Abstract
Background
Neuroimaging studies have made an important contribution to our understanding of headache pathophysiology. This systematic review aims to provide a comprehensive overview and critical appraisal of mechanisms of actions of headache treatments and potential biomarkers of treatment response disclosed by imaging studies.
Main body
We performed a systematic literature search on PubMed and Embase databases for imaging studies investigating central and vascular effects of pharmacological and non-pharmacological treatments used to abort and prevent headache attacks. Sixty-three studies were included in the final qualitative analysis. Of these, 54 investigated migraine patients, 4 cluster headache patients and 5 patients with medication overuse headache. Most studies used functional magnetic resonance imaging (MRI) (n = 33) or molecular imaging (n = 14). Eleven studies employed structural MRI and a few used arterial spin labeling (n = 3), magnetic resonance spectroscopy (n = 3) or magnetic resonance angiography (n = 2). Different imaging modalities were combined in eight studies.
Despite of the variety of imaging approaches and results, some findings were consistent. This systematic review suggests that triptans may cross the blood–brain barrier to some extent, though perhaps not sufficiently to alter the intracranial cerebral blood flow. Acupuncture in migraine, neuromodulation in migraine and cluster headache patients, and medication withdrawal in patients with medication overuse headache could promote headache improvement by reverting headache-affected pain processing brain areas. Yet, there is currently no clear evidence for where each treatment acts, and no firm imaging predictors of efficacy. This is mainly due to a scarcity of studies and heterogeneous treatment schemes, study designs, subjects, and imaging techniques. In addition, most studies used small sample sizes and inadequate statistical approaches, which precludes generalizable conclusions.
Conclusion
Several aspects of headache treatments remain to be elucidated using imaging approaches, such as how pharmacological preventive therapies work, whether treatment-related brain changes may influence therapy effectiveness, and imaging biomarkers of clinical response. In the future, well-designed studies with homogeneous study populations, adequate sample sizes and statistical approaches are needed.
Similar content being viewed by others
Background
In the last decades, the field of headache research has progressed significantly in part due to the use of brain imaging techniques. Neuroimaging provides a means to noninvasively capture central and vascular mechanisms underlying the pathophysiology of headache disorders. Evidence from molecular imaging techniques and magnetic resonance imaging (MRI) approaches support the involvement of the trigeminovascular system, brainstem, diencephalic, visual and pain processing cortical areas during the different phases of migraine [1]. Studies investigating patients suffering from trigeminal autonomic cephalalgias have shown significant activation of the hypothalamus and nociceptive brain areas during and outside the headache attacks [2]. Functional and structural alterations of cortical and subcortical areas responsible for the perception of the pain have also been revealed in patients with secondary headaches, like medication overuse and post-traumatic headache [3, 4].
Along with revealing important insights on the neurobiology of headache, neuroimaging techniques have deepened our comprehension of how acute and preventive headache treatments work [5]. The use of imaging techniques also has the potential to identify biomarkers for treatment response. However, a comprehensive overview of the mechanisms of action of headache treatments and possible predictors of clinical response disclosed by imaging studies, is missing. Furthermore, there is a need to identify gaps in the literature to develop robust imaging biomarkers of treatment effect that might guide future drug development.
This review provides a systematic and critical appraisal of imaging studies investigating brain and vascular changes associated with treatments used to abort and prevent headache attacks and exploring imaging predictors of patients´ response.
Methods
In accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, we performed a systematic literature search using the online PubMed and Embase databases. The used search string is reported in Supplementary Table 1.
The search was performed from the inception date up to 21 December 2022. Articles identified by this search strategy and judged relevant for the topic of the review were also selected.
Inclusion criteria for the search were as follows: original human research; molecular imaging studies; MRI studies; use of English language; studies including patients with primary and secondary headache disorders (migraine, trigeminal autonomic cephalalgias, tension-type headache, post-traumatic headache, medication overuse headache); studies investigating acute and preventive headache treatments including adult and/or pediatric and/or adolescents patients; cross-sectional studies exploring imaging predictors of patients´ response; longitudinal studies exploring central effects of treatments; studies investigating the brain and/or cephalic vascular system; studies including asymptomatic patients; studies performed during spontaneous and/or provoked headache attacks. Exclusion criteria for the search were as follows: conference abstracts; reviews; unpublished data; studies investigating the extracephalic vascular system; studies investigating central effects of acute and preventive headache treatments in healthy controls.
After checking for duplicates, studies obtained from the databases search were divided in three and two investigators (RM and RHC, RM and IC, RHC and IC) independently screened the title, abstract and full text of papers according to the pre-defined criteria. Any possible disagreements were resolved by discussion.
Results
The database search identified 2425 records (PubMed: 948; Embase:1477). Eleven additional studies related to the topic were included. After duplicates were removed, the title and abstract of 2169 studies was screened yielding 84 articles for full-text screening. After full-text screening, 63 studies were included in the final qualitative analysis (Fig. 1).
Of the included studies, 54 (86%) investigated migraine patients, 4 (6%) cluster headache (CH) patients and 5 (8%) patients with medication overuse headache (MOH). No studies investigating central effects of acute and preventive treatments in patients with tension-type headache, hemicrania continua, paroxysmal hemicrania and post-traumatic headache were found.
Non-steroidal anti-inflammatory drugs (NSAIDs), triptans and ergotamine were examined in the included studies. While, no studies investigating steroids, indomethacin, oxygen, gepants or lasmitidan were found. Among preventive treatments, antiepileptics, calcium channel blocker, beta blockers and monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) were investigated. No studies examining gepants, antidepressants, anti-hypertensive or anti serotoninergic as prevention were found.
Figure 2 summarizes imaging modalities employed by the included studies. Fourteen studies applied molecular imaging approaches, including single-photon emission computerized tomography (SPECT) (n = 3) and positron emission tomography (PET) (n = 11). Six studies combined PET with MRI (n = 4) or computed tomography (n= 2) to increase the spatial resolution of the technique [6]. Distribution of MRI modalities used in the included studies was: 33 studies using functional MRI (fMRI) (9 task-related and 25 resting state (RS) fMRI studies); three studies using Arterial Spin Labeling (ASL); three studies using Magnetic Resonance Spectroscopy (MRS); two studies using MR angiography; 10 studies using high resolution T1-weighted (n = 8) or T2-weighted MRI without contrast (n = 2); one study using enhanced structural MRI during ultrasmall superparamagnetic iron oxide (USPIO) administration. Different imaging modalities were combined in eight studies.
Imaging modalities employed by the included studies: 1) SPECT and PET are molecular imaging techniques that rely on the detection and quantification of rays released indirectly by radiolabelled molecules (tracers) injected into the body, thus providing information on the metabolism, perfusion and function of brain tissues [7]; 2A-B) Functional MRI (fMRI) techniques are based on the blood oxygenation level dependent mechanism. When a brain area is activated, the neuronal metabolism and regional cerebral blood flow (CBF) increase. The blood flow change is greater than the oxygen consumption, resulting in an increased ratio between the oxygenated and deoxygenated hemoglobin, which increase the MRI signal [8]. fMRI approaches included task-related fMRI, which provide important information about the degree of activation and functional connectivity of brain regions that are involved in performing a specific task, and resting state (RS) fMRI that provide insight into the patterns of activity of brain networks or single brain areas during a rest condition [9]; 2C) Arterial Spin Labeling is a perfusion MRI technique that employs the arterial water to measure regional CBF changes associated with variations in regional neural activity [10]; 2D) Magnetic resonance spectroscopy is a non-invasive method that allows to identify and quantify metabolites present within a volume of interest based on the magnetic properties of their nuclei, mainly hydrogen and phosphorous. The main metabolites of interest are: N-acetylaspartate (NAA), a marker of neuronal integrity, choline (Cho), a marker of cellular membrane turnover, creatine (Cr), a marker of energy stores and the glutamate-glutamine and gamma-aminobutyric acid (Glx and GABA) neurotransmitters [11]; 2E) Magnetic resonance angiography is an approach that based on the magnetic properties of blood and surrounding tissues highlight the vasculature from the background without the use of contrast [12]; 2F-G) High resolution T1-weighted MRI with voxel-based (VBM) and surface-based morphometric (SBM)approaches provide information regarding the regional grey matter volume and cortical thickness; [13, 14] 2H) T2-weighted images without contrast can provide information regarding the presence of white matter hyperintensities. 2I) T2* and T1-weighted MRI with ultrasmall superparamagnetic iron oxide (USPIO), a cellular MR contrast agent, allows to investigate the macrophage-mediated inflammation [15]. Created by R.M. with BioRender.com
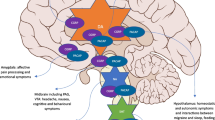
For each individual study, study population and main results are presented in Tables 1, 2, 3 and 4 and Supplementary Tables 2, 3 and 4. Figure 3 outlines the main central structures targeted by treatments described in the included studies.
