Abstract
Background
Percutaneous pedicle screw (PPS) placement is a key step in several minimally invasive spinal surgery (MISS) procedures. Traditional technique for PPS makes use of C-arm fluoroscopy assistance (FA). More recently, newer intraoperative imaging techniques have been developed for PPS, including CT-guided navigation (CTNav). The aim of this study was to compare FA and CTNav techniques for PPS with regard to accuracy, complications, and radiation dosage.
Materials and methods
A total of 192 patients with degenerative lumbar spondylolisthesis and canal stenosis who underwent MISS posterior fusion ± interbody fusion through transforaminal approach (TLIF) were retrospectively reviewed. Pedicle screws were placed percutaneously using either standard C-arm fluoroscopy guidance (FA group) or CT navigation (CTNav group). Intraoperative effective dose (ED, mSv) was measured. Screw placement accuracy was assessed postoperatively on a CT scan using Gertzbein and Robbins classification (grades A–E). Oswestry disability index (ODI) and visual analog scale (VAS) scores were compared in both groups before and after surgery.
Results
A total of 101 and 91 procedures were performed with FA (FA group) and CTNav approach (CTNav group), respectively. Median age was 61 years in both groups, and the most commonly treated level was L4–L5. Median ED received from patients was 1.504 mSv (0.494–4.406) in FA technique and 21.130 mSv (10.840–30.390) in CTNav approach (p < 0.001). Percentage of grade A and B screws was significantly higher for the CTNav group (96.4% versus 92%, p < 0.001), whereas there were 16 grade E screws in the FA group and 0 grade E screws in the CTNav group (p < 0.001). A total of seven and five complications were reported in the FA and CTNav group, respectively (p = 0.771).
Conclusions
CTNav technique increases accuracy of pedicle screw placement compared with FA technique without affecting operative time. Nevertheless, no significant difference was noted in terms of reoperation rate due to screw malpositioning between CTNav and FA techniques. Radiation exposure of patients was significantly higher with CTNav technique.
Level of Evidence: Level 3.
Similar content being viewed by others
Introduction
Minimally invasive spine surgery (MISS) and percutaneous pedicle screw (PPS) placement in degenerative lumbar spondylolisthesis with lumbar canal stenosis (DLSS) is a routinely used and widely accepted technique. Although several MISS techniques are available, the shared goals of these procedures are to offer shorter operative time and hospitalization, minimize blood loss, reduce muscular damage and low back pain, and decrease rate of postoperative complications compared with open surgery [1,2,3,4,5]. Pedicle screw placement is a key step in all MISS procedures. Accurate placement of pedicle screws is of the utmost importance to achieve stable fixation of the spine and avoid any neurological damage. Over the years, new image-guided techniques have been developed to overcome some of the limitations of the traditional fluoroscopy assisted (FA) technique for pedicle screw placement. To date, no definitive study has been performed to compare FA technique with newer systems such as CT-guided technique. The aim of the current study was to compare screw accuracy placement and radiation exposure of fluoroscopy-assisted (FA) versus CT navigation-guided (CTNav) technique for PPS placement in DLSS.
Materials and methods
Patients selection and demographics
Following approval by our local ethical committee (no. 276/2020/CE), we conducted a retrospective review of 192 consecutive patients who underwent lumbar arthrodesis with PPS for DLSS with two different techniques: fluoroscopy-assisted (FA group) and CT navigation (CTNav group). Surgical procedures were performed by the senior author (G.S.) and his assistant (G.L.R.). All patients signed a written informed consent before surgery. Inclusion criteria for the study were: (1) age at surgery between 18 and 75 years, (2) low back pain (LBP) with radicular irradiation in the lower limbs, (3) claudicatio neurogena, and (4) failed conservative treatment for at least 6 months. Diagnosis of DLSS was confirmed with standard standing AP and lateral X-ray of the lumbar spine, flexion–extension X-rays, and lumbar spine magnetic resonance imaging (MRI). Patients with a previous history of instrumented spine surgery as well as osteopenia defined as lumbar T-score < −1 SD on dual energy X-ray absorptiometry were excluded from the study. All patients operated on before March 2020 were assigned to the FA group; after March 2020, all patients were operated on with CT navigation technique. Demographics, intraoperative, clinical outcome, and radiological data were recorded.
