Abstract
Sexual dimorphism has been revealed for many neurological disorders including chronic pain. Prelicinal studies and post-mortem analyses from male and female human donors reveal sexual dimorphism of nociceptors at transcript, protein and functional levels suggesting different mechanisms that may promote pain in men and women. Migraine is a common female-prevalent neurological disorder that is characterized by painful and debilitating headache. Prolactin is a neurohormone that circulates at higher levels in females and that has been implicated clinically in migraine. Prolactin sensitizes sensory neurons from female mice, non-human primates and humans revealing a female-selective pain mechanism that is conserved evolutionarily and likely translationally relevant. Prolactin produces female-selective migraine-like pain behaviors in rodents and enhances the release of calcitonin gene-related peptide (CGRP), a neurotransmitter that is causal in promoting migraine in many patients. CGRP, like prolactin, produces female-selective migraine-like pain behaviors. Consistent with these observations, publicly available clinical data indicate that small molecule CGRP-receptor antagonists are preferentially effective in treatment of acute migraine therapy in women. Collectively, these observations support the conclusion of qualitative sex differences promoting migraine pain providing the opportunity to tailor therapies based on patient sex for improved outcomes. Additionally, patient sex should be considered in design of clinical trials for migraine as well as for pain and reassessment of past trials may be warranted.
Similar content being viewed by others
Background
For reasons that are not well understood, women predominately suffer from chronic pain conditions including migraine, fibromyalgia, irritable bowel syndrome, temporomandibular disorder, rheumatoid arthritis, musculoskeletal disorders, complex regional pain syndrome and some forms of neuropathic pain [1,2,3,4,5]. Increased sensitivity to experimental pain has also been reported in healthy women [6]. Sex differences in pain may be considered as quantitative (i.e., prevalence) or qualitative in which patients of both sexes are diagnosed with the same disorder but the underlying mechanisms promoting pain are different [2, 3]. Qualitative sex differences in pain provide the opportunity to change current treatment paradigms by treating pain based on patient sex, a basic form of precision medicine.
In this review, we focus on two mechanisms that promote migraine and pain selectively in females and provide evidence supporting the conclusion that nociceptors, the fundamental building blocks of pain, are sexually dimorphic. Prolactin is a neurohormone that selectively sensitizes nociceptors from female animals or from female post-mortem human donors. Prolactin increases release of calcitonin gene-related peptide (CGRP), a neuropeptide that is causal in many people with migraine. Both prolactin and CGRP promote migraine-like pain behaviors selectively in female animals. Consistent with preclinical observations, small molecule CGRP-receptor (CGRP-R) antagonists (i.e., gepants) show preferential efficacy in acute migraine therapy in women [7, 8]. Sexual dimorphism therefore suggests that the uniform therapeutic approach for acute treatment of migraine, and pain-related disorders more generally, in men and women requires reassessment, and that opportunities exist for precision medicine based on patient sex.
Introduction – migraine
Migraine is the 2nd leading cause of years lived with disability among both men and women of all ages and the 1st leading cause of years lived with disability among young women [9, 10]. This disorder affects more than 10% of adult population worldwide exceeding 1 billion people, with approximately 700 million being women [10, 11]. For reasons that are not understood, migraine is approximately three times more prevalent in women [12, 13]. Increased disability and medical care utilization has been observed among women with migraine as well as higher reported symptoms and elevated risk of migraine chronification [11, 14]. The high female prevalence of migraine suggests differential contribution of genetic influences and raises the possibility that mechanistic differences may exist in this disorder across sexes.
Characteristics of migraine
Migraine is a multiphasic neurologic disorder that commonly includes a premonitory period, aura, headache, post-drome and interictal stages [15, 16]. A migraine attack can include both painful (i.e., unilateral throbbing headache, cephalic and extra-cephalic allodynia, neck pain) and non-painful symptoms such as visual disturbances, nausea, vomiting, fatigue, mood change, dizziness, enhanced sensitivity to touch (allodynia), light (photophobia), sound (phonophobia), smell (osmophobia) and cognitive dysfunction [17]. The migraine aura that occurs in approximately one-third of people with migraine, is thought to result from a transient wave of cortical depolarization [18,19,20]. Enhanced sensitivity to sensory stimuli likely results from mechanisms of sensory amplification including peripheral nociceptor sensitization, central sensitization and neuronal hyperexcitability. These features often, but not always, present in a time-sequence reflecting distinct, but sometimes overlapping, phases that characterize the complex migraine attack [16]. Collectively, these symptoms significantly affect ability to function, reduce quality of life [21,22,23,24] and impose a huge socio-economic burden [25,26,27]. The International Classification of Headache Disorders (ICHD-3) classifies migraine as episodic (EM) or chronic (CM) based on the clinical progression and frequency of episodes. Chronic migraine is defined as headache on more than 15 days a month for at least three months with at least 8 headache days each month meeting ICHD-3 criteria for migraine [17].
Clinical treatment of migraine
Acute (attack-related) treatments for migraine used to treat migraine pain during an attack include over-the-counter drugs such as acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs) as well as migraine specific medicines such as triptans (i.e., sumatriptan, zolmitriptan, rizatriptan, naratriptan, frovatriptan and eletriptan) that act as agonists at the 5-HT1B/1D receptors and ditans (i.e., lasmitidan) that are agonists at the 5HT1F receptor [28,29,30]. Preventive treatments, designed to reduce the frequency, duration, and severity of attacks and generally initiated for people experiencing at least 4 headache days per month, have included non-specific beta blockers (e.g. propranolol, timolol and metoprolol), other antihypertensives (e.g.lisinopril and candesartan), antidepressants (e.g. pizotifen, amitriptyline, venlafaxine), anticonvulsants (e.g.valproate, gabapentin, topiramate), calcium channel blockers (verapamil, flunarizine), barbiturates (butalbital) and onabotulinumtoxinA for chronic migraine [28,29,30].
