Abstract
Objective
To explore and critically appraise the evidence supporting the role of estrogen withdrawal in menstrual migraine.
Main body
Menstrual migraine, impacting about 6% of reproductive-age women, manifests as migraine attacks closely related to the menstrual cycle. The estrogen withdrawal hypothesis posits that the premenstrual drop in estrogen levels serves as a trigger of migraine attacks. Despite its wide acceptance, the current body of evidence supporting this hypothesis remains limited, warranting further validation. Estrogen is believed to exert a modulatory effect on pain, particularly within the trigeminovascular system – the anatomic and physiologic substrate of migraine pathogenesis. Nevertheless, existing studies are limited by methodologic inconsistencies, small sample sizes, and variable case definitions, precluding definitive conclusions. To improve our understanding of menstrual migraine, future research should concentrate on untangling the intricate interplay between estrogen, the trigeminovascular system, and migraine itself. This necessitates the use of robust methods, larger sample sizes, and standardized case definitions to surmount the limitations encountered in previous investigations.
Conclusion
Further research is thus needed to ascertain the involvement of estrogen withdrawal in menstrual migraine and advance the development of effective management strategies to address unmet treatment needs.
Similar content being viewed by others
Background
Migraine is a disabling neurologic disease that affects more than one billion people worldwide, predominantly females [1]. Characteristic features include recurrent attacks of headache and accompanying symptoms such as photophobia, phonophobia, and nausea or vomiting [2]. Some affected people also experience transient neurologic disturbances, referred to as migraine aura, which tend to precede or accompany the headache [2].
An interesting clinical observation is the significant number of women of reproductive age who report migraine attacks, with and without aura, in relation to their menstruation [3,4,5]. This observation gave rise to the term ‘menstrual migraine’ and provided a foundation for understanding the disorder [6]. There are several hypotheses proposed to explain the etiology of menstrual migraine, with the estrogen withdrawal hypothesis being the most widely accepted. This hypothesis was first introduced by Brian W. Sommerville in 1972 [7], based on his experimental studies suggesting that the precipitous drop in estrogen shortly before menstruation increases the risk of developing a migraine attack [7,8,9]. However, conflicting results have since emerged, and the underlying mechanisms of menstrual migraine are still not entirely clear.
In this review, we undertake a critical appraisal of the estrogen withdrawal hypothesis, a long-standing concept to understanding menstrual migraine. Mindful of the rapidly evolving field of sex differences in migraine, we confine our focus to this particular hypothesis, which has shaped scientific discourse and exploration of menstrual migraine for more than five decades. Through a synthesis of evidence both supporting and contesting the estrogen withdrawal hypothesis, we elucidate existing gaps in understanding, aiming to stimulate further investigation into the exact disease mechanisms at play in menstrual migraine.
Terminology
Menstrual cycle
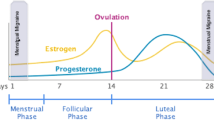
The human menstrual cycle has an average length of 28 days and is divided into a follicular phase and luteal phase, lasting approximately 14 days each [10, 11]. Each phase is furthermore stratified into an early-, mid-, and late-phase. The follicular phase commences on the first day of menstrual bleeding (i.e., day 1 of the menstrual cycle) and ends with ovulation around day 14 [10, 11]. The luteal phase then begins the day after ovulation and ends just before menstrual bleeding [10, 11]. Fluctuating levels of sex hormones regulate the menstrual cycle and include estrogen and progesterone [10, 11]. The concentration of estrogen is at its lowest during menstruation and then rises steadily throughout the follicular phase to peak the day before ovulation [10, 11]. A decline in estrogen levels then follows before a secondary smaller rise occurs during the mid-luteal phase. This concludes with a precipitous drop in estrogen levels, resulting in the onset of menstruation [10, 11]. An overview of the menstrual cycle and the related fluctuating levels of sex hormones are shown in Fig. 1.
Menstrual migraine
The diagnosis of migraine is established based on clinical criteria provided by the International Classification of Headache Disorders (ICHD) [12]. The 1st edition of the ICHD from 1988 did not include distinct diagnostic criteria for menstrual migraine but did provide some commentary [13]. The classification committee suggested that menstrual migraine can be used to describe a condition in which at least 90% of migraine attacks occur on day 1 ± 2 (i.e., days − 2 to + 3) of menstruation [13]. This definition was later revised in the 2nd edition of the ICHD from 2004 [14], in which appendix criteria were outlined for two types of menstrual migraine without aura: “pure menstrual migraine” and “menstrually-related migraine”. The same appendix criteria have been maintained in more recent editions of the ICHD, with the addition of criteria for both types of menstrual migraine in patients with aura symptoms [12]. Table 1 shows the appendix criteria for pure menstrual migraine and menstrually-related migraine, as described in the 3rd edition of the ICHD from 2018 [12]. Of note, the ICHD classifies menstruation as endometrial bleeding due to either the normal menstrual cycle or withdrawal bleeding during hormone-free intervals in regimens with hormonal contraception [12].
Epidemiology
The 1-year prevalence of migraine is estimated to be 15% in the general population, with a 3:1 female to male ratio [15]. The incidence rises steeply during adolescence and early adulthood, with about 50% of affected people reporting onset of migraine before the age of 25 years [16]. A familial aggregation of migraine is now well-established, and genetic factors play a major role in migraine etiology [2].
