and fall-risk than data collected in an in-lab setting [35, 45, 65].
With regard to sensor wearing time, some studies comprised data collection from three up to eight consecutive days. A full week of recording sensor data may cover the range of motor performance of older adults better than a time span of only three days [54], however, drop-out rate may be higher and feasibility may worsen with increasing wearing time. In addition, it may be important to not only take into account sensor data from gait but also from different activities, like sit-to-stand transitions [52].
When assessing sensor data during daily-life, various environmental conditions cannot be controlled. Moreover, movement behaviour in daily-life does not follow a protocol, so the amount of sensor data might differ significantly between study participants [35]. In contrast, in a supervised setting (e. g. nursing homes or hospitals), all participants are assessed in the same facility and environmental conditions are standardized and comparable [48].
The placement of the sensors differed within the included studies. The most-often used sensor location was the lower back for which a high user acceptance was reported in previous studies [66]. However, Howcroft et al. [56] examined different sensor positions and concluded that sensors placed at the head or pelvis provided the best classification capability among single-sensor models. Only one study group used wrist-worn sensors for detection of sit-to-stand transitions, but the performance was comparable to studies using waist-worn devices [63]. An advantage of wrist-worn sensors might be the non-intrusiveness and the similarity to a wristwatch [67].
Several parameters of motor performance identified through sensor data may provide valuable information about motor deficits that are associated with fall risk, as well as indications for further fall prevention programs. Interestingly, sensor-derived parameters that were associated with fall risk were not associated with clinical fall risk assessments (e.g. TUG). This may indicate that not all fall-related movements can be detected by conventional clinical assessments [55], thereby highlighting the importance of body-worn sensors. To overcome the potential limitations of clinical assessments, a combination of daily-life sensor data and outcomes of clinical assessments to improve fall prediction was recommended [43, 44].
Although individuals with CI represent the group with the highest risk of falling in older adults, they are often excluded from studies examining sensor-based methods to assess fall risk. Therefore, the secondary aim of this systematic review was to provide practical recommendations for using sensors in fall risk assessment in individuals with CI. Since recording of data during daily-life provides slightly better results, this may be one approach to consider for individuals with CI. The daily-life recording in the included studies ranged from three to eight days and was considered feasible regardless of the cognitive status of included participants. Previous studies with individuals with CI and dementia also reported good feasibility of sensor-based data collection of up to three days [68,69,70]. Recording of daily-life data should thus be preferred to in-lab data collection as individuals with CI are more likely to be affected from test instructions or external distraction [71].However, individuals with CI may be less active during the day which may hamper collection of high-quality data [72].
Furthermore, it must be noted that both the location and the method of attachment of sensors appear to be of high importance when collecting sensor-based data on individuals with CI. The application of more than one sensor may provide more detailed information but is less practicable in this target group [19]. In addition, particularly in individuals with CI, researchers or instructed nursing staff need to be present to assume or supervise the placement and correct wearing position of the sensor [36, 61]. From a practical point of view, the location of the sensor should be carefully chosen, and clinicians and researchers may want to ensure that participants are not disturbed by the device [67, 73]. Moreover, researchers and/ or clinicians may need to consider technical aspects such as battery life span, data transmission or storage capacity when selecting an appropriate sensor for research or clinical practice [19].
Furthermore, some studies concluded, that additional information concerning other fall-risk related factors (e. g. age) might improve fall prognosis [36], and more studies are needed to examine the interplay between cognitive functioning and motor performance for fall risk assessment [45].
Strength and limitations
To the best of our knowledge, this review was the first to particularly focus on, and to also provide practical implications for using body-worn sensors in fall risk assessment in individuals with CI. However, several limitations must be noted. For example, we included studies with different study designs, which may limit the comparability of findings between studies. Furthermore, regarding our secondary aim, we only identified one study particularly focusing on individuals with CI. Therefore, we also considered studies not explicitly excluding individuals with CI for our practical recommendations. Nevertheless, this limits our ability to make assumptions about the use and practicability of wearable sensors in persons with CI. More research is needed to address this important topic, particularly as individuals with CI exhibit more gait abnormalities such as asymmetry as compared to persons without CI. In addition, besides motor performance, cognitive abilities as well as other factors such as medication intake, mental health, or support from caregivers also play a significant role when assessing risk of falling [11]. However, this review solely focused on sensor-based characteristics of motor performance. Of note, wearable sensors are also widely used in fall detection which we did not address with our systematic review. Combining wearable sensors for both fall risk assessment as well as fall detection may thus be an effective prevention strategy in clinical settings.