More recently, therapy for migraine has evolved to include drugs targeting the CGRP pathway that are used both for acute and preventive therapy [31]. Preventive therapies include monoclonal antibodies (mAbs) that sequester CGRP peptide (eptinezumab, fremanezumab, and galcanezumab) or block CGRP-R (erenumab). Small molecule antagonists of the CGRP-R, i.e., gepants, are used for acute migraine and include ubrogepant and rimegepant that are given orally and zavegepant that is administered intranasally. Atogepant (taken daily) and rimegepant (taken every other day) are gepant drugs that are also used for preventive therapy. CGRP targeting drugs have gained prominence and are often preferred over triptans for acute migraine treatment because of superior tolerability. Unlike triptans, CGRP-R antagonists do not produce vasoconstriction and are not associated with risks of cardiovascular adverse events. Moreover, CGRP targeting drugs are not associated with medication overuse headache (MOH) [28,29,30]. Despite female prevalence of migraine, with the exception of hormonal therapies, at present, migraine is treated with the same drugs in men and women.
Sex differences in migraine
Female sex hormones have long been recognized as factors in promoting migraine across all life stages experienced by women [32,33,34]. The increased female prevalence of migraine begins at the age of menarche, peaks at age 40 and diminish after menopause [13, 35]. Many women experience increased migraine attacks at or around the time of their menstrual periods, in the first trimester of pregnancy and in the perimenopause. The onset of menstrual bleeding coincides with falling levels of hormones including progesterone and estrogen. The influence of gonadal hormones including especially estrogen has been a key focus of studies exploring sex differences in migraine. Female migraineurs show faster estrogen withdrawal during the late luteal phase than controls [36] and the number of migraine attacks has been found to be higher during the phases of falling estrogen and lower during the phases of rising estrogen [37]. These observations support the hypothesis that estrogen withdrawal along with changes in other hormones including oxytocin could trigger migraine attacks [38, 39].
Hormonal therapy consisting of estrogen replacement (i.e., contraceptives) are commonly used for women with migraine [40, 41]. Fluctuations in sex hormones have been shown to influence the release of CGRP and to activate the trigeminovascular system [42]. Women with regular menstrual cycles have higher CGRP concentrations in plasma and tear fluid [43]. Women using estrogen containing oral contraceptive pills have CGRP levels similar to women without migraine supporting the conclusion that stable estrogen levels may be protective for migraine possibly by influencing CGRP.
Prolactin, CGRP and migraine
Recent work has also highlighted the role of prolactin, a circulating neurohormone that is released by the pituitary and that can also be produced locally by multiple cell types, in promoting migraine-like pain [34, 44, 45]. Significantly, this female predominant hormone enhances CGRP release [46]. Prolactin is under control of estrogen as well as stress. Women (and female animals) have higher circulating levels of prolactin than males and exhibit greater stress responses. Prolactin is released from pituitary lactotrophs and is under tight inhibitory control by hypothalamic dopaminergic tuberoinfundibular (TIDA) cells. Preclinical studies show that the TIDA cells are inhibited by stress-related transmitters resulting in increased circulating prolactin [47]. Dopamine D2 receptor agonists are used therapeutically to reduce release of prolactin from the pituitary. In humans, stress increases circulating prolactin, lowers sensory thresholds increasing the likelihood of pain attacks, is associated with painful menstruation (i.e., dysmenorrhea) and importantly, is the most common self-identified migraine trigger [39, 48]. Patients with very high levels of prolactin resulting from pituitary adenomas have increased migraine attacks that have been shown to respond to treatment by dopaminergic agonists that reduce prolactin levels [45, 49]. Estrogen and stress-related influences on prolactin are therefore likely to have a CGRP component in promoting migraine attacks in women.
CGRP and prolactin are profoundly female-selective in their actions
Preclinical studies show that neurotransmitters can be sexually dimorphic in their effects. In preclinical models, CGRP produces female-selective pain and headache responses [50]. While rodent models cannot capture the complex multi-symptom and multi-phasic nature of migraine, they can and do provide mechanistic information that helps to explain migraine symptoms. As migraine headache likely arises from trigeminal nociceptors that innervate the cranial meninges, direct activation of these afferents in animals provides a surrogate measure of headache through assessment of pain behaviors including cutaneous cephalic allodynia, a translational measure that reflects a symptom that is also observed in patients during migraine attacks. Dural stimulants also elicit other surrogate measures including facial grimace in rodents that may be representative of ongoing headache pain. Importantly, application of CGRP directly to the dura mater elicited both evoked and ongoing migraine-like pain behaviors at much lower doses and with longer lasting effects in female, compared to male mice [50].
