Abstract
Background
Due to the special anatomy of the lower leg, tibial diaphyseal fracture causes increased intracompartmental pressure (ICP). Not only is this increased ICP the manifestation of skeletal muscle injury, but it induces further deterioration of the injury. The aim of this study was to assess the association between short-term ICP elevation and long-term skeletal muscle recovery after severe limb trauma.
Methods
In this single-center ambispective cohort study, we retrospectively screened and recruited a cohort of tibial diaphyseal fracture patients with integrated ICP data during the early post-traumatic period, and performed a prospective observational study to evaluate their skeletal muscle recovery through long-term follow-up and MR imaging after the removal of the implants. We analyzed the association between ICP elevation and skeletal muscle recovery using statistical methods.
Results
A total of 46 patients with healed fractures underwent intramedullary nail removal and MR imaging. The absolute values of the Pearson product-moment correlation coefficients between various ICP parameters and the cross-sectional area ratio (CSAR) ranged from 0.588 to 0.793, and the correlation coefficients between the ICP parameters and the average T2-weighted signal intensity ratio (T2SIR) varied from 0.566 to 0.775. Statistically significant associations were observed between the ICP parameters and the MR imaging parameters when simple linear regression analysis was performed. Among the ICP parameters, the accumulated ΔP (ΔP = diastolic blood pressure minus ICP) had the highest determination coefficient and explained 62.1% and 59.1% of the variance in CSAR and T2SIR, respectively.
Conclusions
Short-term ICP elevation was associated with long-term skeletal muscle recovery following tibial diaphyseal fracture, especially for ICP data that integrated time factors.
Level of evidence
Level 3.
Similar content being viewed by others
Introduction
Tibial diaphyseal fracture is a commonly encountered injury, accounting for approximately 2% of all fractures in orthopedic trauma [1,2,3,4]. Anatomically, the lower leg consists of four compartments. In a tibial diaphyseal fracture, bleeding, edema, and exudation are confined within those compartments, resulting in increased intracompartmental pressure (ICP) [5, 6]. In general, the more severe the trauma to the calf, the greater the energy transmitted to the soft tissues, leading to more extensive bleeding, edema, and exudation and hence higher pressure in the compartment [6, 7]. In addition to hindering blood circulation and causing the vicious cycle of ischemia–edema–ischemia aggravation, the increased ICP—the manifestation of skeletal muscle injury—also induces further deterioration of the injury [8]. The skeletal muscle is continuously damaged during the period of elevated ICP [9], such that an extended period of elevated ICP prevents full recovery from the injury [10]. Skeletal muscle that cannot be fully repaired ultimately degenerates and fibrosis repair occurs [11], which manifests as a decrease in skeletal muscle volume and a change in internal structure, affecting the patient’s physical performance in daily life and sporting activities [12]. Magnetic resonance (MR) imaging is recognized as one of the best diagnostic methods for skeletal muscle injury due its excellent resolution and its nonradiographic and noninvasive nature [13, 14]. MR imaging is a powerful tool for evaluating and quantifying skeletal muscle volume and internal structures [15, 16]. However, metal internal fixators such as intramedullary nails can interfere with MR imaging, which limits its application for evaluating the recovery of skeletal muscle in tibial diaphyseal fracture patients with intramedullary nails [17, 18].
Nevertheless, because of the influence of their cultural traditions and religious beliefs, our patients do not want implants in their bodies permanently; thus, in most cases, they volunteer to have the intramedullary nails removed when their fractures have completely healed. This meant that there was an opportunity for us to use MR imaging to assess the recovery of skeletal muscle following tibial diaphyseal fracture. Therefore, we retrospectively screened and recruited a cohort of tibial diaphyseal fracture patients with integrated ICP data in the early post-traumatic period. Furthermore, we conducted a prospective observational study to evaluate their skeletal muscle recovery through long-term follow-up and MR imaging after the removal of the implants. The purpose of the current study was to assess the association between short-term ICP elevation and long-term skeletal muscle recovery after severe limb trauma.
Materials and methods
Ethical approval
The present study was approved by the Ethics Committee of Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital (2017-C-003), and the patients were fully informed about the purpose and procedure of this study before they underwent ICP monitoring, intramedullary nail removal, and MR imaging. They consented to ICP monitoring, MR imaging, and voluntary removal of the intramedullary nail after fracture healing. All patients provided written informed consent before the procedure.