Conclusion
In conclusion, wearable sensors appear to be feasible tools to assess fall risk in older adults regardless of CI, in both an in-lab setting and during daily-life when measured for a period of up to eight days. Overall, sensor-derived data of daily-life were more useful in distinguishing between or predicting groups of faller status, indicating that the wide range of variables from daily-life data provides more valuable information about fall risk as compared to data collected in an in-lab setting. Similar results were observed when focusing on older adults with CI. Nonetheless, there exists a considerable lack of studies particularly examining sensor-based fall risk assessment in individuals with CI. Future research is needed to further specify which sensor-derived parameters of motor performance measured in daily life are most accurate and reliable predictors of fall risk. Furthermore, more research should focus on use of wearable sensors for fall risk assessment in older adults with CI to improve exercise programs for fall prevention.
Abbreviations
- CI:
-
Cognitive impairment
- NOS:
-
Newcastle-Ottawa Scale
- PRISMA:
-
Preferred Reporting Item for Systematic review and Meta-Analysis
- SPPB:
-
Short Physical Performance Battery
- TUG:
-
Timed-Up and Go Test
References
Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51–61. https://doi.org/10.1016/j.maturitas.2013.02.009.
Patel M, Pavic A, Goodwin VA. Wearable inertial sensors to measure gait and posture characteristic differences in older adult fallers and non-fallers: a scoping review. Gait Posture. 2020;76:110–21. https://doi.org/10.1016/j.gaitpost.2019.10.039.
Fernando E, Fraser M, Hendriksen J, Kim CH, Muir-Hunter SW. Risk factors associated with falls in older adults with dementia: a systematic review. Physiother Can. 2017;69(2):161–70. https://doi.org/10.3138/ptc.2016-14.
Joshi A, Rajabali F, Turcotte K, Beaton MD, Pike I. Fall-related deaths among older adults in British Columbia: cause and effect of policy change. Inj Prev. 2020;26:412–6. https://doi.org/10.1136/injuryprev-2019-043280.
Cox C, Vassallo M. Fear of falling assessments in older people with dementia. Rev Clin Gerontol. 2015;25(02):98–106. https://doi.org/10.1017/S0959259815000106.
Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil. 2011;8:2.
Damián J, Pastor-Barriuso R, Valderrama-Gama E, de Pedro-Cuesta J. Factors associated with falls among older adults living in institutions. BMC Geriatr. 2013;13:6.
Mohler MJ, Wendel CS, Taylor-Piliae RE, Toosizadeh N, Najafi B. Motor performance and physical activity as predictors of prospective falls in community-dwelling older adults by frailty level: application of wearable technology. Gerontology. 2016;62(6):654–64. https://doi.org/10.1159/000445889.
Huijben B, van Schooten KS, van Dieen JH, Pijnappels M. The effect of walking speed on quality of gait in older adults. Gait Posture. 2018;65:112–6. https://doi.org/10.1016/j.gaitpost.2018.07.004.
Taylor ME, Delbaere K, Lord SR, Mikolaizak AS, Brodaty H, Close JCT. Neuropsychological, physical, and functional mobility measures associated with falls in cognitively impaired older adults. J Gerontol A Biol Sci Med Sci. 2014;69(8):987–95. https://doi.org/10.1093/gerona/glt166.
Zhang W, Low L-F, Schwenk M, Mills N, Gwynn JD, Clemson L. Review of gait, cognition, and fall risks with implications for fall prevention in older adults with dementia. Dement Geriatr Cogn Disord. 2019;48(1–2):17–29. https://doi.org/10.1159/000504340.