Surgical technique
Surgery was performed under general anesthesia with patients positioned prone on a radiolucent table (TruSystem 7000, TRUMPF Medizin Systeme GmbH, Saalfeld, Germany). Intraoperative neuromonitoring was used for all surgical procedures (NVM5, NuVasive, Memphis, TN, USA). In the FA group, a standard C-arm fluoroscopy (OEC Brivo Plus, GE Healthcare, USA) was used for pedicle screw placement. We proceeded from rostral to caudal, putting both screws of the same level at the same time to reduce intraoperative radiation exposure. In the CTNav group, an AIRO mobile intraoperative CT scanner (v 2.1.0.2, Mobius Imaging LLC, Shirley, MA, USA) was used for pedicle screw placement. In brief, a small midline lumbar incision at the level of the intercristal line [6] was performed, and a spinous process clamp (Brainlab AG, Munich, Germany) was placed. The spinous process clamp was used as a reference guide for CT scanning. Following 3D reconstruction of the surgical area, a navigated drill guide (Brainlab AG, Munich, Germany) was used to drill the holes for PPS placement. After PPS placement, an intraoperative CT scan was performed again to check for the accuracy of screws placement. After screw placement, a midline incision for laminoartrectomy and dural sac/roots decompression was performed in both groups. Transforaminal interbody fusion with TLIF cage (Trabecular Metal, TM Ardis, Zimmer Biomet, IN, USA) was added where deemed appropriate. At the end of the surgical procedure, AP and lateral X-ray was performed to confirm the correct position of the implants in all patients.
Radiological and clinical outcomes
Intraoperative radiation exposure data were collected according to the imaging modality used. In the FA group, radiation exposure was recorded by the C-arm software in terms of: (1) cumulative radiation exposure (mGy), defined as the kinetic energy per unit mass of air provided to a defined point in space, (2) dose–area product (DAP) (Gy·cm2), defined as patient’s dose per area of skin irradiated within the radiation field, and (3) radiation time (seconds) total time of X-ray beam activation [7]. In the CTNav group, radiation exposure was measured using BrainLab Curve 1.2 navigation system software (Brainlab AG, Munich, Germany) provided with the AIRO Mobile intraoperative CT scanner (Mobius Imaging LLC, Shirley, MA, USA). Radiological data from AIRO included (1) dose–length product (DLP) (mGy·cm), a measure of CT tube radiation output, and (2) CT dose index volume (CTDIvol) (mGy), the radiation intensity used to perform a specific CT exam. To compare radiation exposure between the two groups, effective dose (ED) in mSv was calculated. In FA group, ED was calculated by multiplying DAP measurement by the conversion factor 0.27 mSv/Gy·cm2, as previously reported [8, 9]. Similarly, in the CTNav group, ED was calculated by multiplying DLP by the dose–length conversion factor 0.014, as previously reported [10,11,12].
All patients underwent X-ray and CT scan examination at 6 months after surgery. Screw positioning was assessed by a radiologist and a spine surgeon not involved in the surgical care of the patients. Screw placement accuracy was measured according to Gertzbein and Robbins classification and was graded from A to E, according to the extent by which every single screw breached the cortex of the pedicle [13, 14]. Screws were graded as follows: (A) fully intrapedicular position without breach of the pedicle cortex; (B) breach of the pedicle cortex < 2 mm; (C) breach of the pedicle cortex 2–4 mm; (D) breach of the pedicle cortex 4–6 mm; (E) breach of the pedicle cortex > 6 mm or screw outside of the pedicle. Grades A and B were considered satisfactory results, whereas grades C–E were considered unsatisfactory results.
