Abstract
Diabetes mellitus, a chronic metabolic disease, often leads to numerous chronic complications, significantly contributing to global morbidity and mortality rates. High glucose levels trigger epigenetic modifications linked to pathophysiological processes like inflammation, immunity, oxidative stress, mitochondrial dysfunction, senescence and various kinds of cell death. Despite glycemic control, transient hyperglycemia can persistently harm organs, tissues, and cells, a latent effect termed "metabolic memory" that contributes to chronic diabetic complications. Understanding metabolic memory's mechanisms could offer a new approach to mitigating these complications. However, key molecules and networks underlying metabolic memory remain incompletely understood. This review traces the history of metabolic memory research, highlights its key features, discusses recent molecules involved in its mechanisms, and summarizes confirmed and potential therapeutic compounds. Additionally, we outline in vitro and in vivo models of metabolic memory. We hope this work will inform future research on metabolic memory's regulatory mechanisms and facilitate the development of effective therapeutic compounds to prevent diabetic complications.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by elevated blood glucose caused by deficiency or resistance to insulin (Joslin 1946). Chronic hyperglycemia can lead to multiple organ injury, thereby causing various complications, such as diabetic retinopathy (DR), diabetic kidney disease (DKD), and diabetic cardiovascular disorders (Zheng et al. 2018). Epidemiological studies have revealed that DM has emerged as a significant threat to human mortality. At present, the International Diabetes Federation (IDF) estimates that DM affects approximately 536.6 million adults worldwide, and that number is expected to increase to 783.2 million by 2045 (Sun et al. 2022).
In addition to its high incidence, the pathogenesis of diabetic complications is also very complex. In the early stages of DM, hyperglycemia induces oxidative stress and excessive advanced glycation end product (AGE) formation (Domingueti et al. 2016). As the disease progresses, protein glycation and mitochondrial DNA (mtDNA) damage to respiratory chain components can in turn exacerbate oxidative stress injury (Bhatti et al. 2022). Metabolic imbalance then promotes inflammation through binding receptors for glycation products to cause senescence or cell death (Takahashi et al. 2022; Phoenix et al. 2022; Teodoro et al. 2018). These structural changes can lead to various diabetes-related vascular complications (Teodoro et al. 2018). To improve the mechanisms described above, multiple novel hypoglycemic agents, such as sodium glucose co-transporter 2 inhibitor (SGLT2i), dipeptidyl peptidase 4 inhibitors (DPP4i) and glucagon-like peptide 1 receptor agonists (GLP-1RAs), have been applied in clinical practice (Mouhayyar et al. 2020; Nathan et al. 2013; Zhang and Wu 2014) (Mostafa et al. 2016; Mostafa et al. 2015). However, early hyperglycemia can still lead to a variety of diabetic complications. Fortunately, the novel concept of “metabolic memory” may explain this phenomenon. Metabolic memory, also known as hyperglycemic memory, arises from the enduring presence of an underlying driver. The persistence of cellular changes and characteristics represents the organism's recovery of a prior metabolic state, potentially playing a pivotal role in the etiology of DM and its chronic complications (Reddy et al. 2015).
In this comprehensive review, we aim to delve into the research chronology and distinct characteristics of metabolic memory. Additionally, we present a summary of the diverse molecular mechanisms that govern its regulation. By emphasizing its prevalence and profound implications, we highlight the significance of metabolic memory in various chronic diabetes complications. Furthermore, we delve into potential mechanisms and pharmacological advancements related to metabolic memory. Additionally, we consolidate information on various in vitro and in vivo models of metabolic memory. We hope that this review can offer valuable insights into the intricacies of metabolic memory, thereby paving the way for novel therapeutic strategies for the treatment of DM and its complications.