Patient population
The current study was conducted as a single-center ambispective observational cohort study at a trauma center that provides medical care for approximately 1,000,000 community residents from March 2017 to July 2019. Patients with severe lower extremity trauma who were diagnosed with tibial diaphyseal fractures were retrospectively screened for possible recruitment. The inclusion criteria were as follows: (1) tibial diaphyseal fracture as defined by AO/OTA classification code 42; (2) fixation of the tibial diaphyseal fracture with intramedullary nails; (3) complete ICP data for the anterior compartment after the trauma, with the maximum ICP above 30 mmHg and the minimum ΔP (ΔP = diastolic blood pressure minus ICP) below 50 mmHg; (4) aged over 18 at the time of injury; and (5) the patient was willing to have the intramedullary nail removed after fracture healing. Based on the inclusion criteria, 64 patients were found to be eligible for the study.
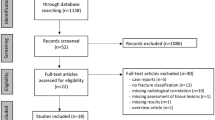
The exclusion criteria were as follows: (1) open tibial diaphyseal fracture; (2) fasciotomy due to a diagnosis of acute compartment syndrome (ACS); (3) open reduction during surgery; (4) MR imaging contraindications (e.g., pacemaker); and (5) vascular and nerve damage to the affected limb. Finally, a total of 51 patients were recruited into the study cohort (Fig. 1).
ICP data collection
In cases of severe calf trauma, such as tibial diaphyseal fractures, the anterior compartment is most frequently affected [8], so we measured and recorded the ICP in the anterior compartment immediately upon each patient’s arrival at the emergency room. A 22-gauge intravenous catheter filled with normal saline was inserted into the anterior compartment of the affected limb and connected to an invasive arterial blood pressure monitor system (IABPMS) to measure and monitor the ICP continuously for 48 h. The patient’s ICP and blood pressure were recorded by nurses each hour [19,20,21,22] (Fig. 2).
Based on these records, we identified the maximum ICP and the minimum ΔP, which is a widely recognized indicator of skeletal muscle perfusion [21]. An ICP elevation of above 30 mmHg is considered clinically significant [5,6,7]. In order to determine the duration of ICP elevation based on continuous monitoring, the accumulated ICP was calculated as the sum of hourly ICP values exceeding 30 mmHg. However, simply adding ΔP values together is meaningless, because the smaller the ΔP, the more severe the obstruction of the blood circulation in the affected limb. According to a previous study, the diastolic blood pressure of patients increased drastically due to stress and pain after lower extremity injury, but gradually decreased with analgesia, immobilization, and hemorrhage, culminating in a mean diastolic blood pressure of about 80 mmHg 48 h after injury [19]. Since an ICP elevation > 30 mmHg was considered clinically significant and ΔP = diastolic blood pressure minus ICP, we determined a reference value of 50 mmHg. We then summed all of the hourly ΔP values below 50 mmHg (Fig. 3).
Radiographic analysis
All patients were treated with closed reduction and intramedullary nail fixation for tibial diaphyseal fractures when the operating conditions permitted, and were subsequently followed up until bone union. Approximately 1 year after bone union was achieved, the patients requested the removal of the intramedullary nail; accordingly, we arranged a surgery after fully informing the patients of the risks involved and obtaining their informed consent. To avoid artifacts in the MR imaging from skeletal muscle edema and hemorrhage caused by the removal surgery, MR imaging was performed about 2 months thereafter using a high field strength (1.5 T) scanner (MAGNETOM Avanto Dot, Siemens Medical Solutions, USA). The patients laid supine in the body coil with their legs extended and relaxed so that images of the affected and normal limbs were obtained. Patients were imaged with axial and coronal turbo spin echo (TSE) T1-weighted sequences (TR 800 ms, TE 20–25 ms, SL 4–5 mm; in-plane resolution; matrix 512 × 512), TSE T2-weighted sequences (TR 2700 ms, TE 86 ms, SL 4–5 mm; in-plane resolution; matrix 512 × 512), and turbo inversion recovery magnitude (TIRM) T2-weighted sequences (TR 5510 ms, TE 32 ms, SL 4–5 mm; in-plane resolution; matrix 512 × 512). The acquired images were stored in Digital Imaging and Communications in Medicine format for further analyses.