Migraine
Acute treatments
Non-steroidal anti-inflammatory drugs
Three fMRI studies [16,17,18] have examined imaging predictors of response to NSAIDs. The first of these used pre-treatment RS fMRI to predict the response to NSAIDs [16]. In 70 patients without aura, they found that the visual network in responders had decreased functional connectivity (FC) with the somatosensory network and increased FC with the auditory network, compared to non-responders. In addition, a support vector machine model based on pre-treatment FC reported a 93% accuracy in predicting responders. In another study [17] based on the same cohort and focused on the FC of the left amygdala, responders had increased FC with the left calcarine, superior frontal, and parietal areas, as well as a decreased FC with the ipsilateral caudate nucleus, compared to non-responders. Pre-treatment RS FC of the amygdala with the caudate, visual and frontoparietal areas predicted patients’ response with an accuracy of 89%. Finally, one study [18] examined whether white matter hyperintensities (WMHs) could predict a consistent response to ibuprofen, defined as pain freedom within two hours in at least four of five treated attacks. Using T2 weighted imaging, the study examined 500 patients with migraine who treated their attacks with ibuprofen 200–400 mg. The study found that the 244 responders less frequently had WMHs, and that their WMHs were of a smaller size and diameter, than the 256 non-responders.
Triptans and ergotamines
Two early SPECT studies with Tc-99 m-HMPAO or Xe-133 [21, 22] demonstrated that treatment of the migraine attack with subcoutanoues sumatriptan was not associated with regional cerebral blood flow (CBF) changes. Similar findigs were found by two later MR angiography studies demonstrating that sumatriptan constricts extracerebral arteries such as the superficial temporal and middle meningeal arteries, but not intracerebral arteries [19, 20].
In one PET study using the 5-HT1B receptor radioligand [11C]AZ10419369, eight patients with migraine without aura were examined during cilostazol-induced migraine attacks before and after receiving subcutaneous sumatriptan 6 mg [23]. Sumatriptan reduced serotonin receptor binding in pain modulating regions, including frontal areas, sensorimotor cortex, insula, and amygdala, by 16.0%. Another study [25] examined the effects of eletriptan on central serotonin synthesis in six participants with migraine without aura and six healthy controls using PET with tracer α-[11C]MTrp, a surrogate marker of cerebral 5-HT synthesis. In patients with migraine, eletriptan reduced the rate of 5-HT synthesis in the entire brain, whereas no change occurred in healthy controls.
There is also evidence showing that in 12 migraine patients examined by USPIO-enhanced MRI sumatriptan attenuated the uptake of USPIO in the anterior cerebral artery perfusion territory after cilostazol induced attack. USPIO uptake may reflect activated macrophages or extravasation, but the finding should be interpreted with caution due to its exploratory nature [24].
One study [27] also explored morphometric brain features associated with a good triptan response, showing a lower volume of the left hippocampus in sumatriptan responders compared to non-responders. Even so, sumatriptan response was defined retrospectively and the between-group comparison was not adjusted for age, gender, or total intracranial volume, which may all influence hippocampal volume. Likewise, the analysis was not corrected for multiple comparisons.
Only one study explored whether dihydroergotamine (DHE) has central effects. Six patients with migraine without aura received 11C-DHE before and after administration of nitroglycerin (GTN) to provoke a migraine attack. At PET-MRI before and 3 h after GTN infusion, 11C-DHE did not pass the BBB [26].
Preventive treatments
Pharmacological approaches
Beta-blockers: Propranolol, nadolol and metoprolol
Two studies have examined whether beta-blockers caused cerebral changes, when used for migraine prevention. The first PET study [28] compared whole-brain serotonin synthesis before and after 12 weeks of propranolol or nadolol treatment in five migraine patients, using the 11C-AMT tracer. The study found that beta-blockers did not change whole-brain serotonin synthesis. In another study [29], Hebestreit et al. examined changes of task-based fMRI in response to trigeminal painful stimulation before and after at least 2 months of treatment with metoprolol 75 mg in 19 patients with migraine. The study found no significant functional brain changes after treatment. When performing an uncorrected exploratory analysis, metoprolol increased the hypothalamic BOLD response after treatment. However, the hypothalamic BOLD response correlated negatively with the reduction in headache days at follow-up. This is difficult to reconcile with a treatment effect.
Antiepileptic medication: Topiramate and levetiracetam
Ahmed and colleagues [18] sought to predict the efficacy of topiramate in migraine patients based on WMHs. The study enrolled 500 patients who underwent T2-weighted MRI prior to treatment with 2–200 mg topiramate for at least 2 months. The same cohort was investigated for patients’ response to Ibuprofen 200–400 mg. Like acute treatments, compared to non-responders, responders to topiramate less frequently had WMHs, and the WMHs were fewer and of smaller diameter. These findings should be replicated in a separate cohort.
Although there is no strong evidence supporting the superiority of levetiracetam over placebo and topiramate for migraine prevention [49], one study [30] examined changes in GABA concentration with MRS before and after 12 weeks of treatment with levetiracetam. This study found decrease GABA levels in the posterior cingulate cortex (PCC) after treatment, whereas anterior cingulate cortex (ACC) and prefrontal cortex levels were unchanged. The PCC is activated during pain, but whether the changes were associated with the treatment response was not examined.
Calcium channel blocker: Flunarizine
One study [31] examined whether differences in D2 receptor occupancy might affect the flunarizine response, using the D2 receptor ligand 123I-Iodobenzamide. The study found no differences in receptor binding between six responders and five non-responders. However, flunarizine still decreased dopamine binding in treated migraine patients compared to untreated healthy controls, suggesting that flunarizine does bind central D2 receptors, but other receptors or channels could mediate the migraine preventive effect.
Botulinum toxin
Dominguez et al. [32] examined whether iron deposition in subcortical structures could predict botox treatment response in chronic migraine. This study found a decreased T2-weighted signal in the periaqueductal grey (PAG) in 47 responders compared to 15 non-responders, suggesting increased iron accumulation in responders. However, it should be noted that the T2 weighted signal is not specific for iron accumulation. Another study [33] examined whether pre-treatment cortical structure and RS FC patterns distinguished botox responders from non-responders. The study found increased cortical thickness in several pain relevant areas, including the right primary somatosensory cortex, anterior insula and left inferior frontal gyrus, in responders compared to non-responders. Further examining the FC of these regions, compared to non-responders, responders showed an altered functional interaction between fronto-parietal pain processing areas and occipital regions implicated in visual processing.
Anti-CGRP monoclonal antibodies
So far, three MRI studies [34,35,36] have examined brain functional changes after treatment with anti-CGRP monoclonal antibodies (mAbs). Two studies [34, 35] used task-based fMRI with noxious trigeminal stimulation and ASL to investigate brain functional changes after 2–3 weeks of galcanezumab, a mAb targeting the CGRP ligand, and erenumab, a mAb targeting the CGRP receptor. In 27 patients, galcanezumab reduced the response to trigeminal stimulation in the right hypothalamus and cerebellum, whereas erenumab reduced the response in both cerebellar hemispheres, the left operculum, right thalamus, middle temporal, and lingual cortex in 26 patients. Comparing galcanezumab to erenumab, it has been shown that the two mAbs decreased the acitivity of different brain areas involved in nociceptive activity [35]. Neither erenumab nor galcanezumab changed the regional CBF. These studies explored also imaging features associated with patients’ response after 3 months of treatment. Galcanezumab treatment decreased the activity of the cerebellum, insula, and hypothalamus in responders compared to non-responders, while treatment with erenumab decreased the activity of many areas, including the parahippocampus, cerebellum, inferior parietal, and precentral cortex. The absolute reduction in monthly headache days correlated with higher pretreatment activity of the spinal trigeminal nucleus for galcanezumab, and with the decreased activity of the right putamen, hypothalamus, cerebellum, and thalamus observed after erenumab treatment.