The prevalence of menstrual migraine is not well understood, primarily due to the scarcity of data and limitations in population-based studies that differ in case definitions and assessment methods used [17,18,19,20,21]. A Dutch study recruited 1181 women aged 13–55 years from the general population and evaluated for the presence of menstrual migraine based on a questionnaire [18]. The study found that 3% of women reported migraine attacks between day − 2 and day + 2 of the menstrual cycle, with 0.9% of women reporting attacks exclusively during this time. A Norwegian population-based study screened 3,514 women aged 30–34 years for possible menstrual migraine using a questionnaire and invited potential cases for a semi-structured interview to confirm adherence with the ICHD-3β appendix criteria [17]. The results showed that among women aged 30–34 years in the general population, 0.8% have pure menstrual migraine without aura, and 0.1% have pure menstrual migraine with aura. The corresponding figures were 5.3% for menstrually-related migraine without aura and 0.6% for menstrually-related migraine with aura. However, the Dutch and Norwegian population-based data must be interpreted with caution [17, 18]. A recent clinic-based study found that the accuracy of self-reported menstrual migraine is poor, compared with a diary-based diagnosis [22]. Future estimates on the prevalence of menstrual migraine should be based on prospectively collected data from headache diaries with daily entries and time stamps over at least three months.
Clinical presentation
Menstrual migraine can present with distinct clinical features that distinguish it from attacks outside the perimenstrual period. Diary-based studies have found that perimenstrual attacks are often more disabling and can persist up to 35% longer than those unrelated to the menstrual cycle [23,24,25,26,27]. Furthermore, some evidence suggests that perimenstrual attacks are associated with more severe pain and accentuated photophobia and phonophobia [23]. The therapeutic response to triptans also seems to differ between perimenstrual attacks and those outside the perimenstrual period, with the former having a higher recurrence rate of attacks after treatment [23]. Taken together, these observations suggest the need for further inquiries into the unique clinical features of perimenstrual migraine attacks.
Migraine pathogenesis
The trigeminovascular system is the anatomic and physiologic framework of migraine [28]. Nociceptive impulses originate from the primary afferents of sensory neurons whose cell bodies are located in the trigeminal and upper cervical ganglia (i.e., first order neurons) [28]. From here, ascending nociceptive information is conveyed to second-order neurons in the brainstem which, in turn, relay the information to third-order neurons in the thalamus [28]. The latter then projects the information to the somatosensory cortex and other cortical and subcortical areas, which are ultimately responsible for the perception of migraine pain and its accompanying symptoms [28].
The initial generation of nociceptive impulses is likely caused by various chemical agents, which are released from primary afferents of the trigeminal and upper cervical ganglia as well as parasympathetic efferents of the sphenopalatine ganglia [2]. These agents are known to promote dilation of meningeal arteries and sensitize perivascular nociceptors [2]. The most well-documented example is calcitonin gene-related peptide (CGRP) [29], albeit it merits emphasis that other agents are also involved.
Estrogen and the trigeminovascular system
Estrogen is a steroid hormone predominantly synthesized in the ovaries of females [30]. The endogenous natural estrogens include estrone (E1), estradiol (E2), estriol (E3), and estetrol (E4), with estradiol being the primary active estrogen in females of reproductive age [30]. There are three main types of estrogen receptors (ER): ERα, ERβ, and G protein-coupled ER (GPER), all of which are expressed at the level of the trigeminal ganglion and trigeminal nucleus caudalis as well as the hypothalamus [31]. Although it has been suggested that activation of ERs might sensitize trigeminovascular neurons [31], the available data to support this assertion is currently limited.
The estrogen withdrawal hypothesis
The estrogen withdrawal hypothesis was first described by Sommerville in 1972 [7]. He conducted an interventional study, in which six women with a “regular premenstrual or menstrual migraine” attack received intramuscular (IM) injection of estradiol before the onset of menstruation [7]. All six participants underwent blood sampling before the intervention. Three participants then had daily blood samples collected throughout the menstrual cycle. In the other three participants, daily blood samples were collected during the “premenstrual and menstrual phases” (without a clear temporal definition) of two consecutive menstrual cycles. The first cycle served as a control without any intervention, whilst the second cycle was preceded by IM injection of estradiol. In four participants, the intervention was 10-mg estradiol valerate in 3–10 days before the expected onset of menstruation. The remaining two participants received two injections of 5-mg estradiol valerate that were separated by a few days. The results showed that the expected onset of migraine had seemingly been delayed by a few days following the injection of estradiol valerate and, in some, even until after the end of menstrual bleeding. The delayed onset of migraine coincided notably with the precipitous drop in plasma levels of estradiol following a period of sustained high levels as a result of the injection. In addition, the injection increased plasma levels of estradiol but did not affect plasma levels of progesterone nor delay the onset of menstruation. Based on these findings, Sommerville proposed the estrogen withdrawal hypothesis, which states that the decline in plasma levels of estrogen shortly before menstruation increases the risk of developing a migraine attack.