Reasons for enhanced CGRP-mediated pain responses in female animals remain unclear but could include sex differences in CGRP expression, distribution and signaling at receptors that bind this peptide. αCGRP, the primary neuronal form, is a potent 37-amino acid neuropeptide widely distributed in the trigeminovascular system including the trigeminal ganglion that is thought to be critical in promoting the headache phase of migraine through both neuronal actions and likely from dilation of meningeal blood vessels [51,52,53,54,55,56]. CGRP signals through the canonical CGRP-R and can also signal through the amylin 1 (AMY1) receptor. The canonical CGRP-R, consists of the calcitonin-like receptor (CLR), a G protein-coupled receptor, and an associated receptor activity modifying protein 1 (RAMP1) [53]. Additionally, CGRP can signal through the amylin 1 (AMY1) receptor that includes RAMP1 along with the G protein-coupled receptor, calcitonin receptor (CTR) [52]. In experimental human studies, activation of AMY1 which expresses RAMP1 but not CLR, can promote migraine in patients with primary headache disorders [53]. In contrast, adrenomedullin receptors 1 and 2 (AM1/2) that express CLR but not RAMP1 have not been implicated in inducing migraine. The female selectivity of CGRP might thus be influenced by sexual dimorphism in the expression of either CLR and/or RAMP1. At present, the relative contributions of these receptors to migraine continue to be an area of active investigation. Nevertheless, possible mechanistic insights may be deduced by a comparison of the relative affinities of the small molecule CGRP receptor antagonists (i.e., gepants) for these receptors. Ubrogepant, rimegepant and zavegepant all show comparable low picomolar affinities for the canonical CGRP receptor but vary significantly in their affinities for the AMY1 receptor [57]. In spite of this, the clinical benefits of gepants are very similar, suggesting that CGRP signaling promoting migraine likely requires interactions with both CLR and RAMP1. Preclinical studies demonstrated that while no sex difference is observed in CLR protein levels [58], administration of inflammatory mediators onto the dura mater of rodents induced increased mRNA expression of CGRP-R components in females when compared to males [59]. Additional studies in preclinical models and in human tissues are thus warranted and may help to clarify whether differences in expression of CGRP signaling components can contribute to female selective actions of CGRP and CGRP-R antagonists in migraine.
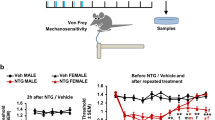
Preclinical studies have also consistently demonstrated that prolactin is selective in sensitizing female nociceptors and associated with increased release of CGRP [44, 60]. Application of prolactin to the mouse dura mater produces headache-like pain behaviors in female, but not male, mice that is blocked by either a prolactin receptor antagonist or by a CGRP receptor antagonist [44, 46]. These studies reveal cross-talk between prolactin and CGRP that is relevant to migraine-like pain in females (Fig. 1) [44, 46]. Stress-induced sensitization of trigeminal ganglion (TG) afferents to a transient receptor potential ankyrin 1 (TRPA1) agonist that has been linked to headache in humans has also been shown to be blocked by dopaminergic agonists or by interference with prolactin signaling at prolactin receptors selectively in female mice [47].
Cross-talk between prolactin and CGRP to produce migraine-like pain selective to females. A Increased CGRP release can be observed following prolactin sensitization of nociceptors selectively in females. B Application of prolactin to the mouse dura mater produces headache-like pain behaviors in female, but not male, mice. Prolactin-induced migraine-like pain behavior in females is blocked by a CGRP receptor antagonist [46]. C Application of CGRP to the mouse dura mater produces female-selective headache-like pain behavior which is blocked by a prolactin receptor antagonist [46]. PRL may be an upstream mechanism for CGRP-related migraine-like pain selectively in females
Sexual dimorphism in rodent and human nociceptors
Recent discoveries have challenged our long-held understanding of somatosensory mechanisms promoting the perception of pain and headache. Nociceptors are sensory fibers with cell bodies residing in dorsal root ganglia (DRG) and TG that detect and transmit high intensity stimuli capable of eliciting actual or potential tissue damage from the peripheral tissues to the central nervous system [61]. Remarkably, analyses of human post-mortem DRG neurons have revealed that these cells are sexually dimorphic [62]. Differences in transcript, protein and function have emerged from analysis of sensory neurons of rodents, non-human primates and humans [62,63,64,65,66]. A key observation is sexual dimorphism in transcript for CGRP that is expressed at higher levels in DRG neurons recovered from human female, compared to male, donors [62]. Additionally, transcriptome analysis of non-neuronal satellite cells in post-mortem human DRG have also been found to differ between male and female tissues suggesting additional sexually dimorphic mechanisms that may promote neuronal activation and signaling [67, 68]. Most recently, sexual dimorphism has been observed in human nociceptors at the protein and functional level [65]. Thus, nociceptors, the fundamental building blocks of pain are different in men and women and, by extension, the mechanisms that may promote pain can also differ between the sexes.
Prolactin signals via homodimers of prolactin receptor (PRLR) long and short isoforms. PRLR homo dimers are composed of isoforms of the receptor. Prolactin (PRL) signaling at the long and short PRLR isoforms (PRLR-L and PRLR-S) have different effects. While signaling at homodimers of the PRLR long isoform activates intracellular gene transcription pathways, signaling at homodimers of the PRLR short isoform is pronociceptive by sensitizing effectors such as transient receptor potential (TRP) channels [47, 69,70,71,72]. Interestingly, PRLR heterodimers composed of two different receptor isoforms result in silencing of signaling [69, 73,74,75]. Thus, the balance of expression of the PRLR long and short isoforms determines the consequences of prolactin signaling. The female selectivity of PRL in promoting migraine-like pain behavior was confirmed by direct dural application in rodents [46]. PRL produced sustained and long-lasting migraine-like behavior in cycling and ovariectomized female, but not male rodents [46]. Consistent with this observation, PRLR are expressed at higher levels in DRG cells recovered from rodents, monkeys and from post-mortem female donors [65]. Furthermore, incubation of nociceptors from female, but not male, DRG cells including from human donors with prolactin produced increased excitability indicating dissociation of the properties of these cells [65].
Collectively, these studies support the conclusion that there are male and female nociceptors that can be distinguished at the transcript, protein and functional levels. While limited by assessment of a very small sample size, it appears that similar conclusions can be drawn from analysis of human TG neurons [76] Sexual dimorphism of nociceptors and the profound female selectivity of both CGRP and prolactin suggests an opportunity for the discovery and implementation of therapies for treatment of pain and migraine based on patient sex. Collectively, the growing body of evidence endorsing the functional distinction between “male” and “female” nociceptors might pave the way for an advanced understanding of mechanisms by which pain is produced in men and in women.