Another study [36] examined functional changes after 2-month treatment with erenumab in 32 patients with migraine, using RS fMRI and fMRI during extracranial nociceptive stimulation. At follow-up, when compared to 14 non-responders, 18 responders had a greater pain-induced response in the left cingulate cortex, PAG and right putamen, as well as increased RS FC of the hypothalamus, fronto-parietal and temporal brain regions. At baseline, responders were distinguished by a decreased activity in the frontal supplemental motor areas in response to painful stimulation compared to non-responders. Finally, one study [37] used MRS to examine changes in ACC and PCC levels of GABA and glutamate. The primary analysis used a mixed population of patients receiving botox and erenumab, precluding firm findings regarding either dug, but a post-hoc analysis reported that the 18 patients who received erenumab had a greater increase in the GABA levels of the ACC, compared to the 10 patients who received botox.
Sphenopalatine ganglion block with local anesthetics
Two studies [38, 39] examined morphometric and functional brain changes after nasal-bupivicaine sphenopalatine ganglion blockade in patients with chronic migraine and MOH. Six weeks after twice weekly treatment, the studies reported an increased volume of the left nucleus accumbens, a decreased volume of the right hippocampus and pallidum, and decreased cortical thickness of the left temporal pole and lateral occipital-temporal sulcus cortex, as well as an altered FC of several regions of the salience and executive networks. However, these studies did not describe their statistical approach in detail, making the findings difficult to interpret.
Non-pharmacological approaches
Acupuncture
Of the reviewed studies, 17 (27%) explored functional and structural brain changes associated with acupuncture treatment. In the included studies, the acupoints selected varied greatly. The duration of each session ranged from 1 up to 30 min, the number of treatments per weeks was inconstant and the treatment period could range from 4 to 16 weeks. Sham acupuncture including inactive acupoints was used as a placebo control only in some studies.
Using RS fMRI, many studies [50,51,52,53,54,55,56,57] demonstrated that, compared to controls, migraine patients experienced widespread functional alterations in brain areas implicated in the processing of the sensory-discriminative, cognitive, and emotional aspects of pain, which were reverted after acupuncture treatment.
A few studies [52, 53, 57, 58] explored whether acupuncture-related functional brain changes were associated with patients´ improvement after treatment, showing an association between changes in brain activity and changes in the severity and frequency of migraine attacks.
Tian and colleagues [50] explored FC patterns associated with a good response to acupuncture, defined as at least 30% reduction in headache intensity or migraine attack frequency, showing that, compared to 29 patients who were non-responders, 19 responders had greater increases of thalamic FC after 4 weeks of treatment. Acupuncture-related thalamic changes have also been described by Gu et al. [59], who, using MRS, showed increased NAA/Cr ratio but unchanged Cho/Cr ratio in the thalamus in patients treated with acupuncture for nine weeks. An increase in the NAA/Cr ratio may reflect higher thalamic neuronal activity and energy metabolism as a result of acupuncture treatment. However, the study did not provide information regarding pre-treatment thalamic metabolism in migraine patients, thus precluding firm conclusions. It should be noted that, these studies did not include a sham group.
Recent studies [60,61,62,63,64,65] investigated neural changes associated with acupuncture comparing the effect of verum acupuncture to sham acupuncture, which included inactive acupoints. RS fMRI studies [60,61,62] described more extensive changes in the function of pain modulatory brain areas in patients receiving verum acupuncture compared to those treated with sham treatment. The study conducted by Li and colleagues [65] showed that only treatment with verum acupuncture could normalize the lower activity of the rostral ventromedial medulla revealed in migraine patients compared to controls before acupuncture initiation. The rostral ventromedial medulla is a pivotal area of the descending pain inhibitory system [66].
Two PET studies [63, 64] examined a small sample of patients with migraine demonstrating that 30 min of verum electro-acupuncture stimulation induced broad modifications in brain metabolism compared to sham stimulation.
Besides fMRI and molecular imaging, a recent study [67] aimed to explore the value of grey matter (GM) volume in predicting migraine patients´ response to acupuncture. Using a machine learning approach, the study showed that a predictive model including the GM volume of the calcarine cortex, precuneus, cuneus, temporal, frontal and parietal brain areas could discriminate responders from non-responders with an accuracy of 83%. This study also showed that, compared to non-responders, responders to 4-week of acupuncture treatment developed an increased GM volume of the left cuneus after treatment. However, these results should be validated in a different cohort.
Non-invasive and invasive neuromodulation
Some studies have explored whether central effects could occur secondary to non-invasive neuromodulation approaches. The study conducted by Russo and colleagues [68] showed an increased activation of the right ACC during trigeminal heat stimulation in 16 migraine patients compared to 16 age and sex-matched healthy controls. In migraine patients treated for two months with external trigeminal stimulation (eTNS), the nociceptive-induced activation of the ACC was reduced after treatment. Similar findings were observed in 14 chronic migraine patients treated with eTNS for three months [69]. Comparing 18-fluorodeoxyglucose (18FDG) uptake to controls, migraine patients initially displayed hypometabolism of the orbitofrontal cortex and ACC, which reverted after treatment. The ACC is known to be involved in the descending antinociceptive pathway and the orbitofrontal cortex is implicated in cognitive aspects of pain modulation. However, there was no difference between responders and non-responders, possibly due to a small sample size.
Using a single-blind, crossover fMRI study design, Luo and colleagues [70] showed that, compared to sham stimulation, eight minutes of verum electrical stimulation of the auricular branch of the vagus nerve (aVNS) reduced the FC between the amygdala and fronto-parietal brain areas largely involved in pain processing and modulation in 27 migraine patients. Central effects of aVNS have also been investigated in a larger study [71] showing that the increased activity of the thalamus, frontal and parietal areas experienced by 60 migraine patients compared to controls could be reversed after 4 weeks of aVNS treatment. It has also been demonstrated that abnormal activity of the trigeminal cervical complex, insula, cingulate cortex, frontal and temporal gyrus could predict patients´ treatment response to 4-week treatment with aVNS [72].
The study conducted by Markin et al. [73] showed that the application of repetitive Transcranial Magnetic Stimulation (rTMS) for five days in 19 migraine patients was associated with FC changes within the default mode, salience and visual networks, which have been implicated in migraine pathophysiology.
Only one study [74] has explored structural brain changes after transcranial direct current stimulation (tDCS), examining 24 patients with migraine who received tDCS or sham stimulation over the visual cortex for 28 days. Compared to sham stimulation, in patients treated with tDCS the frequency of monthly migraine days progressively decreased during the four months after treatment initiation and returned to baseline during the fifth month [75]. Before starting tDCS treatment, migraine patients had decreased GM volume of the left lingual gyrus compared to controls. Five months after treatment, the GM volume of the left lingual gyrus was normalized only in patients who received tDCS but not in those treated with sham treatment. Given the clinical worsening observed after five months from treatment start, these morphometric results are difficult to reconcile with a treatment effect.
The only invasive neurostimulation approach that has been studied using imaging techniques is the occipital nerve stimulation (ONS). Using PET, Matharu and colleagues described an association between pain relief after ONS and regional CBF changes at level of the dorsal rostral pons, ACC, basal ganglia, cuneus, precuneus, cerebellum, frontal, temporal and occipital cortex in a small group of patients with chronic migraine [76].
Behavioural approaches
The study of 19 adolescents with migraine using fMRI showed a greater activation of frontal brain regions and an increased FC of the amygdala with frontal and sensorimotor regions after 8-week treatment with cognitive behavioural therapy (CBT), which was significantly associated with headache improvement in terms of reduction of headache days [77]. The amygdala FC with frontal and sensorimotor regions at baseline could predict headache days reduction after treatment [78].