Chan WC, Yeung JWF, Wong CSM, Lam LCW, Chung KF, Luk JKH, et al. Efficacy of physical exercise in preventing falls in older adults with cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(2):149–54. https://doi.org/10.1016/j.jamda.2014.08.007.
Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424.
Thibaud M, Bloch F, Tournoux-Facon C, Brèque C, Rigaud AS, Dugué B, et al. Impact of physical activity and sedentary behaviour on fall risks in older people: a systematic review and meta-analysis of observational studies. Eur Rev Aging Phys Act. 2012;9(1):5–15. https://doi.org/10.1007/s11556-011-0081-1.
Rapp K, Becker C, Cameron ID, König H-H, Büchele G. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of bavarian nursing homes. J Am Med Dir Assoc. 2012;13(2):187.e1–6.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. https://doi.org/10.1093/geronj/49.2.M85.
Podsiadlo D, Richardson S. The timed "up & go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x.
Trautwein S, Maurus P, Barisch-Fritz B, Hadzic A, Woll A. Recommended motor assessments based on psychometric properties in individuals with dementia: a systematic review. Eur Rev Aging Phys Act. 2019;16(1):20. https://doi.org/10.1186/s11556-019-0228-z.
Rivolta MW, Aktaruzzaman M, Rizzo G, Lafortuna CL, Ferrarin M, Bovi G, et al. Evaluation of the Tinetti score and fall risk assessment via accelerometry-based movement analysis. Artif Intell Med. 2019;95:38–47. https://doi.org/10.1016/j.artmed.2018.08.005.
Montesinos L, Castaldo R, Pecchia L. Wearable inertial sensors for fall risk assessment and prediction in older adults: a systematic review and meta-analysis. IEEE Trans Neural Syst Rehabil Eng. 2018;26(3):573–82. https://doi.org/10.1109/TNSRE.2017.2771383.
Bet P, Castro PC, Ponti MA. Fall detection and fall risk assessment in older person using wearable sensors: a systematic review. Int J Med Inform. 2019;130:103946. https://doi.org/10.1016/j.ijmedinf.2019.08.006.
Kluge F, Gaßner H, Hannink J, Pasluosta C, Klucken J, Eskofier BM. Towards Mobile Gait Analysis: Concurrent Validity and Test-Retest Reliability of an Inertial Measurement System for the Assessment of Spatio-Temporal Gait Parameters. Sensors (Basel). 2017;17(7):1522. https://doi.org/10.3390/s17071522.
Díaz S, Stephenson JB, Labrador MA. Use of wearable sensor Technology in Gait, balance, and range of motion analysis. Appl Sci. 2020;10(1):234.
Chaccour K, Darazi R, El Hassani AH, Andres E. From fall detection to fall prevention: a generic classification of fall-related systems. IEEE Sensors J. 2017;17(3):812–22. https://doi.org/10.1109/JSEN.2016.2628099.
Nooruddin S, Islam MM, Sharna FA, Alhetari H, Kabir MN. Sensor-based fall detection systems: a review. J Ambient Intell Humaniz Comput. 2021. https://doi.org/10.1007/s12652-021-03248-z.
Shu F, Shu J. An eight-camera fall detection system using human fall pattern recognition via machine learning by a low-cost android box. Sci Rep. 2021;11(1):2471. https://doi.org/10.1038/s41598-021-81115-9.
Howcroft J, Kofman J, Lemaire ED. Review of fall risk assessment in geriatric populations using inertial sensors. J Neuroeng Rehabil. 2013;10(1):91. https://doi.org/10.1186/1743-0003-10-91.
Gillain S, Boutaayamou M, Beaudart C, Demonceau M, Bruyère O, Reginster JY, et al. Assessing gait parameters with accelerometer-based methods to identify older adults at risk of falls: a systematic review. Eur Geriatr Med. 2018;9(4):435–48. https://doi.org/10.1007/s41999-018-0061-3.