CGRP is causal in promoting migraine in humans
Three crucial lines of evidence support the contribution of CGRP as a migraine substrate in humans: (a) increased levels of this transmitter were observed in the jugular outflow of people with migraine during attacks and normalization of CGRP levels was observed following relief of headache pain with sumatriptan [77]; (b) infusion of CGRP in people with migraine provokes headache with a migraine phenotype [54] and, as noted above, (c) therapies that prevent CGRP signaling are clinically effective. Critically, however, evidence supporting CGRP was obtained from studies conducted almost exclusively in women limiting conclusions regarding the causal role of CGRP across sexes. The initial study demonstrating elevated CGRP levels during migraine and normalization by sumatriptan was conducted in 8 patients, 7 of whom were women [77]. While all of the female patients showed elevated CGRP levels during migraine, responded to sumatriptan, and returned to baseline CGRP levels after treatment, the one male patient did not exhibit elevated CGRP during migraine, did not respond to sumatriptan and CGRP levels remained unchanged after treatment [77]. Thus, whether the conclusions of this seminal study may apply to men generally remains uncertain. Provocative studies of CGRP infusion to elicit migraine have also been conducted almost exclusively in women [78]. Such studies must be interpreted with caution as the pharmacological dose of CGRP used to elicit migraine may not reflect the contribution of endogenous CGRP in the trigeminal system during naturally occurring migraine. The clinical effectiveness of the gepants for acute migraine therapy provides the most convincing evidence for a causal role of CGRP in initiating a migraine attack.
Sexual dimorphism in acute migraine therapy
The clinical effectiveness of the gepant drugs as well as CGRP-based antibodies strongly support the conclusion of a causal role of CGRP in migraine pathophysiology. However, CGRP-R antagonists are not effective in all patients for acute or preventive migraine treatment. Understanding which patient groups would preferentially respond to CGRP-R antagonists would improve patient care and avoid the use of these drugs in likely non-responders. An important consideration, therefore, is whether CGRP-R antagonists are effective in both men and women [7, 8].
The clinical and statistical reviews conducted by the FDA Center for Drug Evaluation and Research (CDER) concerning the New Drug Applications (NDA) for ubrogepant, rimegepant and zavegepant in migraine therapy are publicly available. These reviews supported the conclusion of efficacy in women based on a sex-specific analysis of pooled pivotal trial data for both CGRP-R antagonists effects on the co-primary endpoints of pain freedom (PF) and absence of the most bothersome symptom (MBS) at the 2-hour time point [73, 74]. However, there was no evidence of efficacy for acute migraine treatment with these drugs in men. For example, ubrogepant demonstrated a treatment benefit effect over placebo of 8.3% for PF and 12.4% for MBS in women while effect over placebo was 0.2% for PF and 0.7% for MBS in men [79]. Similarly, the treatment benefit effect of rimegepant over placebo in women was 8.9% for PF and 10.2% for MBS, while in men, the rimegepant benefit effect over placebo was 1.1% for PF and 6.6% for MBS [80]. These findings highlight the sex-based differences in the impact of CGRP-related migraine treatments and reveal that CGRP-R antagonists demonstrate significant therapeutic benefit in the acute treatment of migraine in women but do not provide evidence of efficacy in men [7, 8].
The reasons for the apparent enhanced efficacy of CGRP-R antagonists for acute migraine in women remain unclear. The current absence of therapeutic benefit in men might be attributed to potential limitations in statistical power as most clinical trials including those with ubrogepant and rimegepant were comprised predominately of women. Other possibilities may also be considered. For example, women might treat attacks earlier resulting in improved effects. However, all patients are required to wait until pain is at least moderate before administering study drug. Also, improved outcomes are not seen across a range of other therapies including triptans [33]. Based on currently available data, it is not possible to conclude that these drugs provide clinical benefit for acute migraine treatment in men. It remains to be determined if this conclusion is supported by future studies. Nevertheless, the female selectivity of CGRP in promoting migraine-like pain and the apparent female selective effects of CGRP-R antagonists support the possibility that fundamental biological differences exist between men and women in mechanisms of migraine, and other, pain conditions.
Data from the CDER reviews of gepant drugs are also consistent with the likely influence of female hormones across life. Possibly diminished efficacy in promoting PF from acute migraine at the 2-hour timepoint after treatment with ubrogepant and rimegepant was observed in a small number of patients over age 65. While the reported data from these trials were not separated by sex, both trials included a high percentage of women (approximately 85%) suggesting that the decreased effect observed in older patients may represent the contributions of a large group of post-menopausal women. Similarly, recent CDER evaluation of zavegepant data reveal diminished effects when studied in patients over the age of 40 [81]. In patients younger than 40, zavegepant was significantly more effective in producing pain freedom at 2 h than in those over age 40 [81]. This trial also did not separate the data by sex but the population over the age of 40 likely includes a larger proportion of post-menopausal women than those under 40 [81]. Possible differences may thus occur in the efficacy of CGRP-R antagonists in postmenopausal women who generally have lower CGRP levels [43]. The smaller effect observed in older patients is therefore consistent with diminished effects of CGRP-R antagonism in postmenopausal women.