Functional changes in brain areas implicated in the emotional and cognitive aspects of pain have also been demonstrated in 11 adult migraine patients treated for 16 weeks with autogenic training, a behavioural approach that includes desensitization-relaxation techniques [79].
Among CBT strategies, enhanced mindfulness-based stress reduction (MBSR) is an approach based on mindfulness practice and self-compassion that trains the ability to respond to distress [80]. Seminowicz and colleagues described distinct patterns of brain activation during a challenging cognitive task and of RS FC of the insula in 50 migraine patients treated with MBSR compared to 48 patients receiving didactic sessions focused on the role of stress and other triggers in headaches, supporting increased cognitive efficiency after MBSR [81].
Cluster headache
Using H215O-PET, May and colleagues reported that 60 s of hypothalamic deep brain stimulation was able to change the activity of the hypothalamus, thalamus, trigeminal nucleus and ganglion, and several cortical areas that are usually active in pain perception and during CH attacks [40]. However, the study examined only 10 patients and results were uncorrected for multiple comparisons.
Another PET study [41] examined cerebral glucose metabolism in 10 patients with drug-resistant and side-locked chronic CH treated with ONS. Before treatment initiation, CH patients had altered glucose metabolism in pain processing cortical and brainstem areas compared to healthy controls, which normalized after at least 6 months of treatment. No short-term changes were observed, suggesting that ONS may work through slow neuromodulatory processes in CH.
Using ASL, Medina and colleagues [42] examined regional CBF changes before and after greater occipital nerve blockade with methylprednisolone 80 mg and 2 ml of lidocaine 2% in 17 interictal chronic CH patients. Seven days after the blockade, the regional CBF increased in the right secondary visual cortices and decreased in the left medial temporal gyrus, cerebellum, caudate and putamen. At baseline, responders had greater CBF in the right lateral occipital cortex and left medial prefrontal cortex, and lower CBF in the right PCC compared to non-responders. The study underlines that a strictly peripheral treatment can induce measurable central changes.
A recent study [43] examined clinical and brain morphometric predictors of verapamil response, in 194 patients treated for at least three months. Compared to responders, non-responders had an increased GM volume of the cerebellar vermis and of bilateral cerebellar lobule VI, when using an uncorrected threshold. The study showed also that a supervised machine learning algorithm can discriminate verapamil responders from non-responders with an accuracy of 66%, based only on the clinical characteristics of the patients. The inclusion of the cerebellar GM volume in the predictive model increased slightly the accuracy of the verapamil responsiveness prediction (from 66 to 68%). Both accuracies are considered “poor” (< 0.7) according to general guidelines [82].
Medication overuse headache
Comparing 16 patients with MOH to 68 healthy controls using [26] FDG PET-MRI, Fumal and colleagues [44] found reduced glucose metabolism in pain-related brain areas of patients, including the cerebellum, right parietal cortex, bilateral insula, orbitofrontal cortex, and thalamus. Except for the orbitofrontal cortex, the hypometabolism was reverted after withdrawal. However, the study did not report any restrictions on analgesic intake prior to the scan. It cannot therefore be possible to exclude that metabolic changes observed could be attributed to direct analgesic effects rather than to the overuse of the medication [83].
Similar findings were observed in a later study [45] that examined the BOLD response to a decision making task in patients with MOH who discontinued or not the overused medication. This study found that, compared to controls and chronic migraine patients without MOH, patients with MOH had an increased activity of the ventral medial prefrontal and PCC, which reverted following the medication withdrawal. While, a decreased activity of the midbrain, including the substantia nigra and ventral tegmental area, was specific for MOH and did not change after withdrawal.
Mehnert and colleagues [46] examined changes in GM volume and fMRI response to noxious trigeminal stimuli in 18 patients with MOH before and after withdrawal. After withdrawal, patients displayed an increased responsiveness to nociceptive stimuli and a decreased volume of the left cuneus, superior temporal gyrus, and cerebellum. However, these results should be interpreted with caution given the analgesic intake prior to the pre-withdrawal and follow-up scans and the lack of significant differences in longitudinal fMRI and volumetric changes between patients and controls.
In the study performed by Ferraro et al. [47] nine patients with MOH had a higher BOLD response to painful stimulation of the left hand in the primary somatosensory, parietal, and supramarginal cortex, compared with healthy controls. At rescan 3 weeks after withdrawal, this difference disappeared. While this could suggest that withdrawal ameliorated the central sensitization, the relevance of this changed response to extra-cephalic pain in MOH is unknown. However, it is possible that similar differences might occur for cephalic pain.
A few studies have also examined structural brain predictors of withdrawal effect. After medication withdrawal, Riederer et al. [48] reported a reduction in midbrain PAG volume specifically in 11 responders. Both Mehnert [46] and Rieder [48] found that a greater volume of the orbitofrontal cortex predicted a better response to withdrawal.
Discussion
The reviewed studies applied many different imaging approaches, treatment schemes and study designs, leading to results that are often incomparable or inconsistent. Despite this, some coherent findings have been reported for triptans as abortive treatments for migraine attacks, non-pharmacological approaches employed in migraine and cluster headache prevention and for central effects of medication withdrawal in patients with MOH.
In the following, we will discuss evidence coming from the included studies highlighting their strength and weakness.
Migraine
Acute treatments
NSAIDs are the first line acute treatment for migraine [84]. They are thought to act both peripherally and centrally through effects on nociceptive pathways [85]. Their site of action in migraine is unknown, but three studies [16,17,18] have examined imaging predictors of their efficacy. The findings of these studies may suggest that differences in the FC of the visual network and left amygdala could have importance for the effects of NSAIDs in migraine. While the right amygdala has been implicated in pain-processing, the role of the left amygdala is less clear [86]. Of note, results concerning the visual network were reported at an uncorrected statistical threshold [16] and the inclusion of RS FC metrics of the left amygdala that have already been shown to differ between responders and non-responders might have skewed prediction models [17]. Moreover, the direction of the amygdala RS FC alterations found in patients who responded to NSAID is difficult to interpret since different brain areas were found when comparing healthy controls to the two subgroups of patients.
Ahmed and colleagues [18] showed an association between a poor response to ibuprofen and the presence of WMHs in migraine patients. However, the percentage of consistent responders reported in the study was remarkably high (48.8%) considering that pain freedom at two hours is 20–26% for ibuprofen 200–400 mg [87]. In addition, the number of WMHs increased with age, and age might also affect the efficacy. The findings should be confirmed in a separate cohort and adjusted for age before clinical inferences can be made.
If NSAIDs are inefficient or not tolerated, triptans are the second line acute treatment for migraine [84]. Triptans are 5-HT1B/1D receptor agonists with both vascular and neural effects. An important question is whether different triptans pass the blood–brain barrier (BBB) to exert central effects and side-effects. Imaging studies have provided important information in this regard. The majority of these have used subcutaneous injections of sumatriptan. Using angiography and SPECT, these studies consistently found that sumatriptan constricts extracerebral arteries but do not alter the intracerebral perfusion [19,20,21,22]. This suggests that sumatriptan is unable to cross the BBB to an extent where it can act upon the abluminal 5-HT1B/1D receptors of cerebral arteries. Even so, some imaging studies suggest triptans may cross the BBB to some extent and bind centrally, though perhaps not sufficiently to alter the CBF. Two PET studies [23, 25] demonstrated that triptans reduce the rate of cerebral serotonin synthesis and its activity. Deen and colleagues [23] found a 16% reduction of central 5-HT1B receptor binding across pain-modulating brain areas in patients treated with sumatriptan. Serotonin is an inhibitory neurotransmitter, but whether this level of binding is sufficient to inhibit nociceptive signaling is unknown. Importantly, this study was not placebo controlled, so it cannot be completely excluded that increased serotonin binding is part of the untreated migraine attack or that the reduced binding occurred indirectly.
The degree to which the triptans pass the BBB, likely depends on their individual lipophilicity. Almotriptan is the least lipophilic, eletriptan the most, and sumatriptan is in between [88]. BBB passage could explain some differences in efficacy and tolerability. In comparison, lasmiditan, which is lipophilic and designed as an agonist of central 5-HT1F receptors, is efficacious for the treatment of migraine attacks but may possess more marked central side effects than triptans [89]. However, neuroimaging studies examining the central or neurovascular effects of other triptans different from sumatriptan and eletriptan are lacking, and none have examined those of lasmiditan.