To further explore the possible association of estrogen with menstrual migraine, Sommerville conducted an interventional study [9], in which women who had exclusively premenstrual or menstrual migraine attacks were enrolled. All participants received IM injection of either long-lasting or short-lasting estradiol formulations after the resolution of their usual menstrual migraine attacks. The results showed that three of four women who received long-lasting estradiol developed migraine attacks after plasma levels of estradiol had decreased following a sustained period of high levels. No migraine attacks were reported by the two women who had received short-lasting estradiol. Based on these observations, Sommerville posited that the development of menstrual migraine attacks is associated with decreasing levels of plasma estrogen after a prolonged period of high plasma levels [9]. He therefore asserted that repeated or continuous treatment with estradiol might prevent menstrual migraine attacks [8]. However, he did not find any therapeutic benefits with various formulations of estradiol [8].
The impact of Sommerville’s experiments has been profound, and his estrogen withdrawal hypothesis remains accepted by many in the migraine field (Fig. 2). However, it merits emphasis that his intervention studies were non-randomized, unblinded, and limited by small samples and inconsistent use of case definitions. It seems more reasonable to regard them as idea-generating, exploratory studies that require further validation through more rigorously designed experiments.
Treating migraine with estradiol
Since the early work by Sommerville [7,8,9], several clinical trials have been performed to evaluate the effectiveness of treating migraine with estradiol [6]. The administration of perimenstrual estradiol gel in small cohorts of women with menstrual migraine yielded a noteworthy reduction in migraine frequency when compared with placebo [32, 33]. Conversely, two studies that used estradiol patches (50 µg/24 h) reported negative results [34, 35]. The largest study to date was conducted in 2006 and enrolled 35 women with pure menstrual or menstrually-related migraine in adherence with the ICHD-2 criteria [36]. The study had a randomized, placebo-controlled, 2-way crossover design, in which participants were treated with estradiol gel for three menstrual cycles, followed by three cycles of placebo, or vice versa. The treatment period ranged from ten days after the peak fertility, as determined by daily measurements of sex hormones, until the second day of menstruation. Compared to placebo, estradiol treatment was associated with a 22% additional reduction of migraine days during the treatment period. However, the proportion of migraine days during estradiol vs. placebo treatment was only numerically in favor of estradiol but without statistical significance, which might be attributed to the small sample size and the study being underpowered. In line with the original observation by Somerville, migraine frequency increased in the five days after estradiol treatment (RR 1.40 compared to placebo).
The above discussion suggests that the effects of estrogen or its withdrawal on migraine depends on several factors, including dose, duration of exposure, and formulation. However, the available studies are limited by small samples and differences in definitions used. Thus, caution must be exercised in interpreting the current evidence, and further investigation in larger and better-defined samples is needed to ascertain the therapeutic promise of treating migraine with estradiol.
Insights from experimental studies
Estrogen can modulate several physiological processes in the body, including pain [37]. The effects of estrogen on pain are context-dependent and timing-specific, as it has been shown to possess both analgesic and hyperalgesic properties [37]. The fluctuations in estrogen levels that occur during the menstrual cycle, menopause, or surgical interventions (e.g., ovariectomy) can also affect pain perception [37].
Insights from animal experiments
Animal studies have produced conflicting findings on the relationship between the estrous cycle and pain perception in rodents [38]. Some studies report reduced pain sensitivity during the proestrus phase when estrogen levels are at their peak [39,40,41,42], and that sensitization of the trigeminal nucleus caudalis occurs in the second half of the cycle when estrogen levels are low [43]. Conversely, other animal studies have showed high-estrogen phases to be associated with increased pain sensitivity [44,45,46,47,48,49,50].
Ovariectomy is a surgical procedure that involves the removal of ovaries, leading to a significant reduction in estrogen levels. Animal models have demonstrated that ovariectomy can result in a decrease in pain threshold for various pain stimuli [51,52,53]. This effect can then be reversed by estrogen supplementation [51, 53,54,55,56]. However, there are also studies reporting no change or an increase in pain perception following ovariectomy in animal models [57, 58]. Therefore, the direction in which estrogen modulates pain perception remains unclear [37]. Some discrepancies might be explained by a site-specific hormonal sensitivity in nociception, with low estrogen states causing increased orofacial pain sensitivity with less effects on extracephalic pain [59].
It is uncertain whether findings from animal studies can be directly applied to migraine pain in humans [37]. This uncertainty stems from several factors. Firstly, the hormonal profile of the rodent estrous cycle differs from that of the human menstrual cycle, which limits comparisons [37]. Secondly, most animal studies have examined pain sensitivity in non-cephalic areas of the body [37], and thus may not reflect the perception of head pain characteristic of migraine. Lastly, migraine is a unique human experience, underscoring the importance of research involving humans to draw conclusive results.
Insights from human experiments
The menstrual cycle’s influence on pain perception was first reported by Herren in 1933 [60]. He studied five young women over 11 menstrual cycles and discovered that pain and touch two-point discrimination thresholds were lowest during the premenstrual phase. Later studies validated these findings by demonstrating that pain sensitivity to different kind of stimuli is higher during low estrogen phases and lower during high estrogen phases [61,62,63,64,65,66,67]. In one study, capsaicin was injected on the forehead of 14 healthy females during both their menstrual and luteal phases [68]. The pain area, as drawn by participants on a face chart, as well as the area of facial redness mapped by the investigators were larger during menstruation, which might indicate increased trigeminal sensitization during this phase. However, some studies have reported pain threshold changes in the opposite direction [69, 70], while others found no cyclical changes of pain thresholds in women [71,72,73,74,75,76,77].