Overview of metabolic memory
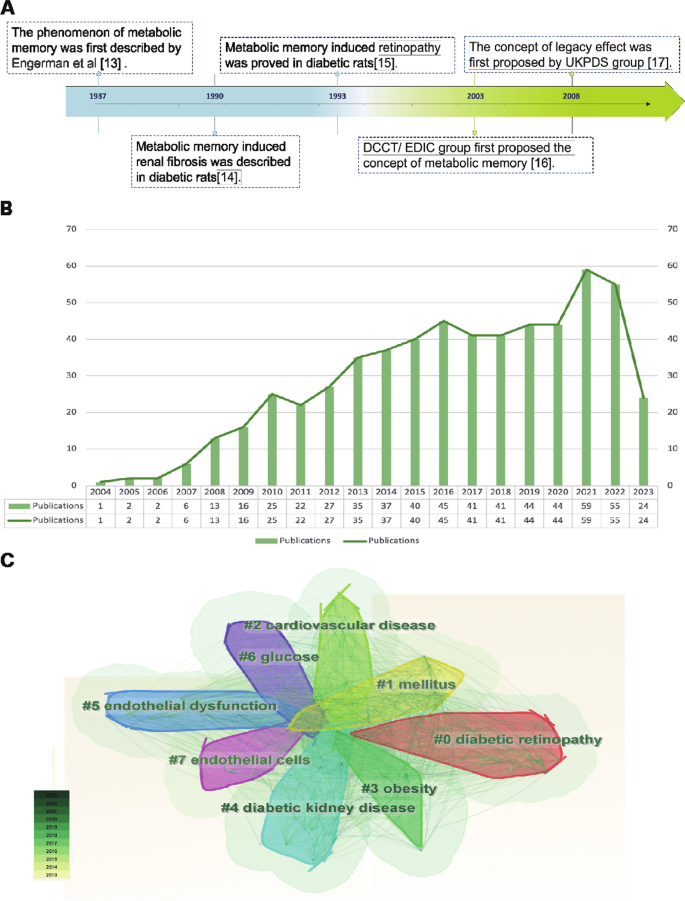
The metabolic memory of diabetes refers to the observation that patients are vulnerable to developing diabetic complications due to early hyperglycemia, even if effective hypoglycemic agents are taken to maintain blood glucose within normal levels in the later stage of DM. As shown in Fig. 1A, in 1987, Engerman et al. (Engerman and Kern 1987) first described the phenomenon of metabolic memory that decreased hyperglycemia to normal levels after 2.5 years of exposure in diabetic dogs, and the incidence of DR was still high (Engerman and Kern 1987). In addition, high glucose caused an increase in fibronectin and collagen IV expression that could not be reversed even after restoration to normal levels in diabetic rats in 1990 (Roy et al. 1990). In 1993, Hammes et al. (Hammes et al. 1993) further described the exposure time more accurately. Their research indicated that islet transplantation to diabetic rats could prevent the occurrence of DR within 6 weeks after onset. However, at 12 weeks after onset, DR still occurred. Later, in 2003, the Diabetes Control and Complications Trial (DCCT) with further follow-up in the Epidemiology of Diabetes Interventions and Complications (EDIC) study (DCCT/EDIC), where the concept of "metabolic memory" was first proposed, demonstrated that initial hyperglycemia still increased the risk of long-term diabetic complications, although the HbA1c of the intensive treatment group and conventional treatment group was maintained at similar levels (Writing Team 2003). In 2008, the United Kingdom Prospective Diabetes Study (UKPDS) again demonstrated the term “legacy effect”, in which early intensive glucose lowering can lead to long-term benefits in patients with newly diagnosed type 2 diabetes (Holman et al. 2008; Ranjit Unnikrishnan et al. 2011). Both "metabolic memory" and the “legacy effect” refer to the long-term effects of blood glucose on macrovascular and microvascular complications of diabetes. However, the concept of metabolic memory may focus on the negative effect of hyperglycemia impairment, while the legacy effect mainly focuses on the positive influence of effective treatments.