Interestingly, findings with DHE, an effective migraine treatment that also activates 5-HT1B receptors, suggests that high efficacy can be reached through peripheral mechanisms of action alone [26]. Ergotamine use, however, is hampered by potentially serious side effects.
Preventive treatments
Pharmacological approaches
Numerous treatments are approved for migraine prevention. The response to these treatments is generally heterogenous and there are few clinical predictors of treatment response. Preventives may work at different levels of the signaling pathways driving migraine pathogenesis, which may, in part, explain variability between patients [90]. Neuroimaging offers the possibility to identify these sites of action, and how they differ between responders and non-responders.
The findings of the two studies [28, 29] investigating central effects of beta-blockers could suggest that these treatments primarily act peripherally in migraine. However, the studies’ small sample sizes preclude firm conclusions. The beta-blockers discussed are all lipophilic and capable of passing the BBB. Future studies might further explore such direct or indirect central effects of beta-blockers.
Flunarizine is a calcium antagonist which also blocks H1, serotonin, and D2 receptors in addition to voltage gated-sodium channels [91]. Because of the multifarious effects, the exact mechanisms of action in migraine are unknown. However, Wöber et al. [31] speculated that the anti-dopaminergic effects could be mainly involved in migraine prevention.
Botulinum toxin is an effective treatment option for chronic migraine. Botox is administered subcutaneously, where it inhibits the release of vasodilatory neurotransmitters involved in migraine [92]. Because of this, its primary site of action is thought to be peripheral, with secondary central effects. Due to the logistical and financial demands of the treatment, predictors of treatment response are highly relevant. Hubbard and colleagues suggested that functional and structural changes in pain and visual processing areas could have a role in determining botox efficacy [33]. However, their study compared only 11 responders to 12 non-responders, which is likely too few for generalizable results.
Several randomized controlled trials (RCT) demonstrated that mAbs targeting the CGRP are effective and well-tolerated migraine preventive treatments [93]. Their site of action is thought to be mainly in the periphery. However, recent fMRI [34,35,36] and MRS [37] studies demonstrated that anti-CGRP mAbs modulate the activity of pain related brain areas. Central effects may occur secondary to peripheral modulation, or directly through the negligible fraction of mAbs that crosses the BBB [94].
Imaging might help to identify responders to anti-CGRP mAbs. This is highly warranted, since their high-cost hampers widespread use. Distinct patterns of brain functional activity have been found in patients treated with erenumab and galcanezumab. Differences between galcanezumab and erenumab in treatment-related functional brain changes are interesting, since they could explain why some patients have distinct responses to mAbs targeting the CGRP ligand and those blocking the receptor [95]. However, the major limitation of these studies are the small sample size and the use of uncorrected statistical comparisons, which have a high risk of false positive findings [96]. As of date, no studies have reported central changes with anti-CGRP mAbs using an appropriately corrected approach, where false positives can be excluded with greater certainty.
Non-pharmacological approaches
The poor compliance of patients to some pharmacological treatments due to adverse effects and contraindications linked to pregnancy or lactation have encouraged the use of non-pharmacological approaches for migraine prevention [97]. Treatments that have been examined using imaging techniques include acupuncture, behavioural and neuromodulation approaches.
Acupuncture involves the stimulation of specific points on the body by the insertion and rotation of filiform needles until a sensation of numbness and distention, called the de-qi sensation, is achieved [98]. Although acupuncture remains one of the most frequently used approaches in Chinese medicine [98], the use of acupuncture in migraine prophylaxis has yielded to contradictory results. A large, multicentre, RCT did not find acupuncture to be superior to sham [99]. This corroborates a Cochrane meta-analysis that identified several differences in methodology and outcome selection [100]. Imaging findings related to acupuncture should therefore be interpreted in light of the uncertain role of acupuncture in migraine prevention.
In recent years, a vast number of neuroimaging studies have explored the neural mechanisms of acupuncture. Some studies [50,51,52,53,54,55,56,57] suggested that acupuncture could promote migraine improvement by modulating the activity of migraine-affected nociceptive regions and enhancing the function of the descending pain inhibitory system. One of the main limitations of these studies was the lack of a sham group, thus not allowing the exclusion of a placebo effect. However, similar evidence were also found when the effects of verum acupuncture was compared to sham acupuncture. Widespread brain functional and metabolic changes and a reinforced pain inhibitory activity of the brainstem was found in patients treated with verum acupuncture compared to those receiving sham acupuncture. These findings may suggest that only verum acupuncture could modulate the activity of pain-related brain areas, thus improving migraine.
Although further larger RCTs on non-invasive neuromodulation techniques are needed, their potential as therapeutic alternatives to standard pharmacological treatments have recently emerged [101]. Many neuromodulation devices have been introduced in the management of migraine patients. They work by stimulating the central or peripheral nervous system with electric or magnetic stimuli, thus modulating central mechanisms involved in migraine [97].
Transcutaneous cranial nerve stimulation, such as the eTNS and aVNS, modulates the nerves activity at the periphery by applying an electrical current [102]. The aVNS stimulates the auricular branch of the vagus nerve at the concha of the outer ear. This branch of the vagus nerve contains less myelinated Aβ fibers compared to the cervical branch [103, 104]. These anatomical differences may explain the different stimulation regimen used for aVNS and cervical VNS [105]. During aVNS electrical pulses at 25 Hz are applied to the skin of the concha for 1–4 hours [106]. While, cervical VNS stimulation lasts for 2 min, it can be performed 6–12 times a day and delivers a maximum output current of 60 mA to the anterolateral surface of the vagus nerve in the neck [105, 107].
Using fMRI and PET, a few studies [68,69,70,71] showed that both eTNS and aVNS could exert their beneficial migraine preventive effect turning the activity of pain modulatory brain areas, including the ACC, thalamus and trigeminal cervical complex, to normal. Even so, this evidence should be confirmed by further larger studies with a sufficient sample of responders and non-responders. rTMS uses a fluctuating magnetic field to produce an electrical current that can change the excitability of brain networks [102]. Another non-invasive neuromodulation method is the tDCS, which modulates the cortical activity by applying an excitatory or inhibitory electric current to the scalp [102]. Only two studies [73, 74] have investigated functional and structural brain changes related to rTMS and tDCS treatments. The small sample size of these studies, the lack of a control group and the use of an uncorrected statistical threshold disallow solid conclusions regarding central modifications related to these treatments.
The ONS involves an implantable device that delivers electrical stimulation to the greater occipital nerve. One PET study [76] including chronic migraine patients with implanted ONS showed that treatment-related pain improvement correlated with CBF changes in regions involved in the affective dimension of pain and migraine pathophysiology. The study's extremely small sample size hinders drawing conclusions that can be applied broadly. Morevoer, its shoud be noted that results from three RCTs examining the efficacy of ONS in migraine prevention have overall not been promising [102].
Behavioural approaches, including relaxation and CBT, have been used in the management of migraine patients with the aim of teaching patients how to cope with the experience of pain and other migraine symptoms [108]. Despite the lack of high quality evidence supporting their effectiveness in migraine prevention, behavioural treatments remain an important choice for many patients [108]. fMRI studies [77,78,79,80,81] showed that behavioural approaches may influence the cognitive and emotional control of pain to aid migraine improvement.
Cluster headache
Imaging data investigating central effects of CH treatments are scarce and with small samples, thus limiting interpretation. Two PET studies [40, 41] examined neural substrates of neurostimulation in CH, showing treatment-related changes in the activity and metabolism of brain areas implicated in pain transmission and CH attacks. Interestingly, Tso and colleagues [43] showed that clinical characteristics of CH have a rather low accuracy (66%) in discriminating patients who respond to verapamil, a calcium channel blocker that is the first-line preventive drug for CH, from non-responders. The accuracy of the verapamil responsiveness prediction was marginally increased (from 66 to 68%) when clinical features were combined with the cerebellar GM volume. These findings suggest that structural MRI has a minimal role in predicting response to verapamil apart from what can be clinically deduced. However, the study was limited by inclusion of patients with probable and post-traumatic CH, retrospective acquisition of data, and missing information on whether patients were in or out of bout. Furthermore, the study also used different scanners with different field strengths. Though the statistical analysis attempted to adjust for this, no harmonization efforts were reported.