Concluding remarks and future perspectives
Despite increasing evidence of sexual dimorphism in neurological diseases, patient sex is usually not considered in the choice of therapies for migraine and other pain conditions. The great majority of historical preclinical studies on pain and migraine have exclusively used male rodents [3, 82]. In the past decades, the funding agencies mandate policies requiring the incorporation of sex as a variable in preclinical research [82]. This mandate has led to important findings revealing sexual dimorphism in human and animal nociceptors, suggesting the significant sex-related distinction in migraine and pain pathophysiology. This emerging perspective underscores the need to factor in patient sex when determining the most appropriate therapeutic approach and to address potential qualitative sex differences in neurological disorders. Information from preclinical studies appear to align well with clinical data and supports the conclusion of a qualitative sex difference in migraine pathophysiology related to female selectivity of prolactin and CGRP. The relative absence of data demonstrating clinical benefits of CGRP-R antagonists for acute migraine treatment in men suggests the need for a careful consideration of decision making in the choice of care and for discussion and patient disclosure. Additionally, to establish efficacy in men, dedicated and appropriately powered clinical trials should be performed. Similarly, efficacy estimates should be established for pre- and postmenopausal women separately. Consequently, addressing the unmet medical need for satisfactory migraine therapy in men calls for approaches that extend beyond CGRP-based mechanisms. The female selective role of prolactin in sensitization of trigeminal nociceptors suggest a therapeutic opportunity for development of novel prolactin targeting therapies including anti-prolactin antibodies [83].
These findings with migraine also raise the possibility that conclusions as to the efficacy of CGRP-based therapies for other, non-migraine, but female prevalent pain conditions such as fibromyalgia, irritable bowel syndrome and others could have been influenced by the relative proportion of men and women, as well as the percentage of pre- vs. postmenopausal women, in any given trial. Overall, our conceptual view of therapy for migraine and other pain conditions requires adjustment so that studies consider patient sex as the most fundamental aspect of precision medicine. The future direction in the field of migraine management should prioritize personalized treatment strategies that take into account the sex-related differences in pathophysiology, thus paving the way for more effective and tailored care for individuals suffering from migraine.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AM1:
-
adrenomedullin receptors 1
- AM2:
-
adrenomedullin receptors 2
- AMY1:
-
amylin 1 receptor
- CDER:
-
Center for Drug Evaluation and Research
- CGRP:
-
calcitonin gene-related peptide
- CGRP-R:
-
CGRP-receptor
- CLR:
-
calcitonin-like receptor
- CM:
-
chronic migraine
- DRG:
-
dorsal root ganglia
- EM:
-
episodic migraine
- ICHD-3:
-
International Classification of Headache Disorders
- NSAIDs:
-
non-steroidal anti-inflammatory drugs
- mAbs:
-
monoclonal antibodies
- MBS:
-
most bothersome symptom
- MOH:
-
medication overuse headache
- NDA:
-
New Drug Applications
- PF:
-
pain freedom
- PRL:
-
prolactin
- PRLR:
-
prolactin receptor
- PRLR-L:
-
prolactin receptor long isoform
- PRLR-S:
-
prolactin receptor short isoform
- RAMP1:
-
receptor activity modifying protein 1
- TIDA:
-
dopaminergic tuberoinfundibular
- TG:
-
trigeminal ganglion
- TRP:
-
transient receptor potential
- TRPA1:
-
transient receptor potential ankyrin 1
References
Canavan C, West J, Card T (2014) The epidemiology of irritable bowel syndrome. Clin Epidemiol 6:71–80. https://doi.org/10.2147/CLEP.S40245
Mogil JS (2012) Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13(12):859–866. https://doi.org/10.1038/nrn3360
Mogil JS (2020) Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 21(7):353–365. https://doi.org/10.1038/s41583-020-0310-6
Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ (2012) Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain 13(3):228–234. https://doi.org/10.1016/j.jpain.2011.11.002
Steingrimsdottir OA, Landmark T, Macfarlane GJ, Nielsen CS (2017) Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. Pain 158(11):2092–2107. https://doi.org/10.1097/j.pain.0000000000001009
Bartley EJ, Fillingim RB (2013) Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111(1):52–58. https://doi.org/10.1093/bja/aet127
Porreca F, Navratilova E, Hirman J, van den Brink AM, Lipton RB, Dodick DW (2024) Evaluation of outcomes of calcitonin gene-related peptide (CGRP)-targeting therapies for acute and preventive migraine treatment based on patient sex. Cephalalgia 44(3):3331024241238153. https://doi.org/10.1177/03331024241238153
Porreca FN, Navratilova E, Hirman J, MaassenVanDenBrink A, Lipton RB, Dodick DW (2023) Translational impact of basic science promotes a new era of precision medicine for migraine. MedRxIV (preprint)
Diseases GBD, Injuries C (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet 396(10258):1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z (2020) Lifting the Burden: the global campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21(1):137. https://doi.org/10.1186/s10194-020-01208-0
Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A et al (2007) The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 27(3):193–210. https://doi.org/10.1111/j.1468-2982.2007.01288.x
Ahmad SR, Rosendale N, Sex, Gender Considerations in Episodic Migraine (2022) Curr Pain Headache Rep 26(7):505–516. https://doi.org/10.1007/s11916-022-01052-8