Sun R, Sosnoff JJ. Novel sensing technology in fall risk assessment in older adults: a systematic review. BMC Geriatr. 2018;18(1):14. https://doi.org/10.1186/s12877-018-0706-6.
Dolatabadi E, van Ooteghem K, Taati B, Iaboni A. Quantitative mobility assessment for fall risk prediction in dementia: a systematic review. Dement Geriatr Cogn Disord. 2018;45(5–6):353–67. https://doi.org/10.1159/000490850.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–93. https://doi.org/10.1126/science.3287615.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.; 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Assessed 2020 Sep 10.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0147601. https://doi.org/10.1371/journal.pone.0147601.
Iluz T, Weiss A, Gazit E, Tankus A, Brozgol M, Dorfman M, et al. Can a body-fixed sensor reduce Heisenberg's uncertainty when it comes to the evaluation of mobility? Effects of aging and fall risk on transitions in daily living. J Gerontol A Biol Sci Med Sci. 2016;71(11):1459–65. https://doi.org/10.1093/gerona/glv049.
Gietzelt M, Feldwieser F, Goevercin M, Steinhagen-Thiessen E, Marschollek M. A prospective field study for sensor-based identification of fall risk in older people with dementia. Inform Health Soc Care. 2014;39(3–4):249–61. https://doi.org/10.3109/17538157.2014.931851 .
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Borson S, Scanlan JM, Chen P, Ganguli M. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–4. https://doi.org/10.1046/j.1532-5415.2003.51465.x.
Wade DT, Vergis E. The short orientation-memory-concentration test: a study of its reliability and validity. Clin Rehabil. 1999;13(2):164–70. https://doi.org/10.1191/026921599673848768.
Bautmans I, Jansen B, van Keymolen B, Mets T. Reliability and clinical correlates of 3D-accelerometry based gait analysis outcomes according to age and fall-risk. Gait Posture. 2011;33(3):366–72. https://doi.org/10.1016/j.gaitpost.2010.12.003.
Hua A, Quicksall Z, Di C, Motl R, LaCroix AZ, Schatz B, et al. Accelerometer-based predictive models of fall risk in older women: a pilot study. NPJ Digit Med. 2018;1(1):25. https://doi.org/10.1038/s41746-018-0033-5.
Zakaria NA, Kuwae Y, Tamura T, Minato K, Kanaya S. Quantitative analysis of fall risk using TUG test. Comput Methods Biomech Biomed Engin. 2015;18(4):426–37. https://doi.org/10.1080/10255842.2013.805211.
Bizovska L, Svoboda Z, Janura M, Bisi MC, Vuillerme N. Local dynamic stability during gait for predicting falls in elderly people: a one-year prospective study. PLoS One. 2018;13(5):e0197091. https://doi.org/10.1371/journal.pone.0197091.
Ihlen EAF, van Schooten KS, Bruijn SM, van Dieen JH, Vereijken B, Helbostad JL, et al. Improved prediction of falls in community-dwelling older adults through phase-dependent entropy of daily-life walking. Front Aging Neurosci. 2018;10:44. https://doi.org/10.3389/fnagi.2018.00044.
Mancini M, Schlueter H, El-Gohary M, Mattek N, Duncan C, Kaye J, et al. Continuous monitoring of turning mobility and its association to falls and cognitive function: a pilot study. J Gerontol A Biol Sci Med Sci. 2016;71(8):1102–8. https://doi.org/10.1093/gerona/glw019.
Wang K, Delbaere K, Brodie MAD, Lovell NH, Kark L, Lord SR, et al. Differences between gait on stairs and flat surfaces in relation to fall risk and future falls. IEEE J Biomed Health Inform. 2017;21(6):1479–86. https://doi.org/10.1109/JBHI.2017.2677901.
Brodie MAD, Menz HB, Smith ST, Delbaere K, Lord SR. Good lateral harmonic stability combined with adequate gait speed is required for low fall risk in older people. Gerontology. 2015;61(1):69–78. https://doi.org/10.1159/000362836.