Overview of metabolic memory. A Chronological depiction of key events in the development of metabolic memory. B, C Bibliometric analysis exploring the intersection of metabolic memory and diabetic complications. Search criteria were set as follows: TS = ((“metabolic memory” OR “hyperglycemic memory”) AND (“diabetes” OR “diabetic”)) with a date range of DOP = (2013–08-01/2023–08-01). B Illustration of the annual trend in the number of published articles. C Clustered view of the key terms and concepts emerging from the literature
The bibliometric analysis of the research published on metabolic memory in the decade following its formal designation in 2004. Based on the information provided by the Web of Science (webofscience.com), we analyzed the scientific output related to metabolic memory and diabetes from 2000 to 2022. In total, 579 articles were identified. The trend of research related to metabolic memory and diabetes is displayed in Fig. 1B, which shows a steady upward trend since its official naming in 2004, particularly in 2021–2022. Cluster analysis of high-frequency keywords related to metabolic memory and diabetes was performed using CiteSpace (Fig. 1C). The clustering outcomes revealed a preponderance of research centering on the interplay between metabolic memory and diabetes, with a focus on DR, cardiovascular disease, endothelial dysfunction, DKD and obesity. Notably, obesity is intricately intertwined with glycemic and metabolic homeostasis, as evident in previous studies (El-Mesallamy et al. 2013; Aboouf et al. 2015; Khella et al. 2017). However, Zapata et al. (2022) also observed that obesity elicits a persistent metabolic imprint that persists despite weight loss, phenotypically resembling metabolic memory. Despite this, the existing literature consistently associates metabolic memory with glycemic fluctuations. This preponderance of findings can be partially attributed to the inherent constraints of bibliometric analysis, including the challenges associated with the precision and breadth of bibliographic databases, the absence of contextual understanding, and potential biases towards high-impact journals or specific research domains. Consequently, there is a pressing need for further exploration in this realm to clarify the intricate relationships among obesity, metabolic memory, and glycemic fluctuations. Our review primarily centered on metabolic memory and the potential long-term health implications of transient abnormalities in glucose metabolism.
The main molecular mechanisms of metabolic memory
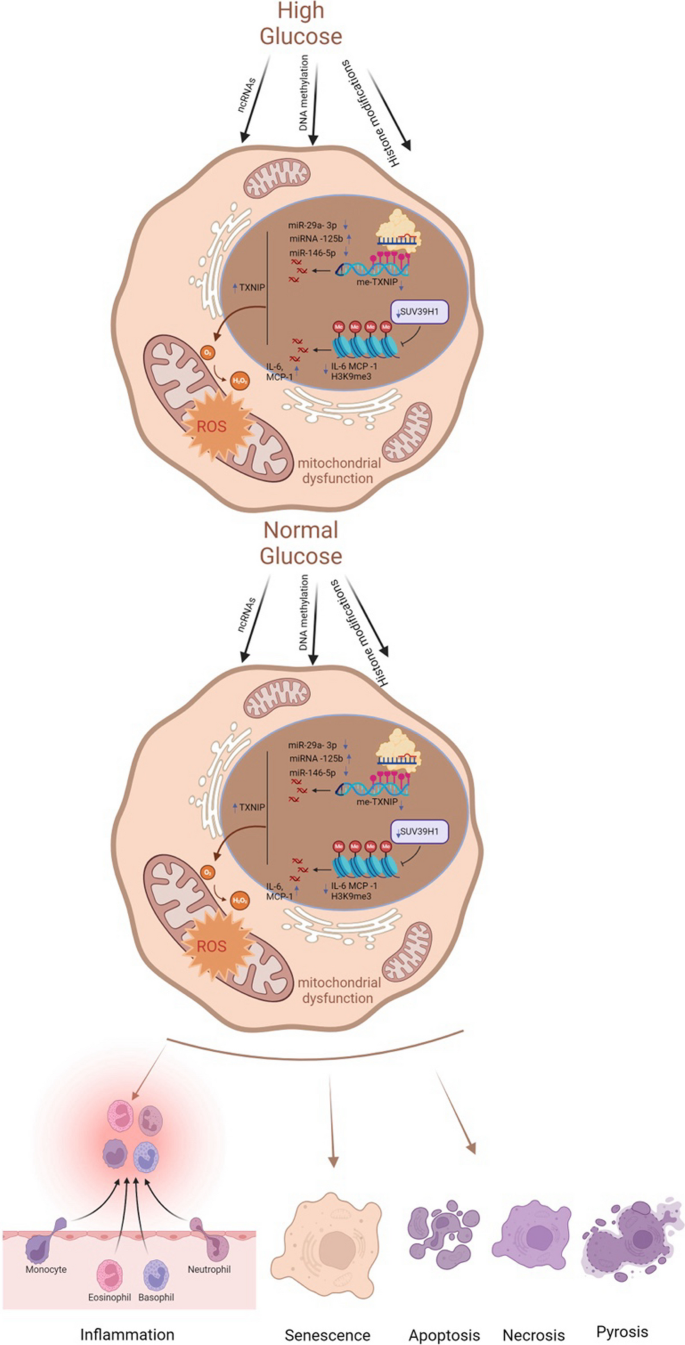
The underlying mechanisms of metabolic memory and diabetic complications include inflammation and immunity, oxidative stress and mitochondrial dysfunction, senescence and various kinds of cell death. In fact, these mechanisms involve crosstalk with each other (Galicia-Garcia, et al. 2020; Berezin 2016). Epigenetic modifications can lead to inflammation, oxidative stress, and senescence, which in turn can be regulated by these mechanisms (Fig. 2).