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF et al (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68(5):343–349. https://doi.org/10.1212/01.wnl.0000252808.97649.21
Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM et al (2013) Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 53(8):1278–1299. https://doi.org/10.1111/head.12150
Brennan KC, Pietrobon D, A Systems Neuroscience Approach to Migraine (2018) Neuron 97(5):1004–1021. https://doi.org/10.1016/j.neuron.2018.01.029
Dodick DW (2018) A phase-by-phase review of Migraine Pathophysiology. Headache 58 Suppl 14–16. https://doi.org/10.1111/head.13300
Olesen J (2018) International classification of Headache disorders. Lancet Neurol 17(5):396–397. https://doi.org/10.1016/S1474-4422(18)30085-1
Close LN, Eftekhari S, Wang M, Charles AC, Russo AF (2019) Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia 39(3):428–434. https://doi.org/10.1177/0333102418774299
Lemale CL, Luckl J, Horst V, Reiffurth C, Major S, Hecht N et al (2022) Migraine aura, transient ischemic attacks, stroke, and dying of the Brain share the same key pathophysiological process in neurons driven by Gibbs-Donnan Forces, namely spreading depolarization. Front Cell Neurosci 16:837650. https://doi.org/10.3389/fncel.2022.837650
Vinogradova LV (2018) Initiation of spreading depression by synaptic and network hyperactivity: insights into trigger mechanisms of migraine aura. Cephalalgia 38(6):1177–1187. https://doi.org/10.1177/0333102417724151
Buse DC, Rupnow MF, Lipton RB (2009) Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc 84(5):422–435. https://doi.org/10.1016/S0025-6196(11)60561-2
Dahlof CG, Dimenas E (1995) Migraine patients experience poorer subjective well-being/quality of life even between attacks. Cephalalgia 15(1):31–36. https://doi.org/10.1046/j.1468-2982.1995.1501031.x
Dueland AN, Leira R, Cabelli ST (2005) The impact of migraine on psychological well-being of young women and their communication with physicians about migraine: a multinational study. Curr Med Res Opin 21(8):1297–1305. https://doi.org/10.1185/030079905X56394
Linde M, Dahlof C (2004) Attitudes and burden of disease among self-considered migraineurs–a nation-wide population-based survey in Sweden. Cephalalgia 24(6):455–465. https://doi.org/10.1111/j.1468-2982.2004.00703.x
Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML (1999) Burden of migraine in the United States: disability and economic costs. Arch Intern Med 159(8):813–818. https://doi.org/10.1001/archinte.159.8.813
Seddik AH, Branner JC, Ostwald DA, Schramm SH, Bierbaum M, Katsarava Z (2020) The socioeconomic burden of migraine: an evaluation of productivity losses due to migraine headaches based on a population study in Germany. Cephalalgia 40(14):1551–1560. https://doi.org/10.1177/0333102420944842
Stewart WF, Lipton RB (1994) The economic and social impact of migraine. Eur Neurol 34 Suppl 2:12 – 7. https://doi.org/10.1159/000119527
Karsan N, Goadsby PJ (2022) New oral drugs for Migraine. CNS Drugs 36(9):933–949. https://doi.org/10.1007/s40263-022-00948-8
Lee MJ, Al-Karagholi MA, Reuter U (2023) New migraine prophylactic drugs: current evidence and practical suggestions for non-responders to prior therapy. Cephalalgia 43(2):3331024221146315. https://doi.org/10.1177/03331024221146315
Puledda F, Silva EM, Suwanlaong K, Goadsby PJ (2023) Migraine: from pathophysiology to treatment. J Neurol 270(7):3654–3666. https://doi.org/10.1007/s00415-023-11706-1
Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A (2022) Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol 21(3):284–294. https://doi.org/10.1016/S1474-4422(21)00409-9
de Vries Lentsch S, Rubio-Beltran E, MaassenVanDenBrink A (2021) Changing levels of sex hormones and calcitonin gene-related peptide (CGRP) during a woman’s life: implications for the efficacy and safety of novel antimigraine medications. Maturitas 145:73–77. https://doi.org/10.1016/j.maturitas.2020.12.012
van Casteren DS, Kurth T, Danser AHJ, Terwindt GM, MaassenVanDenBrink A (2021) Sex differences in response to triptans: a systematic review and Meta-analysis. Neurology 96(4):162–170. https://doi.org/10.1212/WNL.0000000000011216
Szewczyk AK, Ulutas S, Akturk T, Al-Hassany L, Borner C, Cernigliaro F et al (2023) Prolactin and oxytocin: potential targets for migraine treatment. J Headache Pain 24(1):31. https://doi.org/10.1186/s10194-023-01557-6
Stewart WF, Wood C, Reed ML, Roy J, Lipton RB, Group AA (2008) Cumulative lifetime migraine incidence in women and men. Cephalalgia 28(11):1170–1178. https://doi.org/10.1111/j.1468-2982.2008.01666.x
Pavlovic JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS et al (2016) Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology 87(1):49–56. https://doi.org/10.1212/WNL.0000000000002798
MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 67(12):2154–2158. https://doi.org/10.1212/01.wnl.0000233888.18228.19
Bharadwaj VN, Porreca F, Cowan RP, Kori S, Silberstein SD, Yeomans DC (2021) A new hypothesis linking oxytocin to menstrual migraine. Headache 61(7):1051–1059. https://doi.org/10.1111/head.14152
Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB (2014) Trigger factors and premonitory features of migraine attacks: summary of studies. Headache 54(10):1670–1679. https://doi.org/10.1111/head.12468
Lyall M, de Oliveira BR, Mody SK (2023) Considerations for contraceptive use among patients with migraines. Curr Obstet Gynecol Rep. https://doi.org/10.1007/s13669-023-00349-8
van Lohuizen R, Paungarttner J, Lampl C, MaassenVanDenBrink A, Al-Hassany L (2023) Considerations for hormonal therapy in migraine patients: a critical review of current practice. Expert Rev Neurother 24(1):1–21. https://doi.org/10.1080/14737175.2023.2296610
Labastida-Ramirez A, Rubio-Beltran E, Villalon CM, MaassenVanDenBrink A (2019) Gender aspects of CGRP in migraine. Cephalalgia 39(3):435–444. https://doi.org/10.1177/0333102417739584