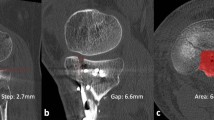
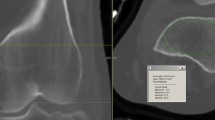
When the MR images were retrieved from the picture archiving and communication system (PACS), radiographic analysis of all cases was performed by a single radiologist who was blinded to the ICP data. Although it is considered to be the most accurate and reliable method for measuring skeletal muscle volume, MR imaging is costly and time-consuming when used for direct measurements [15, 23]. Therefore, a cross-sectional area (CSA) obtained from a single-slice image has been used in many studies as a quantitative indicator to evaluate skeletal muscle volume [12, 23, 24]. In the current study, we selected five slice cross-sectional images of bilateral calves and traced the outlines of the anterior compartments of the affected and normal limbs to measure the CSA of the anterior compartments. Using the CSA of the normal limb as a reference object, we calculated the ratio of the average anterior compartment CSA in the five slice images of the affected and normal limbs as the cross-sectional area ratio (CSAR) to assess the recovery of skeletal muscle volume (Fig. 4a). Furthermore, we selected five coronal plane slice images of the T2-weighted sequence, each of which contained the anterior compartments of the affected and normal limbs. We chose two regions of interest (ROIs) with an area of 1 cm2 for the anterior compartment of each limb in each image, thus selecting a total of 10 ROIs for each limb. As much as possible, the ROIs were chosen such that vascular and fascial structures were outside the ROIs and the ROIs were at identical locations for both limbs [25]. For each ROI, we calculated the average T2-weighted signal intensity (T2SI), after which the average value for 10 ROIs was accepted as the T2SI of the affected or normal limb (Fig. 4b). As in the case of CSAR, we compared the T2SI from the affected limb to that from the normal limb to obtain the average T2-weighted signal intensity ratio (T2SIR), which was used to assess the recovery of the internal structures of the skeletal muscle.
a The traced outlines of anterior compartments of the affected and normal limbs in the axial plane, which were used to measure anterior compartment cross-sectional areas (CSAs). The cross-sectional area ratio (CSAR) of the affected limb to the normal limb was calculated to evaluate the recovery of skeletal muscle volume. b T2-weighted image obtained in the coronal plane. Two regions of interest (ROIs), each with an area of 1 cm2, were chosen at identical locations in the affected and normal limbs . Vascular and fascial structures were kept outside the ROIs. The average T2-weighted signal intensity (T2SI) of the ROI was calculated for the affected limb and for the normal limb; the ratio of these T2SIs—the average T2-weighted signal intensity ratio (T2SIR)—was used to assess the recovery of skeletal muscle internal structures. Note the high-signal lesion in the medullary cavity of the affected tibia after the removal of the intramedullary nail
Statistical analysis
The data were analyzed with SPSS® version 21.0 (SPSS Inc, Chicago, IL, USA). Continuous data are presented here as minimum–maximum (mean ± standard deviation). The Pearson product-moment correlation coefficient (PPCC) was used to investigate bivariate linear relations between the ICP data and the radiographic data. To evaluate the association of ICP elevation with the recovery of skeletal muscle, simple linear regression analyses of various ICP data and MR imaging data were performed. The significance level for all calculations was defined as P < 0.05.
Results
In this ambispective cohort study, 51 patients met the criteria to be recruited, and a total of 46 patients underwent intramedullary nail removal and MR imaging after 11–30 (19.23 ± 5.01) months of follow-up. Five patients failed to meet the study criteria during follow-up because of a new limb injury, coronary stenting, stroke, and contact loss. All patients were treated with close reduction and intramedullary nail fixation when the operating conditions permitted, eventually achieving bone union. The fracture healing process lasted 15–46 (23.17 ± 5.82) weeks (Table 1). Two cases presented delayed union after intramedullary nail fixation; in those cases, the distal interlocking screws were removed and union was achieved after dynamization. The removal of the intramedullary nail was completely successful in all patients; there were no complications such intramedullary nails or screws remaining in the body, vascular or nerve injuries, hematoma, or infection.
The 46 patients underwent MR imaging 6–11 (8.48 ± 1.53) weeks after the removal of the internal fixator. Measurements and calculations of the CSAR (0.59–1.02; 0.85 ± 0.10) and T2SIR (0.98–1.63; 1.17 ± 0.15) of the affected and normal limbs were obtained in all cases (Table 1). These showed that the CSAR presented a downward trend and the T2SIR an upward trend with increasing ICP.