All patients were asked to complete Oswestry disability index (ODI), visual analog scale leg pain (VAS-LP), and visual analog scale back pain (VAS-BP) questionnaires before surgery and at 6-month follow-up.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.01 software (GraphPad Software Inc., San Diego, CA). Radiological and clinical data were expressed as median (range) and count (percentages), as appropriate. Means and percentages between the two groups were compared using the Mann–Whitney U and the χ2 tests, as appropriate. A p value < 0.05 was considered significant.
Results
Demographics and operative data
One-hundred and ninety-two MISS procedures with PPS placement for degenerative lumbar spondylolisthesis with stenosis (DLSS) were performed between August 2019 and March 2021 at our institution. From August 2019 to February 2020, 101 procedures were performed with FA technique (FA group), while from March 2020 to March 2021, 91 cases were performed with CTNav approach (CTNav group). Patients demographics are summarized in Table 1. Median age was 61 years in both groups (p = 0.811), and male-to-female ratio was also similar (p = 0.664). There were no statistically significant differences between the two study groups in terms of body mass index (BMI) or smoking habit. Fourteen (13.9%) and 21 (23.1%) patients had a history of previous uninstrumented spinal surgery in FA and CTNav group, respectively (p = 0.098). Minimum follow-up was 6 months, but length of follow-up after surgery was longer for the FA group because patients in this group were operated on earlier.
Surgical data
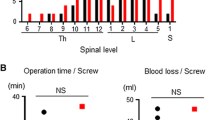
The most commonly treated level was L4–L5, followed by L3–L5 in both groups. There were no statistically significant differences in terms of distribution and number of treated segments between the two study groups. (Table 2).
A total of 952 PPSs were placed during the study period, 502 screws with FA technique and 450 screws with CTNav. Median screw number per patient was 4 (4–8) for both techniques (p = 0.83). A TLIF fusion was added in 40 (39.6%) and 50 (54.9%) patients in FA and CTNav groups, respectively (p = 0.043). No significant difference was observed in terms of total duration of surgery and time per single screw placement between the two groups. The only significant difference was observed for time per screw placement in single-level surgery (6 min in FA group versus 7 min in CTNav group, p = 0.032). A total of seven complications were reported in the FA group (three unintended dural tears during decompression, three cases of postoperative anemia that required transfusion, one TLIF cage mobilization that required revision surgery). In the CTNav group, a total of five complications were reported (two unintended dural tears during decompression, and three cases of postoperative anemia that required transfusion) (p = 0.771). (Table 3).
Data are expressed as median (range) unless stated otherwise. FA fluoroscopy assisted, CTNav CT-navigation assisted, TLIF transforaminal lumbar interbody fusion
Radiological and clinical outcomes
Median ED received from patients was 1.504 (0.494–4.406) mSv in the FA technique and 21.130 (10.840–30.390) mSv in the CTNav approach (p < 0.001). As per Gertzbein and Robbins classification [13], the percentage of grade A and B screws was significantly higher for the CTNav group (96.4% versus 92%, p < 0.001). Numbers of grade C and D screws were not significantly different between the two groups, whereas there were 16 grade E screws in the FA group and 0 grade E screws in the CTNav group (p < 0.001) (Table 4).
Patient reported outcomes measures in terms of ODI, VAS leg pain, and VAS back pain are presented in Table 5. In both groups, a significant improvement in scores was observed after surgery.
Discussion
In recent years, tremendous advancements have been made in the development of new spine stabilization systems together with new image-guidance techniques. Accuracy in pedicle screw placement is of the utmost importance to achieve a stable fixation and avoid neurological damage. Several surgical techniques have been described for pedicle screw placement, the most common ones being: (1) freehand, (2) fluoroscopy-assisted, (3) CT navigation-guided, and (4) robot-assisted [15,16,17,18,19,20,21,22,23,24]. Although no definitive comparative study has been performed on these techniques, more recent techniques are generally perceived as being safer and more accurate. On the other hand, one potential drawback of more recent techniques is that they rely heavily on the use of intraoperative radiation. This has raised some concerns with regard to radiation exposure for patients and surgical teams [25,26,27].