Raffaelli B, Storch E, Overeem LH, Terhart M, Fitzek MP, Lange KS et al (2023) Sex hormones and calcitonin gene-related peptide in women with migraine: a cross-sectional, matched Cohort Study. Neurology 100(17):e1825–e35. https://doi.org/10.1212/WNL.0000000000207114
Al-Karagholi MA, Kalatharan V, Ghanizada H, Dussor G, Ashina M (2023) Prolactin in headache and migraine: a systematic review of preclinical studies. Headache 63(5):577–584. https://doi.org/10.1111/head.14412
Al-Karagholi MA, Kalatharan V, Ghanizada H, Gram C, Dussor G, Ashina M (2023) Prolactin in headache and migraine: a systematic review of clinical studies. Cephalalgia 43(2):3331024221136286. https://doi.org/10.1177/03331024221136286
Avona A, Mason BN, Burgos-Vega C, Hovhannisyan AH, Belugin SN, Mecklenburg J et al (2021) Meningeal CGRP-Prolactin Interaction Evokes Female-Specific Migraine Behavior. Ann Neurol. 2021;89(6):1129-44. https://doi.org/10.1002/ana.26070
Watanabe M, Kopruszinski CM, Moutal A, Ikegami D, Khanna R, Chen Y et al (2022) Dysregulation of serum prolactin links the hypothalamus with female nociceptors to promote migraine. Brain 145(8):2894–2909. https://doi.org/10.1093/brain/awac104
Lipton RB, Buse DC, Hall CB, Tennen H, Defreitas TA, Borkowski TM et al (2014) Reduction in perceived stress as a migraine trigger: testing the let-down headache hypothesis. Neurology 82(16):1395–1401. https://doi.org/10.1212/WNL.0000000000000332
Oliveira MDC, Barea LM, Horn APK, Ongaratti BR, Soares JOD, Araujo B et al (2020) Resolution of headache after reduction of prolactin levels in hyperprolactinemic patients. Arq Neuropsiquiatr 78(1):28–33. https://doi.org/10.1590/0004-282X20190143
Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G (2019) Dural Calcitonin Gene-related peptide produces female-specific responses in Rodent Migraine models. J Neurosci 39(22):4323–4331. https://doi.org/10.1523/JNEUROSCI.0364-19.2019
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313(5997):54–56. https://doi.org/10.1038/313054a0
Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L (2010) Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169(2):683–696. https://doi.org/10.1016/j.neuroscience.2010.05.016
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L (2013) Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 14(11):1289–1303. https://doi.org/10.1016/j.jpain.2013.03.010
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J (2002) CGRP may play a causative role in migraine. Cephalalgia 22(1):54–61. https://doi.org/10.1046/j.1468-2982.2002.00310.x
Mulderry PK, Ghatei MA, Bishop AE, Allen YS, Polak JM, Bloom SR (1985) Distribution and chromatographic characterisation of CGRP-like immunoreactivity in the brain and gut of the rat. Regul Pept 12(2):133–143. https://doi.org/10.1016/0167-0115(85)90194-6
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4):1099–1142. https://doi.org/10.1152/physrev.00034.2013
Garelja ML, Walker CS, Hay DL (2022) CGRP receptor antagonists for migraine. Are they also AMY(1) receptor antagonists? Br J Pharmacol 179(3):454–459. https://doi.org/10.1111/bph.15585
Ji Y, Rizk A, Voulalas P, Aljohani H, Akerman S, Dussor G et al (2019) Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus. Neurobiol Pain 6:100031. https://doi.org/10.1016/j.ynpai.2019.100031
Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE et al (2011) Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache 51(5):674–692. https://doi.org/10.1111/j.1526-4610.2011.01882.x
Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN et al (2006) Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci 26(31):8126–8136. https://doi.org/10.1523/JNEUROSCI.0793-06.2006
Dubin AE, Patapoutian A (2010) Nociceptors: the sensors of the pain pathway. J Clin Invest 120(11):3760–3772. https://doi.org/10.1172/JCI42843
Tavares-Ferreira D, Shiers S, Ray PR, Wangzhou A, Jeevakumar V, Sankaranarayanan I et al (2022) Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci Transl Med 14(632):eabj8186. https://doi.org/10.1126/scitranslmed.abj8186
Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV et al (2020) Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep 10(1):15278. https://doi.org/10.1038/s41598-020-72285-z
Ray PR, Shiers S, Caruso JP, Tavares-Ferreira D, Sankaranarayanan I, Uhelski ML et al (2023) RNA profiling of human dorsal root ganglia reveals sex differences in mechanisms promoting neuropathic pain. Brain 146(2):749–766. https://doi.org/10.1093/brain/awac266
Stratton HDM, Dumaire N, Moutal A, ghetti A, Cotta T, Mitchell S, Yue X, Navratilova E, Porreca F (2023) Functional Sexual Dimorphism in Human Nociceptors. BioRxiv
Tavares-Ferreira D, Ray PR, Sankaranarayanan I, Mejia GL, Wangzhou A, Shiers S et al (2022) Sex differences in Nociceptor Translatomes Contribute to Divergent Prostaglandin Signaling in male and female mice. Biol Psychiatry 91(1):129–140. https://doi.org/10.1016/j.biopsych.2020.09.022
Shen BQ, Sankaranarayanan I, Price TJ, Tavares-Ferreira D (2023) Sex-differences in prostaglandin signaling: a semi-systematic review and characterization of PTGDS expression in human sensory neurons. Sci Rep 13(1):4670. https://doi.org/10.1038/s41598-023-31603-x
Reichling DB, Green PG, Levine JD (2013) The fundamental unit of pain is the cell. Pain 154 Suppl 1S2–9. https://doi.org/10.1016/j.pain.2013.05.037
Belugin S, Diogenes AR, Patil MJ, Ginsburg E, Henry MA, Akopian AN (2013) Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons. J Biol Chem 288(48):34943–34955. https://doi.org/10.1074/jbc.M113.486571
Ikegami D, Navratilova E, Yue X, Moutal A, Kopruszinski CM, Khanna R et al (2022) A prolactin-dependent sexually dimorphic mechanism of migraine chronification. Cephalalgia 42(3):197–208. https://doi.org/10.1177/03331024211039813
Kopruszinski CM, Porreca F, Chichorro JG (2022) Editorial: chronic orofacial pain. Front Pain Res (Lausanne) 3:1086256. https://doi.org/10.3389/fpain.2022.1086256