Key molecular mechanisms of metabolic memory. Despite the normalization of glucose levels, epigenetic modifications, inflammatory and immune responses, oxidative stress, mitochondrial dysfunction, cellular senescence, and apoptosis persist. These processes constitute the core molecular mechanisms underlying metabolic memory. ncRNAs noncoding RNAs, TXNIP thioredoxin-interacting protein, me-TXNIP thioredoxin-interacting protein, IL-6 interleukin-6, MCP-1 monocyte chemotactic protein 1, H3K9me3 trimethylated histone H3 at lysine 9, ROS reactive oxygen species
Epigenetic mechanisms involved in metabolic memory
Epigenetic mechanisms, including DNA methylation, histone modifications and noncoding RNAs (ncRNAs), can influence transcription activity and the generation of a heritable phenotype without changing DNA sequences (Goldberg et al. 2007). Emerging studies have indicated a key role for epigenetic modifications in the regulation of physiological and pathological processes associated with diabetic complications and metabolic memory (Chen and Natarajan 2022). Thus, this section mainly focuses on various modifications involved in hyperglycemic memory.
DNA methylation
DNA methylation, the most stable and widely reported epigenetic mechanism, is considered the primary transcriptional regulator. To investigate the relationship between hyperglycemic memory and DNA methylation, Chen et al. (2016) selected patients with type 1 diabetes mellitus (T1DM) from DCCT and EDIC studies. They discovered twelve distinctively annotated differentially methylated loci that exhibited a strong association with hyperglycemia and were intricately linked to diabetic complications. Notably, among these loci, thioredoxin-interacting protein (TXNIP) is a pivotal gene in the pathogenesis of diabetic complications. Transient hyperglycemic episodes were found to trigger hypomethylation at the 3’ untranslated region (3′ UTR) of TXNIP, leading to persistently elevated expression of this protein in peripheral blood cells (Thielen and Shalev 2018). This, in turn, triggered oxidative stress and triggered apoptotic and pyroptotic processes (Choi and Park 2023). Moreover, Park et al. (2014) derived foot fibroblasts from patients with diabetes with or without ulcers and from nondiabetic subjects without foot ulcers. Then, foot fibroblasts from patients with DM were cultured for four passages under normoglycemic conditions, and global and genome-wide DNA methylation profiles were used to identify alterations in DNA methylation. Their results illustrated that DNA methylation and metabolic memory were associated with poor wound healing outcomes in patients with diabetic foot ulceration. Similarly, proximal tubular epithelial cells (PTECs) derived from patients with or without diabetes were cultured via normoglycemic culture for four passages. After integrative omics analysis, multiple changes in DNA methylation sites were detected; among these changes, HNF4A may regulate epigenetic and hyperglycemic memory in DKD (Bansal et al. 2020).
In summary, these studies suggest that DNA methylation plays a vital role in metabolic memory and diabetic complications. In addition, as DNA methylation is involved in hyperglycemic memory, a review speculated that emerging m6A RNA methylation may also be a potential mechanism (Kumari et al. 2021). However, this theory remains to be confirmed in the future.