Buckinx F, Beaudart C, Slomian J, Maquet D, Demonceau M, Gillain S, et al. Added value of a triaxial accelerometer assessing gait parameters to predict falls and mortality among nursing home residents: a two-year prospective study. Technol Health Care. 2015;23(2):195–203. https://doi.org/10.3233/THC-140883.
Ihlen EAF, Weiss A, Bourke A, Helbostad JL, Hausdorff JM. The complexity of daily life walking in older adult community-dwelling fallers and non-fallers. J Biomech. 2016;49(9):1420–8. https://doi.org/10.1016/j.jbiomech.2016.02.055.
Qiu H, Rehman RZU, Yu X, Xiong S. Application of wearable inertial sensors and a new test battery for distinguishing retrospective fallers from non-fallers among community-dwelling older people. Sci Rep. 2018;8(1):16349. https://doi.org/10.1038/s41598-018-34671-6.
Senden R, Savelberg H, Grimm B, Heyligers IC, Meijer K. Accelerometry-based gait analysis, an additional objective approach to screen subjects at risk for falling. Gait Posture. 2012;36(2):296–300. https://doi.org/10.1016/j.gaitpost.2012.03.015.
van Schooten KS, Pijnappels M, Rispens SM, Elders PJM, Lips P, van Dieen JH. Ambulatory fall-risk assessment: amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(5):608–15. https://doi.org/10.1093/gerona/glu225.
Weiss A, Herman T, Plotnik M, Brozgol M, Giladi N, Hausdorff JM. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol Meas. 2011;32(12):2003–18. https://doi.org/10.1088/0967-3334/32/12/009.
Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, et al. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair. 2013;27(8):742–52. https://doi.org/10.1177/1545968313491004.
Greene BR, Doheny EP, Walsh C, Cunningham C, Crosby L, Kenny RA. Evaluation of falls risk in community-dwelling older adults using body-worn sensors. Gerontology. 2012;58(5):472–80. https://doi.org/10.1159/000337259.
Howcroft J, Lemaire ED, Kofman J. Wearable-sensor-based classification models of faller status in older adults. PLoS One. 2016;11(4):e0153240. https://doi.org/10.1371/journal.pone.0153240.
Howcroft J, Lemaire ED, Kofman J, WE MI. Dual-Task Elderly Gait of Prospective Fallers and Non-Fallers: A Wearable-Sensor Based Analysis. Sensors (Basel). 2018;18(4):1275. https://doi.org/10.3390/s18041275.
Sample RB, Kinney AL, Jackson K, Diestelkamp W, Bigelow KE. Identification of key outcome measures when using the instrumented timed up and go and/or posturography for fall screening. Gait Posture. 2017;57:168–71. https://doi.org/10.1016/j.gaitpost.2017.06.007.
Brodie MA, Coppens MJ, Ejupi A, Gschwind YJ, Annegarn J, Schoene D, et al. Comparison between clinical gait and daily-life gait assessments of fall risk in older people. Geriatr Gerontol Int. 2017;17(11):2274–82. https://doi.org/10.1111/ggi.12979.
Ejupi A, Brodie M, Lord SR, Annegarn J, Redmond SJ, Delbaere K. Wavelet-based sit-to-stand detection and assessment of fall risk in older people using a wearable pendant device. IEEE Trans Biomed Eng. 2017;64(7):1602–7. https://doi.org/10.1109/TBME.2016.2614230.
Marschollek M, Nemitz G, Gietzelt M, Wolf KH, Meyer Zu Schwabedissen H, Haux R. Predicting in-patient falls in a geriatric clinic: a clinical study combining assessment data and simple sensory gait measurements. Z Gerontol Geriatr. 2009;42(4):317–21. https://doi.org/10.1007/s00391-009-0035-7.
Marschollek M, Rehwald A, Wolf K-H, Gietzelt M, Nemitz G, zu Schwabedissen HM, et al. Sensors vs. experts - A performance comparison of sensor-based fall risk assessment vs. conventional assessment in a sample of geriatric patients. BMC Med Inform Decis Mak. 2011;11:48. https://doi.org/10.1186/1472-6947-11-48.