Medication overuse headache
MOH is a secondary headache disorder attributed to overuse of acute headache treatments in patients with a pre-existing headache disorder. Medication withdrawal is crucial in the management of MOH, since it reverts the condition in most patients. The exact mechanisms underlying MOH are unknown. Possible pathophysiological mechanisms may involve the interaction between central sensitization, altered descending pain modulation, biopsychosocial and genetic factors, that affect a state of vulnerability [109]. Imaging before and after withdrawal is instrumental because it could provide information regarding central mechanisms predisposing to the condition and those that are secondary to the frequent intake of acute treatments.
Findings from MRI and PET studies [44,45,46,47,48] indicate that the abnormal function and metabolism of pain processing regions tend to normalize following the discontinuation of the overused treatment, suggesting that these alterations may be secondary to the frequent intake of acute therapies. Whereas, abnormalities of brain regions implicated also in drug dependence, such as the orbitofrontal cortex and ventral tegmental area, tend to persist despite the medication withdrawal, thus reflecting an underlying liability to medication overuse. Curiously, all studies [44, 46, 48] investigating MOH susceptibility and predictors of withdrawal effect implicated the orbitofrontal gyrus, possibly reflecting that the more susceptible patients are also less effective at withdrawing.
Conclusions
In recent years, an increasing number of imaging studies have sought to clarify central mechanisms of action of pharmacological and nonpharmacological treatments commonly used to treat headache patients. It is not unexpected that most of the studies were focused on migraine, being the most frequently studied form of headache. However, if we look at the individual type of acute and preventive migraine treatment, there are only a few studies available, except for acupuncture.
The results of this systematic review suggest that triptans may cross the BBB to some extent, though perhaps not sufficiently to alter the intracranial CBF. An interesting goal of future imaging studies would be to examine how triptans with different efficacy and tolerability cross the BBB. Furthermore, central and vascular mechanisms of action of novel migraine abortive medications, the gepants and lasmiditan, remain unexamined.
In migraine prevention, there is a great need for imaging studies on established treatment, such as anti-hypertensives and anti-epileptics, to further our understanding of their mechanism of action. Whereas imaging studies have provided important information about the anti-CGRP monoclonal antibodies, large-scale studies with robust statistical inferences are needed to consolidate and verify prior findings. This may, in the future, facilitate development of clinically useful predictors of efficacy that can personalize treatment of headache patients.
Acupuncture in migraine, neuromodulation in migraine and cluster headache patients, and medication withdrawal in patients with MOH could lead to headache improvement by reverting headache-affected pain processing brain areas. The way in which neuromodulation devices acting at the periphery could exert their central effects need to be clarified. Moreover, future studies should explore the potential effects of combined pharmacological and non-pharmacological approaches on the brain. Yet, there are no clearly defined brain regions in which each treatment acts, and there are no imaging patterns that could firmly predict the effectiveness of medications.
It should be noted that the studies included in the present review were extremely heterogeneous regarding treatment schemes, study designs, included subjects, and imaging techniques employed. Other limitations of the currently available literature are the small sample size and the frequent use of inadequate statistical approaches that introduce a considerable risk of false positive findings. For many treatment approaches, this excludes robust conclusions. Future studies with adequate sample size, reproducible study paradigms and homogeneous study populations are needed. Moreover, in the future more efforts should be made to study patients with trigeminal autonomic cephalalgias or post-traumatic headache.
A better understanding of how headache treatments work along with the identification of biomarkers of patients’ response could yield crucial insights into the biological mechanisms underlying the pathophysiology of headaches.
Availability of data and materials
Data are available from the corresponding author upon reasonable request.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- ASL:
-
Arterial spin labeling
- aVNS:
-
Auricular vagus nerve stimulation
- BBB:
-
Blood–brain barrier
- BOLD:
-
Blood oxygenation level dependent
- CBF:
-
Cerebral blood flow
- CBT:
-
Cognitive behavioural therapy
- CGRP:
-
Calcitonin gene-related peptide
- CH:
-
Cluster headache
- Cho:
-
Choline
- Cr:
-
Creatine
- DHE:
-
Dihydroergotamine
- eTNS:
-
External trigeminal nerve stimulation
- FC:
-
Functional connectivity
- 18FDG:
-
18-Fluorodeoxyglucose
- fMRI:
-
Functional magnetic resonance imaging
- GABA:
-
Gamma-aminobutyric acid
- Glx:
-
Glutamate-glutamine
- GM:
-
Grey matter
- GTN:
-
Nitroglycerin
- mAbs:
-
Monoclonal antibodies
- MBSR:
-
Mindfulness-based stress reduction
- MOH:
-
Medication overuse headache
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- NAA:
-
N-acetylaspartate
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- ONS:
-
Occipital nerve stimulation
- PAG:
-
Periaqueductal grey
- PCC:
-
Posterior cingulate cortex
- PET:
-
Positron emission tomography
- RCT:
-
Randomized controlled trial
- RS:
-
Resting state
- rTMS:
-
Repetitive transcranial magnetic stimulation
- SBM:
-
Surface-based morphometry
- SPECT:
-
Single-photon emission computerized tomography
- tDCS:
-
Transcranial direct current stimulation
- USPIO:
-
Ultrasmall superparamagnetic iron oxide
- VBM:
-
Voxel-based morphometry
- WMHs:
-
White matter hyperintensities
References
Messina R, Gollion C, Christensen RH, Amin FM (2022) Functional MRI in migraine. Curr Opin Neurol 35(3):328–335
Chong CD, Schwedt TJ, Hougaard A (2017) Brain functional connectivity in headache disorders: A narrative review of MRI investigations. J Cereb Blood Flow Metab 39(4):650–669
Ashina H, Porreca F, Anderson T et al (2019) Post-traumatic headache: epidemiology and pathophysiological insights. Nat Rev Neurol 15(10):607–617
Schwedt TJ, Chong CD (2017) Medication Overuse Headache: Pathophysiological Insights from Structural and Functional Brain MRI Research. Headache 57(7):1173–1178
Messina R, Filippi M, Goadsby PJ (2018) Recent advances in headache neuroimaging. Curr Opin Neurol 31(4):379–385
Fink JR, Muzi M, Peck M, Krohn KA (2015) Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. J Nucl Med 56(10):1554–1561
Miletich RS (2016) Positron Emission Tomography and Single-Photon Emission Computed Tomography in Neurology. Continuum 22(5, Neuroimaging):1636–54
Ogawa S, Menon RS, Tank DW et al (1993) Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 64(3):803–12
Biswal BB (2012) Resting state fMRI: a personal history. Neuroimage 62(2):938–944
Wong EC (2014) An introduction to ASL labeling techniques. J Magn Reson Imaging 40(1):1–10
Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M (2017) Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol 30(3):246–262
Kiruluta AJM, Gonzalez RG (2016) Magnetic resonance angiography: physical principles and applications. Handb Clin Neurol 135:137–149
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97(20):11050–11055
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11(6 Pt 1):805–821
Stoll G, Bendszus M (2010) New approaches to neuroimaging of central nervous system inflammation. Curr Opin Neurol 23(3):282–286
Wei HL, Yang WJ, Zhou GP et al (2022) Altered static functional network connectivity predicts the efficacy of non-steroidal anti-inflammatory drugs in migraineurs without aura. Front Mol Neurosci 15:956797
Wei HL, Xu CH, Wang JJ et al (2022) Disrupted Functional Connectivity of the Amygdala Predicts the Efficacy of Non-steroidal Anti-inflammatory Drugs in Migraineurs Without Aura. Front Mol Neurosci 15:819507
Ahmed SR, Mohamed AAM, Salem HH, Helmy S, Moustafa RR, Borham SMF. Association of white matter hyperintensities with migraine phenotypes and response to treatment. Acta Neurol Belg 2022.