The absolute values of the Pearson product-moment correlation coefficients between various ICP data and CSAR ranged between 0.588 and 0.793, while the correlation coefficients between various ICP data and T2SIR ranged between 0.566 and 0.775. Among all the ICP data, the accumulated ΔP showed the strongest correlations with CSAR and T2SIR (Table 2). Simple linear regression analysis showed that the maximum ICP, the minimum ΔP, the accumulated ICP, and the accumulated ΔP were statistically significantly associated with CSAR and T2SIR. The determination coefficients for the prediction of CSAR and T2SIR from the accumulated ΔP were the highest among all the ICP data (Table 3, Fig. 5).
Discussion
Movement is produced by the transmission of forces generated by skeletal muscles along a biomechanical chain from muscles to tendons to bones [14, 26,27,28]. The weakest link in this chain determines the level of function of the limb [14, 29]. As it is a driver of limb movement, skeletal muscle plays an important role in motor function [30]. In the current study, we conducted an ambispective observational cohort study based on MR imaging after the removal of implants to investigate the relationship between short-term ICP elevation and long-term skeletal muscle recovery after severe limb trauma. If a diagnosis of ACS is not established, it is believed that skeletal muscles will eventually repair themselves and regain good condition. Actually, this is a misunderstanding. Injured skeletal muscle goes through a series of coordinated and interrelated phases of healing, including degeneration, inflammation, regeneration, and fibrosis, which is an area that continues to present a challenge [31]. Clinically, a large number of fracture cases achieve bone union, but incomplete recovery of skeletal muscles leads to a partial loss of function and susceptibility to reinjury [31, 32]. In the presence of implants, however, it is difficult to make a correct evaluation of skeletal muscle recovery using the examination methods available. Thus, there are just a few clinical studies on skeletal muscle recovery, and the recovery was either derived from experimental studies or tested empirically in those studies [33, 34].
Several factors, both iatrogenic and noniatrogenic, can affect skeletal muscle recovery. The extent of skeletal muscle disturbance in open reduction varies with the surgical approach used, and an open fracture can have catastrophic consequences for skeletal muscle recovery in the event of infection. To focus on the impact of ICP and exclude interfering factors, we developed strict inclusion and exclusion criteria to limit our research subjects to those who had a closed tibial diaphyseal fracture and were treated with closed reduction and intramedullary nail fixation. In so doing, we not only excluded the iatrogenic impact of open reduction and internal fixation on skeletal muscle recovery but also avoided the noniatrogenic impact of infection caused by an open fracture. Furthermore, fasciotomy was reported to be a traumatic procedure associated with a set of complications including chronic pain, muscle weakness, and nerve injury [35]. Since fasciotomy for compartment decompression is an independent iatrogenic factor affecting skeletal muscle recovery, it was included as an exclusion criterion for our study.
Recently, however, some researchers have emphasized the importance of monitoring ICP, as indicated in a previously reported study where McQueen et al. [20] estimated the sensitivity and specificity of continuous ICP monitoring for the diagnosis of ACS, and proved—as expected—that it had high sensitivity and specificity for the diagnosis of ACS. Monitoring ICP can reduce both the delay before fasciotomy and the development of sequelae, as the appearance of clinical symptoms and signs lags behind the pressure changes. Long-term ischemia and hypoxia will cause serious consequences; however, skeletal muscle can tolerate short-term ischemia and hypoxia, which can, for instance, be caused by the application of a tourniquet to temporarily block limb blood flow for surgery. Prayson et al. [36] reported that 63% of participants had at least one compartment measurement that exceeded a single threshold of 45 mmHg, but none of them developed compartment syndrome or required a fasciotomy. Egol et al. [37] observed a similar dynamic, fluctuating process for ICP in their prospective cohort study. Through a retrospective analysis of the ICP parameters collected in our study, we found that ICP increased rapidly because of local bleeding and tissue edema after tibial diaphyseal fractures, and that ICP tended to stabilize or even recede with immobilization, the application of a cold compress, dehydration, and other medical approaches [32]. Similar to the results reported by Prayson and Egol, ICP reached an extremely high level (ICP > 45 mmHg) in some patients, but so long as the ICP quickly decreased, no typical symptoms of ACS were observed. As indicated by the MR imaging performed after long-term follow-up, there was no significant impact on the recovery of skeletal muscle. Thus, it is important for the evaluation of the impact of ICP on skeletal muscle to take time factors into account.
In addition to the maximum ICP and minimum ΔP, we organized and calculated the accumulated ICP and accumulated ΔP based on the duration of ICP elevation. The correlation analysis showed strong correlations of the two accumulative parameters with skeletal muscle recovery. Linear regression analysis reconfirmed the importance of the ICP elevation duration, as indicated by the evidence that the maximum ICP and minimum ΔP (which do not take time into account) were less strongly associated with skeletal muscle recovery, while the accumulated ΔP (which takes time into account) produced the highest determination coefficients for CSAR and T2SIR, explaining 62.1% and 59.1% of the variance in CSAR and T2SIR, respectively.