In a recent meta-analysis by Perdomo-Pantoja et al., the use of CTNav guidance for PPS placement showed the highest accuracy (95.5%) compared with freehand (93.1%), fluoroscopy-assisted (91.5%), and robot-assisted (90.5%) techniques. According to the authors, robot-assisted CTNav showed the highest accuracy rate in thoracic spine compared with freehand technique [23]. In the present study, we compared two of the most commonly used techniques for PPS placement in DLSS: traditional C-arm fluoroscopy versus intraoperative CT guided. Our data show that the two techniques are similar in terms of operative time and time per screw in two- and three-level operations, while in one-level surgery screw placement time was lower for FA technique. This can be explained by the fact that the C-arm does not need any setup for reuse during the same procedure, whereas CT-guided systems need some extra setup time between scans (e.g., pre- and post-screw placement) for multiple-level surgery. Accuracy of screw placement was significantly higher for the CTNav group (96.4% A and B) than the FA group (92% A and B) [23, 29]. Furthermore, 16 grade E screws were detected in FA group, although none of them resulted in any new-onset neurological deficit or required revision surgery. Interestingly, in CTNav group, six patients underwent a third intraoperative CT scan because six screws were classified as grade E on the first intraoperative scan and required replacement (after screw replacement, three screws were graded as B and three as C).
With regard to radiation exposure, our findings are in line with similar reports available in literature for both FA and CTNav technique [24]. ED was significantly higher for CTNav technique, although the surgical team was not exposed to any additional radiation. FA technique exposes the patient to a lower radiation dose, but the surgical team cannot leave the operating room during fluoroscopy operation and thus is exposed to the same amount of radiation of the patients. One additional factor to consider when comparing these two techniques is the expertise of the surgeon: experienced surgeons tend to use lower fluoroscopy, and this can significantly impact the total effective dose. Intraoperative CT is fixed, and radiation dose depends on patient features only.
Our study has some limitations. The first limitation is its retrospective nature. FA procedures were performed before CTNav procedures, and some learning curve effect should be taken into account when analyzing our data. Finally, although large enough for the current study, our sample size is insufficient to draw general conclusions with regard to longer fusions (i.e., > three levels). Although it is not possible, on the basis of our data, to strictly recommend one technique over the other, we believe that, if CT navigation technology, is available it makes total sense to use it for every routine case. Furthermore, the technique can be particularly useful in patients with obesity, patients with dysplastic pedicles, or in revision cases where normal anatomy has been disrupted by previous surgery.
In conclusion, CTNav technique is a safe adjunct to spinal surgery. It reduces surgeon and staff radiation exposure (although it does increase radiation dose for patients) and increases the accuracy of screw placement without affecting operation time. Nevertheless, no significant difference was noted in terms of reoperation rate due to screw malpositioning between CTNav and FA techniques.
Availability of data and materials
The datasets generated during the current study are available from the corresponding authors upon request.