Asghar MS, Hansen AE, Amin FM et al (2011) Evidence for a vascular factor in migraine. Ann Neurol 69(4):635–645
Khan S, Amin FM, Christensen CE et al (2019) Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain 142(1):93–102
Ferrari MD, Haan J, Blokland JA et al (1995) Cerebral blood flow during migraine attacks without aura and effect of sumatriptan. Arch Neurol 52(2):135–139
Friberg L, Olesen J, Iversen HK, Sperling B (1991) Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet 338(8758):13–17
Deen M, Hougaard A, Hansen HD et al (2019) Association Between Sumatriptan Treatment During a Migraine Attack and Central 5-HT1B Receptor Binding. JAMA Neurol 76(7):834–840
Khan S, Amin FM, Fliedner FP et al (2019) Investigating macrophage-mediated inflammation in migraine using ultrasmall superparamagnetic iron oxide-enhanced 3T magnetic resonance imaging. Cephalalgia 39(11):1407–1420
Sakai Y, Nishikawa M, Diksic M, Aube M (2014) alpha-[11C] methyl-L tryptophan-PET as a surrogate for interictal cerebral serotonin synthesis in migraine without aura. Cephalalgia 34(3):165–173
Schankin CJ, Maniyar FH, Seo Y et al (2016) Ictal lack of binding to brain parenchyma suggests integrity of the blood-brain barrier for 11C-dihydroergotamine during glyceryl trinitrate-induced migraine. Brain 139(Pt 7):1994–2001
Wu JW, Lai PY, Chen YL et al (2022) The Use of Neuroimaging for Predicting Sumatriptan Treatment Response in Patients With Migraine. Front Neurol 13:798695
Chugani DC, Niimura K, Chaturvedi S et al (1999) Increased brain serotonin synthesis in migraine. Neurology 53(7):1473–1479
Hebestreit JM, May A (2017) The enigma of site of action of migraine preventives: no effect of metoprolol on trigeminal pain processing in patients and healthy controls. J Headache Pain 18(1):116
Li Q, Chen C, Gong T (2018) High-field MRS study of GABA+ in patients with migraine: response to levetiracetam treatment. NeuroReport 29(12):1007–1010
Wober C, Brucke T, Wober-Bingol C, Asenbaum S, Wessely P, Podreka I (1994) Dopamine D2 receptor blockade and antimigraine action of flunarizine. Cephalalgia 14(3):235–240
Dominguez C, Lopez A, Ramos-Cabrer P et al (2019) Iron deposition in periaqueductal gray matter as a potential biomarker for chronic migraine. Neurology 92(10):e1076–e1085
Hubbard CS, Becerra L, Smith JH et al (2016) Brain Changes in Responders vs. Non-Responders in Chronic Migraine: Markers of Disease Reversal. Front Hum Neurosci 10:497
Ziegeler C, Mehnert J, Asmussen K, May A (2020) Central effects of erenumab in migraine patients: An event-related functional imaging study. Neurology 95(20):e2794–e2802
Basedau H, Sturm LM, Mehnert J, Peng KP, Schellong M, May A (2022) Migraine monoclonal antibodies against CGRP change brain activity depending on ligand or receptor target - an fMRI study. Elife 11:e77146
Schwedt TJ, Nikolova S, Dumkrieger G, Li J, Wu T, Chong CD (2022) Longitudinal changes in functional connectivity and pain-induced brain activations in patients with migraine: a functional MRI study pre- and post- treatment with Erenumab. J Headache Pain 23(1):159
Peek AL, Leaver AM, Foster S et al (2021) Increase in ACC GABA+ levels correlate with decrease in migraine frequency, intensity and disability over time. J Headache Pain 22(1):150
Newman-Norlund RD, Rorden C, Maleki N, Patel M, Cheng B, Androulakis XM (2020) Cortical and subcortical changes following sphenopalatine ganglion blocks in chronic migraine with medication overuse headache: a preliminary longitudinal study. Womens Midlife Health 6:7
Krebs K, Rorden C, Androulakis XM (2018) Resting State Functional Connectivity After Sphenopalatine Ganglion Blocks in Chronic Migraine With Medication Overuse Headache: A Pilot Longitudinal fMRI Study. Headache 58(5):732–743
May A, Leone M, Boecker H et al (2006) Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci 26(13):3589–3593
Magis D, Bruno MA, Fumal A et al (2011) Central modulation in cluster headache patients treated with occipital nerve stimulation: an FDG-PET study. BMC Neurol 11:25
Medina S, Bakar NA, O’Daly O et al (2021) Regional cerebral blood flow as predictor of response to occipital nerve block in cluster headache. J Headache Pain 22(1):91
Tso AR, Brudfors M, Danno D et al (2021) Machine phenotyping of cluster headache and its response to verapamil. Brain 144(2):655–664
Fumal A, Laureys S, Di Clemente L et al (2006) Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 129(Pt 2):543–550
Ferraro S, Grazzi L, Muffatti R et al (2012) In medication-overuse headache, FMRI shows long-lasting dysfunction in midbrain areas. Headache 52(10):1520–1534
Mehnert J, Hebestreit J, May A (2018) Cortical and Subcortical Alterations in Medication Overuse Headache. Front Neurol 9:499
Ferraro S, Grazzi L, Mandelli ML et al (2012) Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med 13(2):255–262
Riederer F, Gantenbein AR, Marti M, Luechinger R, Kollias S, Sandor PS (2013) Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci 33(39):15343–15349
Linde M, Mulleners WM, Chronicle EP, McCrory DC (2013) Antiepileptics other than gabapentin, pregabalin, topiramate, and valproate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 2013(6):CD010608
Tian Z, Guo Y, Yin T et al (2021) Acupuncture Modulation Effect on Pain Processing Patterns in Patients With Migraine Without Aura. Front Neurosci 15:729218
Zhang Y, Li KS, Liu HW et al (2016) Acupuncture treatment modulates the resting-state functional connectivity of brain regions in migraine patients without aura. Chin J Integr Med 22(4):293–301
Chen Y, Kang Y, Luo S et al (2022) The cumulative therapeutic effect of acupuncture in patients with migraine without aura: Evidence from dynamic alterations of intrinsic brain activity and effective connectivity. Front Neurosci 16:925698
Li Z, Liu M, Lan L et al (2016) Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep 6:20298
Zou Y, Tang W, Li X, Xu M, Li J (2019) Acupuncture Reversible Effects on Altered Default Mode Network of Chronic Migraine Accompanied with Clinical Symptom Relief. Neural Plast 2019:5047463
Li K, Zhang Y, Ning Y et al (2015) The effects of acupuncture treatment on the right frontoparietal network in migraine without aura patients. J Headache Pain 16:518
Ishiyama S, Shibata Y, Ayuzawa S, Matsushita A, Matsumura A, Ishikawa E (2022) The Modifying of Functional Connectivity Induced by Peripheral Nerve Field Stimulation using Electroacupuncture for Migraine: A Prospective Clinical Study. Pain Med 23(9):1560–1569
Li Z, Lan L, Zeng F et al (2017) The altered right frontoparietal network functional connectivity in migraine and the modulation effect of treatment. Cephalalgia 37(2):161–176
Liu S, Luo S, Yan T et al (2021) Differential Modulating Effect of Acupuncture in Patients With Migraine Without Aura: A Resting Functional Magnetic Resonance Study. Front Neurol 12:680896
Gu T, Lin L, Jiang Y et al (2018) Acupuncture therapy in treating migraine: results of a magnetic resonance spectroscopy imaging study. J Pain Res 11:889–900
Zhang Y, Wang Z, Du J et al (2021) Regulatory Effects of Acupuncture on Emotional Disorders in Patients With Menstrual Migraine Without Aura: A Resting-State fMRI Study. Front Neurosci 15:726505
Zhao L, Liu J, Zhang F et al (2014) Effects of long-term acupuncture treatment on resting-state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. PLoS One 9(6):e99538
Liu L, Lyu TL, Fu MY et al (2022) Changes in brain connectivity linked to multisensory processing of pain modulation in migraine with acupuncture treatment. NeuroImage Clinical 36:103168
Yang M, Yang J, Zeng F et al (2014) Electroacupuncture stimulation at sub-specific acupoint and non-acupoint induced distinct brain glucose metabolism change in migraineurs: a PET-CT study. J Transl Med 12:351
Yang J, Zeng F, Feng Y et al (2012) A PET-CT study on the specificity of acupoints through acupuncture treatment in migraine patients. BMC Complement Altern Med 12:123