The primary limitation of our study was the small sample size, especially the small number of cases with sustained elevated ICP. Most of the cases with with sustained elevated ICP were diagnosed with ACS, meaning that they were excluded from the study cohort due to fasciotomy decompression. This could make the reliability of the estimated specificity of using ICP elevation to predict skeletal muscle recovery questionable in patients with high accumulated ICP and ΔP. MR imaging can assess skeletal muscle recovery, but the results may not always be accurate. Further investigations that focus on larger cohorts and use more accurate and comprehensive approaches to investigate skeletal muscle recovery are needed to explore the underlying mechanism for the impact of elevated ICP on skeletal muscle recovery as well as the functional implications for limbs.
Conclusions
In summary, this ambispective cohort study demonstrated that short-term ICP elevation was associated with long-term skeletal muscle recovery, and that the accumulated ΔP, which accounts for both the ICP data and time, was the ICP parameter most closely associated with the recovery of skeletal muscle. Firm conclusions such as the accumulated ΔP threshold for skeletal muscle recovery could not be drawn from this study due to its size, but the study can provide ideas and thoughts for future research.
Availability of data and materials
All data generated or analyzed during this study are included in this published article’s supplementary information files.
Abbreviations
- ICP:
-
Intracompartmental pressure
- MR:
-
Magnetic resonance
- ACS:
-
Acute compartment syndrome
- IABPMS:
-
Invasive arterial blood pressure monitoring system
- TSE:
-
Turbo spin echo
- TIRM:
-
Turbo inversion recovery magnitude
- PACS:
-
Picture archiving and communication system
- CSA:
-
Cross-sectional area
- CSAR:
-
Cross-sectional area ratio
- T2SI:
-
Average T2-weighted signal intensity
- T2SIR:
-
Average T2-weighted signal intensity ratio
- PPCC:
-
Pearson product-moment correlation coefficient
References
Grutter R, Cordey J, Buhler M, Johner R, Regazzoni P (2000) The epidemiology of diaphyseal fractures of the tibia. Injury 31(Suppl 3):C64-67
Court-Brown CM, Caesar B (2006) Epidemiology of adult fractures: a review. Injury 37:691–697
Wennergren D, Bergdahl C, Ekelund J, Juto H, Sundfeldt M, Moller M (2018) Epidemiology and incidence of tibia fractures in the Swedish fracture register. Injury 49:2068–2074
Larsen P, Elsoe R, Hansen SH, Graven-Nielsen T, Laessoe U, Rasmussen S (2015) Incidence and epidemiology of tibial shaft fractures. Injury 46:746–750
Donaldson J, Haddad B, Khan WS (2014) The pathophysiology, diagnosis and current management of acute compartment syndrome. Open Orthop J 8:185–193
Via AG, Oliva F, Spoliti M, Maffulli N (2015) Acute compartment syndrome. Muscles Ligaments Tendons J 5:18–22
Frink M, Hildebrand F, Krettek C, Brand J, Hankemeier S (2010) Compartment syndrome of the lower leg and foot. Clin Orthop Relat Res 468:940–950
von Keudell AG, Weaver MJ, Appleton PT, Bae DS, Dyer GSM, Heng M, Jupiter JB, Vrahas MS (2015) Diagnosis and treatment of acute extremity compartment syndrome. Lancet 386:1299–1310
Oyster N, Witt M, Gharaibeh B, Poddar M, Schneppendahl J, Huard J (2015) Characterization of a compartment syndrome-like injury model. Muscle Nerve 51:750–758
Xu Y, Bai Y, Li Q, Shen X, Jiang L (2010) Experimental study of prognosis of chronic compartment syndrome. Connect Tissue Res 51:419–425
Serrano AL, Munoz-Canoves P (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316:3050–3058
Tanaka NI, Kanehisa H (2014) Applicability of single muscle CSA for predicting segmental muscle volume in young men. Int J Sports Med 35:608–614
Noseworthy MD, Davis AD, Elzibak AH (2010) Advanced MR imaging techniques for skeletal muscle evaluation. Semin Musculoskelet Radiol 14:257–268
Costa AF, Di Primio GA, Schweitzer ME (2012) Magnetic resonance imaging of muscle disease: a pattern-based approach. Muscle Nerve 46:465–481
Commean PK, Tuttle LJ, Hastings MK, Strube MJ, Mueller MJ (2011) Magnetic resonance imaging measurement reproducibility for calf muscle and adipose tissue volume. J Magn Reson Imaging 34:1285–1294