Another line of experiments evaluated the laser-evoked potential (LEP) in the premenstrual period (i.e., 1–2 days prior to menstruation) and periovulatory period (i.e., 13–15 days prior to menstruation) of nine women with migraine and ten healthy controls [78]. Trigeminal nociception was tested by stimulating the supraorbital zone, while non-trigeminal nociception was assessed by stimulating the right hand. The results showed that women with migraine had an increased amplitude and reduced habituation of LEP in both phases, compared with healthy controls at both sites of testing. In addition, both groups had an increased amplitude and decreased habituation of LEP in the premenstrual phase. The authors suggested that reduced habituation of pain-relevant cortical structures prior to menstruation might increase the likelihood of developing migraine attacks. However, a study involving 32 women with migraine and 20 healthy controls did not find any differences in conditioned pain modulation across the menstrual cycle [79].
Two studies have provided insight into the possible association of nociceptive changes with menstrual migraine. In one study, 11 women were enrolled and all experienced migraine attacks exclusively during the hormone-free period when on a combined oral contraceptive regimen. This regimen entailed 21 days of hormone intake, followed by a 7-day hormone-free period (i.e., 21/7 regimen) [80]. The authors measured the nociceptive withdrawal reflex after sural nerve stimulation during the third week of active treatment and during the estrogen withdrawal period. A decrease in the reflex threshold was observed during the hormone-free interval, which underscores the potential roles of estrogen in modulating pain perception in menstrual migraine. In the other study involving 31 patients with pure menstrual or menstrually-related migraine, the trigeminovascular reflex was evaluated during menstruation and in the headache-free postmenstrual phase [81]. The authors observed shorter reflex latencies during menstruation, indicating a hormonal modulation in brainstem excitability. However, these changes might reflect differences between ictal and interictal phase of migraine rather than being related to hormonal factors, and further investigation is thus needed.
The human experimental studies into estrogen-dependent pain sensitivity remain inconclusive, likely due to differences in characteristics of the study populations and methodology. Despite this, some evidence does indicate that estrogen withdrawal facilitates pro-nociceptive responses, thereby potentially increasing the susceptibility to develop migraine attacks. To arrive at conclusive results, further research with larger sample sizes and standardized methodology is required.
Calcitonin gene-related peptide
The available evidence indicate that estrogen might modulate nociception in migraine, possibly by affecting chemical agents implicated in the genesis of cephalic pain. Among these, CGRP is the most extensively studied agent of interest [82]. The role of CGRP within the trigeminovascular system has been extensively investigated for almost four decades [29]. CGRP is released from primary afferents in the trigeminovascular system [83], and intravenous infusion of CGRP induces migraine attacks in people with migraine [84]. Furthermore, blocking CGRP signaling has proven effective for the acute and preventive treatment of migraine [29].
In some in vitro animal studies, estradiol has been found to reduce CGRP expression in the trigeminal ganglion and trigeminal nucleus caudalis of rodents, whereas ovariectomy led to increased CGRP gene expression [82]. It does however merit mention that other animal studies failed to detect any effect of estrogen on the trigeminovascular system [82].
The relationship between estrogen and CGRP in humans remains unclear, as studies have produced conflicting evidence. One small study reported higher CGRP plasma immunoreactivity in 24 females than in 13 males, with women using combined contraception having the highest CGRP concentrations [85]. However, the study did not account for menstrual cycle phase or hormonal levels. Another study with 55 women in different pregnancy states reported higher estrogen concentrations during pregnancy that decreased postpartum [86]. In healthy women, application of capsaicin to the forehead led to a higher increase in CGRP-dependent dermal blood flow measured by laser doppler perfusion imaging during menstruation compared to ovulation or other times of the menstrual cycle [87]. Other studies have, however, suggested the opposite relationship between estrogen and CGRP [88, 89]. Of note, a recent study reported higher interictal CGRP concentrations in the plasma and tear fluid of women with menstrually-related migraine during menstruation in comparison to healthy women, which could explain their heightened susceptibility to migraine during the perimenstrual period [90]. Further studies are, nonetheless, required to validate these findings. Interpretation of CGRP findings in humans must account for the potential for discrepancies and uncertainties between studies, which can arise due to differences in populations, biomaterials, and methodology. Overall, the current evidence suggests that a dysfunction in the modulation of the CGRP pathway might play a role in the development of menstrual migraine, albeit this assertion is based on preliminary findings. The intricate relationship between CGRP and estrogen offers a compelling direction for future research. It seems pertinent to determine if estrogen can modulate the responsiveness of meningeal nociceptors in rodents, both with and without the administration of CGRP. In addition to this, human experimental studies might evaluate if pre-treatment with estrogen can reduce the incidence of migraine attacks after intravenous infusion of CGRP. Such insights might advance our understanding of migraine pathogenesis and, in particular, the origins of perimenstrual attacks.
Genetic insights
The role of genetics in migraine is an active area of research [91], with some studies suggesting that genetic factors might be involved in the neurobiologic underpinnings of menstrual migraine [92]. Genetics might indeed regulate individual-level sensitivity to estrogen fluctuations, making some women more susceptible to develop menstrual migraine [92]. The current evidence is however conflicting, with, one meta-analysis finding two estrogen receptor polymorphisms (ESR-1 594 G > A and ESR-1 325 C > G) linked to migraine [93], whilst a recent genome-wide association study did not identify any specific hormonal factors among 123 loci connected to migraine [94]. These data derive from pooled populations of patients with all subtypes of migraine. Therefore, conclusive remarks cannot be made for those individuals with menstrual migraine based on these observations.