Abbreviations
- MISS:
-
Minimally invasive spine surgery
- PPS:
-
Percutaneous pedicle screw
- DLSS:
-
Degenerative lumbar spondylolisthesis and lumbar canal stenosis
- FA:
-
Fluoroscopy assisted
- CTNav:
-
CT-navigation assisted
- LBP:
-
Low back pain
- MRI:
-
Magnetic resonance imaging
- TLIF:
-
Transforaminal lumbar interbody fusion
- DAP:
-
Dose–area product
- DLP:
-
Dose–length product
- CTDIvol:
-
CT dose index volume
- ED:
-
Effective dose
- ODI:
-
Oswestry disability index
- VAS-LP:
-
Visual analog scale leg pain
- VAS-BP:
-
Visual analog scale back pain
- BMI:
-
Body mass index
References
Cawley DT, Alexander M, Morris S (2014) Multifidus innervation and muscle assessment post-spinal surgery. Eur Spine J 23:320–327
Peng H, Tang G, Zhuang X, Lu S, Bai Y, Xu L (2019) Minimally invasive spine surgery decreases postoperative pain and inflammation for patients with lumbar spinal stenosis. Exp Ther Med 18:3032–3036
Wu MH, Dubey NK, Li YY, Lee CY, Cheng CC, Shi CS, Huang TJ (2017) Comparison of minimally invasive spine surgery using intraoperative computed tomography integrated navigation, fluoroscopy, and conventional open surgery for lumbar spondylolisthesis: a prospective registry-based cohort study. Spine J 17:1082–1090
Della Pepa GM, Mattogno PP, La Rocca G, Sabatino G, Olivi A, Ricciardi L, Polli FM (2018) Real-time intraoperative contrast-enhanced ultrasound (CEUS) in vascularized spinal tumors: a technical note. Acta Neurochir 160:1259–1263
Visocchi M, La Rocca G, Signorelli F, Roselli R, Jun Z, Spallone A (2015) 10 Levels thoracic no-intrumented laminectomy for huge spontaneous spinal subdural hematoma removal. Report of the first case and literature review. Int J Surg Case Rep 15:57–62
Chakraverty R, Pynsent P, Isaacs K (2007) Which spinal levels are identified by palpation of the iliac crests and the posterior superior iliac spines? J Anat 210:232–236
Dusad T, Kundnani V, Dutta S, Patel A, Mehta G, Singh M (2018) Comparative prospective study reporting intraoperative parameters, pedicle screw perforation, and radiation exposure in navigation-guided versus non-navigated fluoroscopy-assisted minimal invasive transforaminal lumbar interbody fusion. Asian Spine J 12:309–316
Le Heron JC (1992) Estimation of effective dose to the patient during medical x-ray examinations from measurements of the dose-area product. Phys Med Biol 37:2117–2126
O’Donnell C, Maertens A, Bompadre V, Wagner TA, Krengel W 3rd (2014) Comparative radiation exposure using standard fluoroscopy versus cone-beam computed tomography for posterior instrumented fusion in adolescent idiopathic scoliosis. Spine 39:E850–E855
Lange J, Karellas A, Street J, Eck JC, Lapinsky A, Connolly PJ, Dipaola CP (2013) Estimating the effective radiation dose imparted to patients by intraoperative cone-beam computed tomography in thoracolumbar spinal surgery. Spine 38:E306–E312
Abul-Kasim K, Soderberg M, Selariu E, Gunnarsson M, Kherad M, Ohlin A (2012) Optimization of radiation exposure and image quality of the cone-beam O-arm intraoperative imaging system in spinal surgery. J Spinal Disord Tech 25:52–58
Van de Kelft E, Costa F, Van der Planken D, Schils F (2012) A prospective multicenter registry on the accuracy of pedicle screw placement in the thoracic, lumbar, and sacral levels with the use of the O-arm imaging system and StealthStation Navigation. Spine 37:E1580–E1587
Gertzbein SD, Robbins SE (1990) Accuracy of pedicular screw placement in vivo. Spine 15:11–14
Fan Y, Du Peng J, Liu JJ, Zhang JN, Liu SC, Hao DJ (2018) Radiological and clinical differences among three assisted technologies in pedicle screw fixation of adult degenerative scoliosis. Sci Rep 8:890
Gelalis ID, Paschos NK, Pakos EE, Politis AN, Arnaoutoglou CM, Karageorgos AC, Ploumis A, Xenakis TA (2012) Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J 21:247–255