Kopruszinski CM, Watanabe M, Martinez AL, de Souza LHM, Dodick DW, Moutal A et al (2023) Kappa opioid receptor agonists produce sexually dimorphic and prolactin-dependent hyperalgesic priming. Pain 164(6):e263–e73. https://doi.org/10.1097/j.pain.0000000000002835
Gadd SL, Clevenger CV (2006) Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol 20(11):2734–2746. https://doi.org/10.1210/me.2006-0114
Qazi AM, Tsai-Morris CH, Dufau ML (2006) Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol 20(8):1912–1923. https://doi.org/10.1210/me.2005-0291
Xie YL, Hassan SA, Qazi AM, Tsai-Morris CH, Dufau ML (2006) Intramolecular disulfide bonds of the prolactin receptor short form are required for its inhibitory action on the function of the long form of the receptor. Mol Cell Biol 29(10):2546–2555. https://doi.org/10.1128/MCB.01716-08
Yang L, Xu M, Bhuiyan SA, Li J, Zhao J, Cohrs RJ et al (2022) Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron 110(11):1806–1821. https://doi.org/10.1016/j.neuron.2022.03.003
Goadsby PJ, Edvinsson L (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33(1):48–56. https://doi.org/10.1002/ana.410330109
Christensen CE, Younis S, Deen M, Khan S, Ghanizada H, Ashina M (2018) Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J Headache Pain 19(1):105. https://doi.org/10.1186/s10194-018-0927-2
Ubrogepant (2019) New drug application (NDA); Center for Drug Evaluation and Research (CDER); 211765 – Orig1s000 – Clinical Review(s), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211765Orig1s000MedR.pdf (Accessed 3 January 2024)
Rimegepant JL (2020) New drug application (NDA); Center for Drug Evaluation and Research (CDER); 212728 – Orig1s000 – Clinical Review(s), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212728Orig1s000MedR.pdf (Accessed 4 January 2024)
R. K (2023) New drug application (NDA); Center for Drug Evaluation and Research (CDER); 216386 – Orig1s000 – Clinical Review(s), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216386Orig1s000MedR.pdf (accessed January 4 2024)
Bolay H, Berman NE, Akcali D (2011) Sex-related differences in animal models of migraine headache. Headache 51(6):891–904. https://doi.org/10.1111/j.1526-4610.2011.01903.x
Maciuba S, Bowden GD, Stratton HJ, Wisniewski K, Schteingart CD, Almagro JC et al (2023) Discovery and characterization of prolactin neutralizing monoclonal antibodies for the treatment of female-prevalent pain disorders. MAbs 15(1):2254676. https://doi.org/10.1080/19420862.2023.2254676
Acknowledgements
Not applicable.
Funding
This review was supported by NIH fundings (R01NS1295, R01NS114888, P01DA041307 and P30DA051355). The funding source did not influence the work.
Author information
Authors and Affiliations
Contributions
EN and FP designed the concept of the review. SS, CMK, MW, EN, and FP drafted, wrote, revised, and approved the manuscript. DWD read, revised, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable. The image from Fig. 1 was made by the scientific illustration software Biorender. “Confirmation of Publication and Licensing Rights” of “Biorender” is included.
Competing interests
Shagun Singh, Caroline M. Kopruszinski, Moe Watanabe and Edita Navratilova declare that they have no personal, financial, or relational conflicts of interest with this work. Frank Porreca has served as a consultant or received research funding from Amgen, Acadia, Blackthorn, Teva, Abbvie, Eli Lilly, Hoba, Allergan, Ipsen, and Proximagen and is a founder of Catalina Pharma and Axon Therapeutics. David W. Dodick reports the following conflicts within the past 12 36 months: Consulting: Amgen, Atria, CapiThera Ltd., Cerecin, Ceruvia Lifesciences LLC, CoolTech, Ctrl M, Allergan, AbbVie, Biohaven, GlaxoSmithKline, Lundbeck, Eli Lilly, Novartis, Impel, Satsuma, Theranica, WL Gore, Genentech, Nocira, Perfood, Praxis, AYYA Biosciences, Revance, Pfizer. Honoraria: American Academy of Neurology, Headache Cooperative of the Pacific, Canadian Headache Society, MF Med Ed Research, Biopharm Communications, CEA Group Holding Company (Clinical Education Alliance LLC), Teva (speaking), Amgen (speaking), Eli Lilly (speaking), Lundbeck (speaking), Pfizer (speaking), Vector Psychometric Group, Clinical Care Solutions, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, Medica Communications LLC, MJH Lifesciences, Miller Medical Communications, WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Non-profit board membership: American Brain Foundation, American Migraine Foundation, ONE Neurology, Precon Health Foundation, International Headache Society Global Patient Advocacy Coalition, Atria Health Collaborative, Arizona Brain Injury Alliance, Domestic Violence HOPE Foundation/Panfila. Research support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Henry Jackson Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock options/shareholder/patents/board of directors: Aural analytics (options), Axon Therapeutics (shares/board), ExSano (options), Palion (options), Man and Science, Healint (options), Theranica (options), Second Opinion/Mobile Health (options), Epien (options), Nocira (options), Matterhorn (shares), Ontologics (shares), King-Devick Technologies (options/board), EigenLyfe (shares), AYYA Biosciences (options), Cephalgia Group (shares/board), Atria Health (options/employee). Patent 17189376.1-1466:vTitle: Onabotulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis (Non-royalty bearing). Patent application submitted: Synaquell® (Precon Health).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Singh, S., Kopruszinski, C.M., Watanabe, M. et al. Female-selective mechanisms promoting migraine. J Headache Pain 25, 63 (2024). https://doi.org/10.1186/s10194-024-01771-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-024-01771-w