Limited evidence exists for the role of genetics in menstrual migraine which, in part, is attributed to the available data coming from small cohorts that use different case definitions. A British study reported no significant difference in functional polymorphisms of estrogen synthesis and metabolism genes (COMT, CYP1A1, and CYP19A1) between 268 women with menstrual migraine and 142 healthy controls [95]. This finding aligns well with an Italian study finding no association between COMT polymorphisms and pure menstrual or menstrually-related migraine in a cohort of 380 participants with migraine and 132 healthy individuals [96]. However, an American study identified one COMT polymorphism (rs4680) and two tyrosine hydroxylase gene polymorphisms (TH rs2070762 and TH rs6356) linked to self-reported menstrual migraine [97]. Moreover, two ESR-1 polymorphisms (rs2234693 and rs726281) were associated with menstrually-related migraine in Chinese and Turkish cohorts [98, 99]. A British analysis of 37 genetic variants in 14 genes also showed a significant association between menstrual migraine and genetic polymorphisms in the TNF and SYNE1 genes, while NRP1 is another potentially involved gene, with one polymorphism linked to menstrual migraine in the same British cohort [100]. It should be noted that each identified variant is likely to account for modest effects in increasing the risk of developing menstrual migraine. More research is therefore warranted to fully ascertain the role of genetics in menstrual migraine.
Conclusions
The estrogen-withdrawal hypothesis has been the leading theory to explain menstrual migraine since it was first proposed in the 1970s. However, a critical reappraisal reveals limited and conflicting evidence to support this hypothesis based on the available animal and human experimental studies. While it seems evident that estrogen fluctuations are linked to migraine pathophysiology, the exact mechanisms are still a subject to debate. Future research should focus on elucidating the pathogenesis of menstrual migraine in larger and well-defined human cohorts using consistent methodological procedures.
Availability of data and materials
Not applicable.
Abbreviations
- CGRP:
-
Calcitonin Gene-Related Peptide
- ER:
-
Estrogen receptor
- GPER:
-
G protein-coupled estrogen receptor
- ICHD:
-
International Classification of Headache Disorders
- IM:
-
Intramuscular
- LEP:
-
Laser-evoked potential
References
Ashina M (2020) Migraine. N Engl J Med 383(19):1866–1876
Ashina M, Terwindt GM, Al-Karagholi MA, de Boer I, Lee MJ, Hay DL, Schulte LH, Hadjikhani N, Sinclair AJ, Ashina H et al (2021) Migraine: disease characterisation, biomarkers, and precision medicine. Lancet 397(10283):1496–1504
Stewart WF, Lipton R, Chee E, Sawyer J, Silberstein SD (2000) Menstrual cycle and headache in a population sample of migraineurs. Neurology 55(10):1517–1523
MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 67(12):2154–2158
van Casteren DS, Verhagen IE, Onderwater GL, MaassenVanDenBrink A, Terwindt GM (2021) Sex differences in prevalence of migraine trigger factors: a cross-sectional study. Cephalalgia 41(6):643–648
Vetvik KG, MacGregor EA (2021) Menstrual migraine: a distinct disorder needing greater recognition. Lancet Neurol 20(4):304–315
Somerville BW (1972) The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 22(4):355–365
Somerville BW (1975) Estrogen-withdrawal migraine. II. Attempted prophylaxis by continuous estradiol administration. Neurology 25(3):245–250
Somerville BW (1975) Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology 25(3):239–244
Martin VT, Behbehani M (2006) Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis–part 2. Headache 46(3):365–386
Martin VT, Behbehani M (2006) Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis–part I. Headache 46(1):3–23
Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1-211
Headache Classification Committee of the International Headache Society (IHS) (1988) Diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classif Comm Int Headache Soc Cephalalgia 8(Suppl 7):1–96
Headache Classification Committee of the International Headache Society (IHS) (2004) The International Classification of Headache Disorders: 2nd edition. Cephalalgia 24 Suppl 1:9-160
Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, Krymchantowski AV, Lebedeva ER, Ravishankar K, Yu S et al (2021) Migraine: epidemiology and systems of care. Lancet 397(10283):1485–1495
Victor TW, Hu X, Campbell JC, Buse D, Lipton RB (2010) Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 30(9):1065–1072
Vetvik KG, MacGregor EA, Lundqvist C, Russell MB (2014) Prevalence of menstrual migraine: a population-based study. Cephalalgia 34(4):280–288
Couturier EGM, Bomhof MAM, Neven AK, van Duijn NP (2003) Menstrual migraine in a representative dutch population sample: prevalence, disability and treatment. Cephalalgia 23(4):302–308
Mattsson P (2003) Hormonal factors in migraine: a population-based study of women aged 40 to 74 years. Headache 43(1):27–35
Granella F, Sances G, Pucci E, Nappi RE, Ghiotto N, Napp G (2000) Migraine with aura and reproductive life events: a case control study. Cephalalgia 20(8):701–707