Li Z, Zeng F, Yin T et al (2017) Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. NeuroImage Clinical 15:367–375
Akerman S, Holland PR, Goadsby PJ (2011) Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12(10):570–584
Yang XJ, Liu L, Xu ZL et al (2020) Baseline Brain Gray Matter Volume as a Predictor of Acupuncture Outcome in Treating Migraine. Front Neurol 11:111
Russo A, Tessitore A, Esposito F et al (2017) Functional Changes of the Perigenual Part of the Anterior Cingulate Cortex after External Trigeminal Neurostimulation in Migraine Patients. Front Neurol 8:282
Magis D, D’Ostilio K, Thibaut A et al (2017) Cerebral metabolism before and after external trigeminal nerve stimulation in episodic migraine. Cephalalgia 37(9):881–891
Luo W, Zhang Y, Yan Z et al (2020) The Instant Effects of Continuous Transcutaneous Auricular Vagus Nerve Stimulation at Acupoints on the Functional Connectivity of Amygdala in Migraine without Aura: A Preliminary Study. Neural Plast 2020:8870589
Feng M, Zhang Y, Wen Z et al (2022) Early Fractional Amplitude of Low Frequency Fluctuation Can Predict the Efficacy of Transcutaneous Auricular Vagus Nerve Stimulation Treatment for Migraine Without Aura. Front Mol Neurosci 15:778139
Fu C, Zhang Y, Ye Y et al (2022) Predicting response to tVNS in patients with migraine using functional MRI: A voxels-based machine learning analysis. Front Neurosci 16:937453
Markin K, Trufanov A, Frunza D et al (2022) fMRI Findings in Cortical Brain Networks Interactions in Migraine Following Repetitive Transcranial Magnetic Stimulation. Front Neurol 13:915346
Schading S, Pohl H, Gantenbein A et al (2021) Tracking tDCS induced grey matter changes in episodic migraine: a randomized controlled trial. J Headache Pain 22(1):139
Pohl H, Moisa M, Jung HH et al (2021) Long-Term Effects of Self-Administered Transcranial Direct Current Stimulation in Episodic Migraine Prevention: Results of a Randomized Controlled Trial. Neuromodulation 24(5):890–898
Matharu MS, Bartsch T, Ward N, Frackowiak RS, Weiner R, Goadsby PJ (2004) Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 127(Pt 1):220–230
Nahman-Averbuch H, Schneider VJ 2nd, Chamberlin LA et al (2020) Alterations in Brain Function After Cognitive Behavioral Therapy for Migraine in Children and Adolescents. Headache 60(6):1165–1182
Nahman-Averbuch H, Schneider VJ 2nd, Chamberlin LA et al (2021) Identification of neural and psychophysical predictors of headache reduction after cognitive behavioral therapy in adolescents with migraine. Pain 162(2):372–381
Dobos D, Szabo E, Baksa D et al (2021) Regular Practice of Autogenic Training Reduces Migraine Frequency and Is Associated With Brain Activity Changes in Response to Fearful Visual Stimuli. Front Behav Neurosci 15:780081
Parsons CE, Crane C, Parsons LJ, Fjorback LO, Kuyken W (2017) Home practice in Mindfulness-Based Cognitive Therapy and Mindfulness-Based Stress Reduction: A systematic review and meta-analysis of participants’ mindfulness practice and its association with outcomes. Behav Res Ther 95:29–41
Seminowicz DA, Burrowes SAB, Kearson A et al (2020) Enhanced mindfulness-based stress reduction in episodic migraine: a randomized clinical trial with magnetic resonance imaging outcomes. Pain 161(8):1837–1846
Hosmer Jr. DW, SL, Sturdivant RX (2013) Applied lLogistic regression, 3rd Edition. Wiley; ISBN: 978-0-470-58247-3
Lorenz IH, Egger K, Schubert H et al (2008) Lornoxicam characteristically modulates cerebral pain-processing in human volunteers: a functional magnetic resonance imaging study. Br J Anaesth 100(6):827–833
Eigenbrodt AK, Ashina H, Khan S et al (2021) Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17(8):501–514
Vanegas H, Vazquez E, Tortorici V (2010) NSAIDs, Opioids, Cannabinoids and the Control of Pain by the Central Nervous System. Pharmaceuticals (Basel) 3(5):1335–1347
Ji G, Neugebauer V (2009) Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 102(4):2253–2264
Rabbie R, Derry S, Moore RA, McQuay HJ (2010) Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev 10:CD008039
Pascual J, Munoz P (2005) Correlation between lipophilicity and triptan outcomes. Headache 45(1):3–6
Yang CP, Liang CS, Chang CM et al (2021) Comparison of New Pharmacologic Agents With Triptans for Treatment of Migraine: A Systematic Review and Meta-analysis. JAMA Netw Open 4(10):e2128544
Migraine AM (2020) N Engl J Med 383(19):1866–1876
Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS (1984) Flunarizine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs 27(1):6–44
Burstein R, Blumenfeld AM, Silberstein SD, Manack Adams A, Brin MF (2020) Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 60(7):1259–1272
Messina R, Huessler EM, Puledda F, Haghdoost F, Lebedeva ER, Diener HC (2023) Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia 43(3):3331024231152169
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S (2017) Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev 97(2):553–622
Overeem LH, Peikert A, Hofacker MD et al (2022) Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: A multi-center retrospective cohort study. Cephalalgia 42(4–5):291–301
Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 113(28):7900–7905
Puledda F, Goadsby PJ (2016) Current Approaches to Neuromodulation in Primary Headaches: Focus on Vagal Nerve and Sphenopalatine Ganglion Stimulation. Curr Pain Headache Rep 20(7):47
Zheng H, Chen M, Wu X, Li Y, Liang FR (2010) Manage migraine with acupuncture: a review of acupuncture protocols in randomized controlled trials. Am J Chin Med 38(4):639–650
Diener HC, Kronfeld K, Boewing G et al (2006) Efficacy of acupuncture for the prophylaxis of migraine: a multicentre randomised controlled clinical trial. Lancet Neurol 5(4):310–316
Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR (2009) Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev 1:CD001218
Moisset X, Pereira B, de CiampiAndrade D, Fontaine D, Lanteri-Minet M, Mawet J (2020) Neuromodulation techniques for acute and preventive migraine treatment: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 21(22):142
Puledda F, Shields K (2018) Non-Pharmacological Approaches for Migraine. Neurotherapeutics 15(2):336–345
Safi S, Ellrich J, Neuhuber W (2016) Myelinated Axons in the Auricular Branch of the Human Vagus Nerve. Anat Rec (Hoboken) 299(9):1184–1191
Butt MF, Albusoda A, Farmer AD, Aziz Q (2020) The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat 236(4):588–611
Wang Y, Zhan G, Cai Z et al (2021) Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci Biobehav Rev 127:37–53
Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R (2015) Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain 16:543
Silberstein SD, Calhoun AH, Lipton RB et al (2016) Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 87(5):529–538
Ashina M, Buse DC, Ashina H et al (2021) Migraine: integrated approaches to clinical management and emerging treatments. Lancet 397(10283):1505–1518
Ashina S, Terwindt GM, Steiner TJ et al (2023) Medication overuse headache. Nat Rev Dis Primers 9(1):5
Acknowledgements
Not applicable
Funding
None.
Author information
Authors and Affiliations
Contributions
RM contributed to the conception of the review, drafting and revising the work. RHC and IC contributed to drafting and revising the work. MA and MF critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Search string used for PubMed and Embase databases. Supplementary Table 2. Acupuncture for migraine prophylaxis. Supplementary Table 3. Non-invasive and invasive neuromodulation techniques for migraine prophylaxis. Supplementary Table 4. Behavioral approaches for migraine prophylaxis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Messina, R., Christensen, R.H., Cetta, I. et al. Imaging the brain and vascular reactions to headache treatments: a systematic review. J Headache Pain 24, 58 (2023). https://doi.org/10.1186/s10194-023-01590-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01590-5