Histone modifications
Histones, including the corehistones H2A, H2B, H3, and H4 and the linker histone H1, can bind tightly to DNA to form nucleosome structures. Histone posttranslational modifications (HPTMs) refer to covalent modifications in which different modifications are added to one or several amino acid residues on the tails of histones. The modified histones change the loose or tight binding state between histones and DNA to effectively regulate gene transcription. The most common HPTMs are acetylation (Kac) and methylation (Kme) (Jin and Jeong 2023; Sun et al. 2023). Filgueiras et al. (2017) demonstrated that STAT1/MyD88 mRNA and protein levels remained elevated for a minimum of six days in macrophages from diabetic mice. This upregulation could be attenuated by the histone acetyltransferase (HAT) inhibitor anacardic acid. Furthermore, in the skeletal muscle tissue of diabetic mice, persistent enhanced Ped/Pea-15 expression was related to histone H3 lysine 4 monomethylation (H3K4me1) but not histone H3 Lys27 acetylation (H3K27Ac). The high expression of H3K4me1 remained stable even after re-exposure to 5 mM glucose-containing medium. However, there was a prompt loss of acetylation at K27 on histone H3 and a reduction in p300 recruitment at Ped/Pea-15 (Vastolo et al. 2018). In addition to H3K4me1, H3K9me3, a crucial repressive and relatively stable epigenetic chromatin mark, also contributes to metabolic memory in vascular smooth muscle cells (VSMCs) derived from db/db mice. The persistent downregulation of H3K9me3 and the inflammatory phenotype could be reversed by overexpressing suppressor of variegation 3–9 homolog 1 (Suv39h1), which is a histone methyltransferase (Sun et al. 2023). H3K4me1 and H3K9me3 also regulate metabolic memory in CMs and vascular endothelial cells, respectively (Yu et al. 2012; Okabe et al. 2012; Mao et al. 2019). Regrettably, relatively few studies on HPTMs and metabolic memory, especially some emerging HPTMs, such as lactylation, ubiquitination and glycosylation.
ncRNAs
ncRNAs, which mainly include microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), play a vital role in diabetes and its complications as well as multidrug resistance (Li et al. 2022a; Mahmoud et al. 2021). As another major mechanism of epigenetic regulation, ncRNAs can regulate gene expression by modulating protein synthesis at the posttranscriptional and translational levels (Taft et al. 2010). miRNAs, a class of endogenous single-stranded RNAs composed of 20–22 nucleotides, can participate in regulating posttranscriptional gene expression by binding to target mRNAs (Krol et al. 2010). Currently, various miRNAs have been reported to participate in metabolic memory and diabetic complications. To identify hyperglycemic memory-related miRNAs in human aortic endothelial cells, Zhong et al. (2015) used a miRCURY LNA array to screen for transcriptional changes in the normal glucose, high glucose and metabolic memory groups. After validation in vitro and in vivo, miR-125b, miR-29a-3p, and miR-146a-5p were shown to potentially be important for metabolic memory. Notably, miR-125b was the only miRNA confirmed to be related to metabolic memory, specifically targeting Suv39h1 to promote inflammation in VSMCs from diabetic mice (Villeneuve et al. 2010). Subsequently, Costantino et al. (2016) screened 268 miRNAs that remained significantly altered after 3 weeks of intensive glycemic control with insulin from heart samples. The majority of miRNAs related to metabolic memory effects, according to an ingenuity pathway analysis, regulate the myocardial pathways of apoptosis, autophagy, oxidative stress, fibrosis, hypertrophy and heart failure. Regrettably, they verified miRNA expression in left ventricular samples from controls, diabetic mice, and diabetic mice treated with insulin without further exploring the underlying mechanisms involved. In addition, miR-23b-3p has been proven to regulate high glucose-induced metabolic memory via the SIRT1-dependent signaling pathway in DR (Zhao et al. 2016). However, in-depth studies of the links between key lncRNAs and the crosstalk between lncRNAs and miRNAs in metabolic memory and diabetic complications still need further exploration.