Vetvik KG, MacGregor EA, Lundqvist C, Russell MB (2010) Self-reported menstrual migraine in the general population. J Headache Pain 11(2):87–92
Verhagen IE, Spank HA, van der Arend BW, van Casteren DS, MaassenVanDenBrink A, Terwindt GM (2022) Validation of diagnostic ICHD-3 criteria for menstrual migraine. Cephalalgia 42(11–12):1184–1193
van Casteren DS, Verhagen IE, van der Arend BWH, van Zwet EW, MaassenVanDenBrink A, Terwindt GM (2021) Comparing perimenstrual and nonperimenstrual migraine attacks using an e-Diary. Neurology 97(17):e1661–e1671
Granella F, Sances G, Allais G, Nappi RE, Tirelli A, Benedetto C, Brundu B, Facchinetti F, Nappi G (2004) Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 24(9):707–716
Pinkerman B, Holroyd K (2010) Menstrual and nonmenstrual migraines differ in women with menstrually-related migraine. Cephalalgia 30(10):1187–1194
Vetvik KG, Benth J, MacGregor EA, Lundqvist C, Russell MB (2015) Menstrual versus non-menstrual attacks of migraine without aura in women with and without menstrual migraine. Cephalalgia 35(14):1261–1268
MacGregor EA, Victor TW, Hu X, Xiang Q, Puenpatom RA, Chen W, Campbell JC (2010) Characteristics of menstrual vs nonmenstrual migraine: a post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache 50(4):528–538
Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA (2019) Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol 18(8):795–804
Edvinsson L, Haanes KA, Warfvinge K, Krause DN (2018) CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 14(6):338–350
Nappi RE, Tiranini L, Sacco S, De Matteis E, De Icco R, Tassorelli C (2022) Role of Estrogens in Menstrual Migraine. Cells 11(8):1355. https://doi.org/10.3390/cells11081355
Warfvinge K, Krause DN, Maddahi A, Edvinsson JCA, Edvinsson L, Haanes KA (2020) Estrogen receptors α, β and GPER in the CNS and trigeminal system - molecular and functional aspects. J Headache Pain 21(1):131
de Lignières B, Vincens M, Mauvais-Jarvis P, Mas JL, Touboul PJ, Bousser MG (1986) Prevention of menstrual migraine by percutaneous oestradiol. Br Med J (Clin Res Ed) 293(6561):1540
Dennerstein L, Morse C, Burrows G, Oats J, Brown J, Smith M (1988) Menstrual migraine: a double-blind trial of percutaneous estradiol. Gynecol Endocrinol 2(2):113–120
Smite MG, van der Meer YG, Pfeil JPJM, Rijnierse JJMM, Vos AJM (1994) Perimenstrual Migraine: Effect of Estraderm TTS(r) and the value of Contingent negative variation and Exteroceptive Temporalis muscle suppression test. Headache: The Journal of Head and Face Pain 34(2):103–106
Almén-Christensson A, Hammar M, Lindh-Åstrand L, Landtblom AM, Brynhildsen J (2011) Prevention of menstrual migraine with perimenstrual transdermal 17-β-estradiol: a randomized, placebo-controlled, double-blind crossover study. Fertil Steril 96(2):498–500e491
MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover study. Neurology 67(12):2159–2163
Martin VT (2009) Ovarian hormones and pain response: a review of clinical and basic science studies. Gend Med 6(Suppl 2):168–192
Sherman JJ, LeResche L (2006) Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol 291(2):R245–256
Leer MN, Bradbury A, Maloney JC, Stewart CN (1988) Elevated shock threshold in sexually receptive female rats. Physiol Behav 42(6):617–620
Martínez-Gómez M, Cruz Y, Salas M, Hudson R, Pacheco P (1994) Assessing pain threshold in the rat: changes with estrus and time of day. Physiol Behav 55(4):651–657
Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vecchiet L (1997) Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain 71(2):187–197
Fischer L, Torres-Chávez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de Arruda Veiga MCF, Tambeli CH (2008) The influence of sex and ovarian hormones on Temporomandibular Joint Nociception in rats. J Pain 9(7):630–638
Martin VT, Lee J, Behbehani M (2007) Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache 47(4):552–563
Frye CA, Bock BC, Kanarek RB (1992) Hormonal milieu affects tailflick latency in female rats and may be attenuated by access to sucrose. Physiol Behav 52(4):699–706
Frye CA, Cuevas CA, Kanarek RB (1993) Diet and estrous cycle influence pain sensitivity in rats. Pharmacol Biochem Behav 45(1):255–260
Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A (1996) Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res 742(1–2):10–16
Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G (1996) Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res 742(1–2):352–354
Vincler M, Maixner W, Vierck CJ, Light AR (2001) Estrous cycle modulation of nociceptive behaviors elicited by electrical stimulation and formalin. Pharmacol Biochem Behav 69(3–4):315–324
Okamoto K, Hirata H, Takeshita S, Bereiter DA (2003) Response properties of TMJ units in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol 89(3):1467–1477
Ji Y, Tang B, Traub RJ (2008) The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience 154(4):1562–1567
Bradshaw HB, Berkley KJ (2002) Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas 41(2):157–165
Sanoja R, Cervero F (2005) Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: a model of functional abdominal pain. Pain 118(1):243–253