Flores DV, Mejia Gomez C, Estrada-Castrillon M, Smitaman E, Pathria MN (2018) MR imaging of muscle trauma: anatomy, biomechanics, pathophysiology, and imaging appearance. Radiographics 38:124–148
Laakman RW, Kaufman B, Han JS, Nelson AD, Clampitt M, O’Block AM, Haaga JR, Alfidi RJ (1985) MR imaging in patients with metallic implants. Radiology 157:711–714
Singh DR, Chin MS, Peh WC (2014) Artifacts in musculoskeletal MR imaging. Semin Musculoskelet Radiol 18:12–22
Tian S, Lu Y, Liu J, Zhu Y, Cui Y, Lu J (2016) Comparison of 2 available methods with Bland-Altman analysis for measuring intracompartmental pressure. Am J Emerg Med 34:1765–1771
McQueen MM, Duckworth AD, Aitken SA, Court-Brown CM (2013) The estimated sensitivity and specificity of compartment pressure monitoring for acute compartment syndrome. J Bone Joint Surg Am 95:673–677
McQueen MM, Court-Brown CM (1996) Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br 78:99–104
Duckworth AD, McQueen MM (2014) Continuous intracompartmental pressure monitoring for acute compartment syndrome. JBJS Essent Surg Tech 3:e13
Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer C-C, Bosy-Westphal A, Müller MJ (2015) What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 102:58–65
Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T (1994) Cross-sectional areas of fat and muscle in limbs during growth and middle age. Int J Sports Med 15:420–425
van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C (2005) The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med 33:699–704
Exeter D, Connell DA (2010) Skeletal muscle: functional anatomy and pathophysiology. Semin Musculoskelet Radiol 14:97–105
Huijing PA, Jaspers RT (2005) Adaptation of muscle size and myofascial force transmission: a review and some new experimental results. Scand J Med Sci Sports 15:349–380
Cutlip RG, Baker BA, Hollander M, Ensey J (2009) Injury and adaptive mechanisms in skeletal muscle. J Electromyogr Kinesiol 19:358–372
Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M (2005) Muscle injuries: biology and treatment. Am J Sports Med 33:745–764
Huard J, Lu A, Mu X, Guo P, Li Y (2016) Muscle injuries and repair: what’s new on the horizon! Cells Tissues Organs 202:227–236
Best TM, Gharaibeh B, Huard J (2013) Stem cells, angiogenesis and muscle healing: a potential role in massage therapies? Br J Sports Med 47:556–560
Riede U, Schmid MR, Romero J (2007) Conservative treatment of an acute compartment syndrome of the thigh. Arch Orthop Trauma Surg 127:269–275
Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M (2005) Muscle injuries: biology and treatment. Am J Sports Med 33:745–764
Järvinen TA, Järvinen M, Kalimo H (2013) Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J 3:337–345
Schmidt AH (2016) Acute compartment syndrome. Orthop Clin North Am 47:517–525
Prayson MJ, Chen JL, Hampers D, Vogt M, Fenwick J, Meredick R (2006) Baseline compartment pressure measurements in isolated lower extremity fractures without clinical compartment syndrome. J Trauma 60:1037–1040
Egol KA, Bazzi J, McLaurin TM, Tejwani NC (2008) The effect of knee-spanning external fixation on compartment pressures in the leg. J Orthop Trauma 22:680–685
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
ST performed the literature review, intracompartmental pressure monitoring, data analysis, and drafted the manuscript. SC designed the study and helped to draft the manuscript. YL and JZ completed all intramedullary nail fixation operations for tibial diaphyseal fractures and nail removal operations. XK carried out the radiologic studies. All authors edited the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhoupu Hospital, Pudong New Area, Shanghai (2017-C-003). The authors state that this study conforms to the ethical standards laid down in the Declaration of Helsinki. All patients were fully informed about the purpose and procedure of this study and consented to participate.
Consent for publication
All patients gave consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, S., Chang, S., Lu, Y. et al. The association between intracompartmental pressure and skeletal muscle recovery after tibial diaphyseal fractures: an ambispective cohort study. J Orthop Traumatol 22, 18 (2021). https://doi.org/10.1186/s10195-021-00579-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-021-00579-7