Inflammation, immunity, oxidative stress and mitochondrial dysfunction
High blood glucose can induce chronic metabolic inflammation, which contributes to the development of various complications (Nedosugova et al. 2022). Monocytes and macrophages, crucial components of immunity, participate in inflammation in diabetic complications. The proinflammatory activation of macrophages within the liver and adipose tissue can initiate the recruitment and promotion of macrophage polarization, thereby inducing these cells to secrete inflammatory cytokines, including IL-1β, IL-6, and TNF-α. This, in turn, results in immune imbalance, highlighting the critical role of macrophage activation in the pathogenesis of inflammatory conditions (Bleriot et al. 2023; Ding et al. 2022). To further investigate the intricate relationships among inflammation, immunity, and metabolic memory, Mossel et al. (2020) investigated metabolic memory in primary human macrophages. Their findings revealed that even after normalizing glucose levels, the expression of S100A9 and S100A12 remained elevated, potentially due to transient hyperglycemia-induced histone methylation at the promoters of these genes. In addition, innate immune cells, which are integral to diabetes-related complications, can establish nonspecific immunological memory (trained immunity) through epigenetic regulation. Thiem et al. (2021) established both in vitro and in vivo trained immunity models using bone marrow cell transplantation and monocyte isolation. Their study demonstrated that glucose modulation of innate immune cell histone methylation levels can persist, leading to increased glycolysis and exacerbated inflammatory responses even after glucose normalization. Given these insights, diabetes and its complications related to oxidative stress and inflammation, as well as immunity, can significantly benefit from vitamin E intake (Hamdy et al. 2009).
In addition to the aforementioned factors, oxidative stress and mitochondrial dysfunction play essential roles in metabolic memory (Peng, et al. 2020). An imbalance between oxidative and antioxidative processes gives rise to oxidative stress, which can trigger lipid accumulation, inflammation, and fibrosis in diabetic complications (Zhang et al. 2020). Reactive oxygen species (ROS), a hallmark of oxidative stress, encompass a range of free radicals, including superoxide anions, hydroxyl and peroxyl radicals, and other compounds capable of generating free radicals (Halliwell 2006). Since mitochondria are key intracellular sources of ROS, mitochondrial dysfunction is intimately linked to oxidative stress (Cojocaru, et al. 2023). Multiple studies have established that oxidative stress and mitochondrial dysfunction are integral to the mechanism of metabolic memory in diabetic complications, particularly in the progression of DR (Wang et al. 2018; Zhong and Kowluru 2013; Voronova et al. 2017; Drzewoski et al. 2009). Sirtuin-1 (SIRT-1) functions as a modulator of antioxidant defense, energy metabolism, and organelle homeostasis, making it a key player in oxidative stress and mitochondrial dysfunction in various diseases (Kung, et al. 2021; Li et al. 2022b). Lee et al. (2022) demonstrated that SIRT-1 was a link between hyperglycemic memory and oxidative stress and mitochondrial dysfunction in DR. Additionally, Kowluru et al. (2023) provided evidence that transient hyperglycemia results in a persistent imbalance in mitochondrial fission, mitophagy, and new mitochondrial formation, ultimately leading to oxidative stress in DR. Beyond mitochondrial dysfunction, oxidative stress intersects with other organelle dysfunctions, including endoplasmic reticulum (ER) stress, Golgi apparatus stress, and lysosomal homeostasis (Maamoun et al. 2019; Gong et al. 2022; Jiang et al. 2011). However, the intricate relationships between these processes remain largely unexplored and require further investigation.
Senescence and cell death
Cellular senescence, a type of permanent proliferative arrest without cell death, is divided into epigenetically induced senescence, oxidative stress-induced senescence and DNA damage-induced senescence (Hernandez-Segura et al. 2018). The process of senescence is closely related to programmed cell death (PCD) (Galluzzi and Myint 2023). When cellular damage cannot be efficiently repaired, irreversible dysfunction of cells can lead to PCD, including apoptosis, autophagy, pyroptosis and ferroptosis (Moujalled et al. 2021). Recently, a p21-dependent pathway was identified that contributes to senescence and hyperglycemic memory in DKD (Al-Dabet et al. 2022). Furthermore, Mansour et al. (2023) demonstrated that overexpressed p21 can lead to senescence and increase the expression of BAX, a pro-apoptotic gene, to alleviate apoptosis. These results indirectly illustrate that p21, a key gene in metabolic memory, also participates in senescence and apoptosis and may be a promising target. Moreover, in DR, temporary high glucose could lead to consistent upregulation of miR-195 to decrease the expression of its target gene Bcl-2, which is an antiapoptotic gene (Liu et al. 2019a). This research suggested that epigenetic mechanisms, as representative ncRNAs, may interact with senescence and cell death in hyperglycemic memory. Nevertheless, how do other types of cell death regulate metabolic memory in diabetic complications? This question is still unanswered.