Pozaic T, Lindemann U, Grebe A-K, Stork W. Sit-to-stand transition reveals acute fall risk in activities of daily living. IEEE J Transl Eng Health Med. 2016;4:2700211.
Buisseret F, Catinus L, Grenard R, Jojczyk L, Fievez D, Barvaux V, et al. Timed up and go and six-minute walking tests with wearable inertial sensor: one step further for the prediction of the risk of fall in elderly nursing home people. Sensors (Basel). 2020;20(11):3207. https://doi.org/10.3390/s20113207.
Rispens SM, van Schooten KS, Pijnappels M, Daffertshofer A, Beek PJ, van Dieën JH. Identification of fall risk predictors in daily life measurements: gait characteristics' reliability and association with self-reported fall history. Neurorehabil Neural Repair. 2015;29(1):54–61. https://doi.org/10.1177/1545968314532031.
Giansanti D, Morelli S, Maccioni G, Costantini G. Toward the design of a wearable system for fall-risk detection in telerehabilitation. Telemed J E Health. 2009;15(3):296–9. https://doi.org/10.1089/tmj.2008.0106.
Hassan L, Swarbrick C, Sanders C, Parker A, Machin M, Tully MP, et al. Tea, talk and technology: patient and public involvement to improve connected health 'wearables' research in dementia. Res Involv Engagem. 2017;3(1):12. https://doi.org/10.1186/s40900-017-0063-1.
Abel B, Pomiersky R, Werner C, Lacroix A, Schäufele M, Hauer K. Day-to-day variability of multiple sensor-based physical activity parameters in older persons with dementia. Arch Gerontol Geriatr. 2019;85:103911. https://doi.org/10.1016/j.archger.2019.103911.
Fleiner T, Haussermann P, Mellone S, Zijlstra W. Sensor-based assessment of mobility-related behavior in dementia: feasibility and relevance in a hospital context. Int Psychogeriatr. 2016;28(10):1687–94. https://doi.org/10.1017/S1041610216001034.
Schwenk M, Hauer K, Zieschang T, Englert S, Mohler J, Najafi B. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology. 2014;60(6):483–92. https://doi.org/10.1159/000363136.
Fernandez-Duque D, Black SE. Selective attention in early dementia of Alzheimer type. Brain Cogn. 2008;66(3):221–31. https://doi.org/10.1016/j.bandc.2007.08.003.
Hartman YAW, Karssemeijer EGA, van Diepen LAM, Olde Rikkert MGM, Thijssen DHJ. Dementia patients are more sedentary and less physically active than age- and sex-matched cognitively healthy older adults. Dement Geriatr Cogn Disord. 2018;46(1–2):81–9. https://doi.org/10.1159/000491995.
Shany T, Redmond SJ, Marschollek M, Lovell NH. Assessing fall risk using wearable sensors: a practical discussion. A review of the practicalities and challenges associated with the use of wearable sensors for quantification of fall risk in older people. Z Gerontol Geriatr. 2012;45(8):694–706. https://doi.org/10.1007/s00391-012-0407-2.
Acknowledgements
We would like to thank Frieder Krafft for proofreading of the manuscript. We acknowledge support by the KIT-Publication Fund of the Karlruhe Institute of Technology.
Availability of supporting data
Not applicable.
Funding
This project is financially supported by the Dietmar Hopp Stiftung. The sponsor does not have any role in the design of the study, neither in its execution, the data collec-tion, or the analysis and interpretation of the data. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Concept and design of the review: JB, JKR. Literature research: JB, JKR. Analysis and interpretation of data: JB, JKR, TE. Drafting the manuscript: JB. Revision of the manuscript: JB, JKR, TE, DJ, AW.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bezold, J., Krell-Roesch, J., Eckert, T. et al. Sensor-based fall risk assessment in older adults with or without cognitive impairment: a systematic review. Eur Rev Aging Phys Act 18, 15 (2021). https://doi.org/10.1186/s11556-021-00266-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11556-021-00266-w