Sanoja R, Cervero F (2008) Estrogen modulation of ovariectomy-induced hyperalgesia in adult mice. Eur J Pain 12(5):573–581
Forman LJ, Tingle V, Estilow S, Cater J (1989) The response to analgesia testing is affected by gonadal steroids in the rat. Life Sci 45(5):447–454
Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE (2007) Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain 8(4):334–342
Tsao CM, Ho CM, Tsai SK, Lee TY (1999) Effects of estrogen on autotomy in normal and ovariectomized rats. Pharmacology 59(3):142–148
Beatty WW, Fessler RG (1977) Gonadectomy and sensitivity to electric shock in the rat. Physiol Behav 19(1):1–6
Gaumond I, Arsenault P, Marchand S (2002) The role of sex hormones on formalin-induced nociceptive responses. Brain Res 958(1):139–145
Pajot J, Ressot C, Ngom I, Woda A (2003) Gonadectomy induces site-specific differences in nociception in rats. Pain 104(1):367–373
Herren RY (1933) The effect of high and low female sex hormone concentration on the two-point threshold of pain and touch and upon tactile sensitivity. J Exp Psychol 16:324–327
Tedford WH, Warren DE, Flynn WE (1977) Alteration of shock aversion thresholds during the menstrual cycle. Percept Psychophys 21(2):193–196
Hellström B, Lundberg U (2000) Pain perception to the cold pressor test during the menstrual cycle in relation to estrogen levels and a comparison with men. Integr Physiol Behav Sci 35(2):132–141
Isselée H, Laat A, Bogaerts K, Lysens R (2001) Long-term fluctuations of pressure pain thresholds in healthy men, normally menstruating women and oral contraceptive users. Eur J Pain 5(1):27–37
Stening K, Eriksson O, Wahren L, Berg G, Hammar M, Blomqvist A (2007) Pain sensations to the cold pressor test in normally menstruating women: comparison with men and relation to menstrual phase and serum sex steroid levels. Am J Physiology-Regulatory Integr Comp Physiol 293(4):R1711–R1716
Tousignant-Laflamme Y, Marchand S (2009) Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain 146(1):47–55
Teepker M, Peters M, Vedder H, Schepelmann K, Lautenbacher S (2010) Menstrual variation in Experimental Pain: correlation with gonadal hormones. Neuropsychobiology 61(3):131–140
Rezaii T, Hirschberg AL, Carlström K, Ernberg M (2012) The influence of Menstrual Phases on Pain Modulation in Healthy Women. J Pain 13(7):646–655
Gazerani P, Andersen OK, Arendt-Nielsen L (2005) A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain 118(1–2):155–163
Bajaj P, Arendt-Nielsen L, Bajaj P, Madsen H (2001) Sensory changes during the ovulatory phase of the menstrual cycle in healthy women. Eur J Pain 5(2):135–144
Drobek W, Schoenaers J, De Laat A (2002) Hormone-dependent fluctuations of pressure pain threshold and tactile threshold of the temporalis and masseter muscle. J Oral Rehabil 29(11):1042–1051
Veith JL, Anderson J, Slade SA, Thompson P, Laugel GR, Getzlaf S (1984) Plasma beta-endorphin, pain thresholds and anxiety levels across the human menstrual cycle. Physiol Behav 32(1):31–34
Hapidou EG, Rollman GB (1998) Menstrual cycle modulation of tender points. Pain 77(2):151–161
Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD (2006) Sex differences and hormonal influences on response to Cold Pressor Pain in humans. J Pain 7(3):151–160
Klatzkin RR, Mechlin B, Girdler SS (2010) Menstrual cycle phase does not influence gender differences in experimental pain sensitivity. Eur J Pain 14(1):77–82
Bartley EJ, Rhudy JL (2013) Comparing pain sensitivity and the nociceptive flexion reflex threshold across the mid-follicular and late-luteal menstrual phases in healthy women. Clin J Pain 29(2):154–161
de Brito Barbosa M, de Oliveira Guirro EC, Nunes FR (2013) Evaluation of sensitivity, motor and pain thresholds across the menstrual cycle through medium-frequency transcutaneous electrical nerve stimulation. Clinics 68(7):901–908
Rhudy JL, Bartley EJ, Palit S, Kerr KL, Kuhn BL, Martin SL, DelVentura JL, Terry EL (2013) Do sex hormones influence emotional modulation of pain and nociception in healthy women? Biol Psychol 94(3):534–544
de Tommaso MV, Sardaro M, Serpino M, Fruscolo C, Vecchio OD, Cerbo E, Livrea R (2009) Pain perception and laser evoked potentials during menstrual cycle in migraine. J Headache Pain 10(6):423–429
Teepker M, Kunz M, Peters M, Kundermann B, Schepelmann K, Lautenbacher S (2014) Endogenous pain inhibition during menstrual cycle in migraine. Eur J Pain 18(7):989–998
De Icco R, Cucinella L, De Paoli I, Martella S, Sances G, Bitetto V, Sandrini G, Nappi G, Tassorelli C, Nappi RE (2016) Modulation of nociceptive threshold by combined hormonal contraceptives in women with oestrogen-withdrawal migraine attacks: a pilot study. J Headache Pain 17(1):70
Varlibas A, Erdemoglu AK (2009) Altered trigeminal system excitability in menstrual migraine patients. J Headache Pain 10(4):277–282
Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A (2019) Gender aspects of CGRP in migraine. Cephalalgia 39(3):435–444
Goadsby PJ, Edvinsson L, Ekman R (1990) Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28(2):183–187
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J (2002) CGRP may play a causative role in migraine. Cephalalgia 22(1):54–61