Laine T, Schlenzka D, Makitalo K, Tallroth K, Nolte LP, Visarius H (1997) Improved accuracy of pedicle screw insertion with computer-assisted surgery. A prospective clinical trial of 30 patients. Spine 22:1254–1258
Amiot LP, Lang K, Putzier M, Zippel H, Labelle H (2000) Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine 25:606–614
Castro WH, Halm H, Jerosch J, Malms J, Steinbeck J, Blasius S (1996) Accuracy of pedicle screw placement in lumbar vertebrae. Spine 21:1320–1324
Schwarzenbach O, Berlemann U, Jost B, Visarius H, Arm E, Langlotz F, Nolte LP, Ozdoba C (1997) Accuracy of computer-assisted pedicle screw placement. An in vivo computed tomography analysis. Spine 22:452–458
Tian NF, Xu HZ (2009) Image-guided pedicle screw insertion accuracy: a meta-analysis. Int Orthop 33:895–903
Kosmopoulos V, Schizas C (2007) Pedicle screw placement accuracy: a meta-analysis. Spine 32:E111–E120
Verma R, Krishan S, Haendlmayer K, Mohsen A (2010) Functional outcome of computer-assisted spinal pedicle screw placement: a systematic review and meta-analysis of 23 studies including 5,992 pedicle screws. Eur Spine J 19:370–375
Perdomo-Pantoja A, Ishida W, Zygourakis C, Holmes C, Iyer RR, Cottrill E, Theodore N, Witham TF, Lo SL (2019) Accuracy of current techniques for placement of pedicle screws in the spine: a comprehensive systematic review and meta-analysis of 51,161 screws. World Neurosurg. 126(664–678):e3
Mirza SK, Wiggins GC, C.t. Kuntz, J.E. York, C. Bellabarba, M.A. Knonodi, J.R. Chapman, and C.I. Shaffrey. (2003) Accuracy of thoracic vertebral body screw placement using standard fluoroscopy, fluoroscopic image guidance, and computed tomographic image guidance: a cadaver study. Spine 28:402–413
Riis J, Lehman RR, Perera RA, Quinn JR, Rinehart P, Tuten HR, Kuester V (2017) A retrospective comparison of intraoperative CT and fluoroscopy evaluating radiation exposure in posterior spinal fusions for scoliosis. Patient Saf Surg 11:32
Park MS, Lee KM, Lee B, Min E, Kim Y, Jeon S, Huh Y, Lee K (2012) Comparison of operator radiation exposure between C-arm and O-arm fluoroscopy for orthopaedic surgery. Radiat Prot Dosimetry 148:431–438
Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de Gonzalez A, Miglioretti DL (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169:2078–2086
Mendelsohn D, Strelzow J, Dea N, Ford NL, Batke J, Pennington A, Yang K, Ailon T, Boyd M, Dvorak M, Kwon B, Paquette S, Fisher C, Street J (2016) Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J 16:343–354
Rajasekaran S, Bhushan M, Aiyer S, Kanna R, Shetty AP (2018) Accuracy of pedicle screw insertion by AIRO((R)) intraoperative CT in complex spinal deformity assessed by a new classification based on technical complexity of screw insertion. Eur Spine J 27:2339–2347
Acknowledgements
None.
Funding
No specific funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: G.L.R., G.S.; data collection: E.M., F.P., G.G., P.R.; (2) data analysis and interpretation: F.P., G.G., L.A.N.; article drafting and revision: G.L.R., E.M., L.A.N., G.S., E.P., V.D.S., A.O.; study supervision G.L.R., G.S., A.O., V.D.S., E.P., P.R. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by our local ethical committee with approval no. 276/2020/CE. All patients signed a written consent for surgery; owing to its retrospective nature, consent for the study was waived by our local ethical committee.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing financial interests with the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
La Rocca, G., Mazzucchi, E., Pignotti, F. et al. Intraoperative CT-guided navigation versus fluoroscopy for percutaneous pedicle screw placement in 192 patients: a comparative analysis. J Orthop Traumatol 23, 44 (2022). https://doi.org/10.1186/s10195-022-00661-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-022-00661-8