Valdemarsson S, Edvinsson L, Hedner P, Ekman R (1990) Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest 50(4):385–388
Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI (1986) Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed) 293(6558):1329–1330
Ibrahimi K, Vermeersch S, Frederiks P, Geldhof V, Draulans C, Buntinx L, Lesaffre E, MaassenVanDenBrink A, de Hoon J (2017) The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia 37(12):1164–1172
Wyon Y, Frisk J, Lundeberg T, Theodorsson E, Hammar M (1998) Postmenopausal women with vasomotor symptoms have increased urinary excretion of calcitonin gene-related peptide. Maturitas 30(3):289–294
Gupta P, Harte A, Sturdee DW, Sharma A, Barnett AH, Kumar S, McTernan PG (2008) Effects of menopausal status on circulating calcitonin gene-related peptide and adipokines: implications for insulin resistance and cardiovascular risks. Climacteric 11(5):364–372
Raffaelli B, Storch E, Overeem LH, Terhart M, Fitzek MP, Lange KS, Reuter U (2023) Sex hormones and calcitonin gene-related peptide in women with migraine: a cross-sectional, matched Cohort Study. Neurology 100(17):e1825–e1835
Grangeon L, Lange KS, Waliszewska-Prosół M, Onan D, Marschollek K, Wiels W, Mikulenka P, Farham F, Gollion C, Ducros A et al (2023) Genetics of migraine: where are we now? J Headache Pain 24(1):12
Colson N, Fernandez F, Griffiths L (2010) Genetics of menstrual migraine: the molecular evidence. Curr Pain Headache Rep 14(5):389–395
Schurks M, Rist PM, Kurth T (2010) Sex hormone receptor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia 30(11):1306–1328
Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AVE, Kogelman LJA, Thomas LF, Noordam R, Benner C, Gormley P et al (2022) Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet 54(2):152–160
Sutherland HG, Champion M, Plays A, Stuart S, Haupt LM, Frith A, MacGregor EA, Griffiths LR (2017) Investigation of polymorphisms in genes involved in estrogen metabolism in menstrual migraine. Gene 607:36–40
De Marchis ML, Barbanti P, Palmirotta R, Egeo G, Aurilia C, Fofi L, Piroso S, Ialongo C, Della-Morte D, D’Andrea G, Ferroni P, Guadagni F (2015) Look beyond Catechol-O-Methyltransferase genotype for cathecolamines derangement in migraine: the BioBIM rs4818 and rs4680 polymorphisms study. J Headache Pain 16:520
Sullivan AK, Atkinson EJ, Cutrer FM (2013) Hormonally modulated migraine is associated with single-nucleotide polymorphisms within genes involved in dopamine metabolism. Open J Genet 03(02):38–45
An X, Fang J, Qing L, Lu C, Ma Q, Hongli Q (2017) New evidence for involvement of ESR1 gene in susceptibility to chinese migraine. J Neurol 264(1):81–87
CoŞkun S, Yucel Y, Çim A, Cengiz B, Oztuzcu S, Varol S, Özdemir HH, Uzar E (2016) Contribution of polymorphisms in ESR1, ESR2, FSHR, CYP19A1, SHBG, and NRIP1 genes to migraine susceptibility in turkish population. J Genet 95(1):131–140
Rodriguez-Acevedo AJ, Smith RA, Roy B, Sutherland H, Lea RA, Frith A, MacGregor EA, Griffiths LR (2014) Genetic association and gene expression studies suggest that genetic variants in the SYNE1 and TNF genes are related to menstrual migraine. J Headache Pain 15(1):62
Acknowledgements
None.
Funding
B.R. was supported by a research grant of the German Research Foundation (GZ: RA 3907/1–1). M.A. was supported by the Lundbeck Foundation Professor Grant (R310-2018-3711).
Author information
Authors and Affiliations
Contributions
B.R., T.P.D., M.A. and H.A. contributed to the conception and design of the review. All authors contributed to drafting the text or preparing the figures.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
B.R. reports personal fees from AbbVie, Eli Lilly, Lundbeck, Novartis, and Teva, and research grants from Novartis, all outside of the submitted work. B.R. reports serving as junior associate editor of The Journal of Headache and Pain, and associate editor of Frontiers In Neurology. T.P.D. has nothing to disclose. B.A.C. has nothing to disclose. M.A. reports receiving personal fees from AbbVie, Amgen, Eli Lilly, Lundbeck, Novartis, Pfizer and Teva Pharmaceuticals, all outside of the submitted work. MA reports serving as associate editor of Cephalalgia, associate editor of The Journal of Headache and Pain, and associate editor of Brain. F.M.A. reports personal fees from Teva, AbbVie, Pfizer, Lundbeck, Novartis and Eli Lilly, all outside of the submitted work. F.M.A. is the current President of the Danish Headache Society and a member of the Board of Directors in the European Headache Federation. F.M.A. serves as associate editor for Frontiers In Pain Research, Frontiers In Neurology, Headache Medicine, and Acta Neurologica Scandinavica; as junior associate editor for Cephalalgia, and as a member of the editorial board of the European Journal of Headache and Pain. H.A. reports personal fees from Lundbeck and Teva, outside of the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Raffaelli, B., Do, T.P., Chaudhry, B.A. et al. Menstrual migraine is caused by estrogen withdrawal: revisiting the evidence. J Headache Pain 24, 131 (2023). https://doi.org/10.1186/s10194-023-01664-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01664-4