Metabolic memory and chronic complications of DM
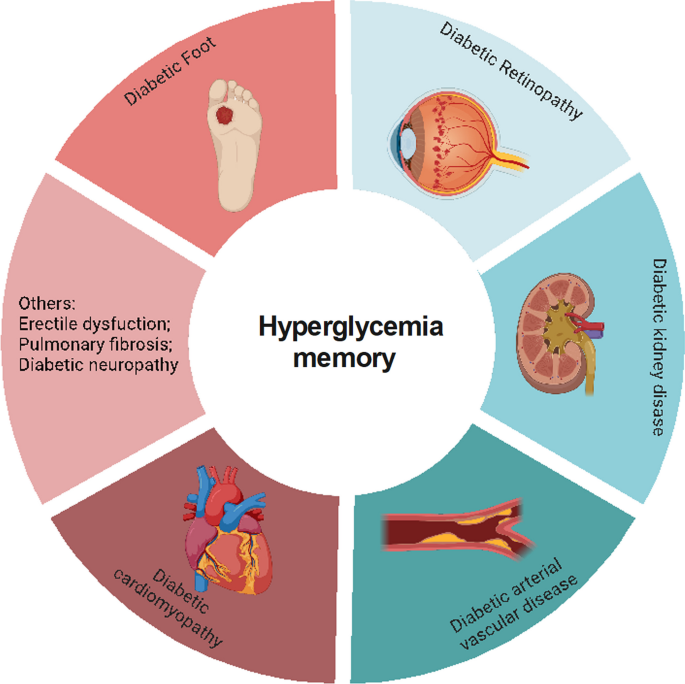
Multiple large-scale clinical trials have verified that early intensive glycemic control can reduce the incidence and progression of macrovascular and microvascular complications of diabetes, including diabetic cardiovascular disorders, DKD, DR, and diabetic foot disease (DF) (C., I. 2003; Cuore et al. 2023; Brown et al. 2010; Nathan et al. 2014; Aiello et al. 2014), which is basically consistent with the results of our bibliometric analysis (Fig. 1C). Numerous studies have also used experiments to elucidate the mechanisms underlying this clinical phenomenon in diabetic complications (Yamagishi et al. 2017; Zhong et al. 2023; Kato and Natarajan 2019). Thus, in this section, we will discuss the relationship between metabolic memory and chronic complications of DM (Fig. 3).
Metabolic memory and chronic complications of diabetes. Hyperglycemia can trigger a range of diabetic complications, including diabetic cardiomyopathy, diabetic arterial vascular disease, diabetic kidney disease, diabetic retinopathy, and diabetic foot. This figure illustrates the intricate relationship between metabolic memory and these chronic conditions
Diabetic cardiovascular disorders and metabolic memory
Diabetic cardiovascular disorders, including diabetic cardiomyopathy (DCM) and arterial vasculopathy, are the leading causes of death among patients with diabetes (Fang et al. 2004). Elevated blood glucose stimulates inflammation, regulates immune cells, and promotes the production of cytotoxic free radicals, thereby attacking myocardial cells and vascular endothelial cells (Johnson et al. 2022; Xie et al. 2022). Under the action of these mechanisms triggered by high glucose, damaged cells further secrete harmful irritants, which promote the transdifferentiation of other cell types into cardiac fibroblasts (Cheng et al. 2023). Subsequently, various adhesion molecules and adipokines, such as adiponectin, influence these fibroblasts, activating them to migrate and aggregate, thus exacerbating myocardial and vascular injury (El-Mesallamy et al. 2011). However, evidence from clinical trials has indicated that even with intensified blood glucose control, patients with diabetes are still at risk for diabetic cardiovascular diseases due to metabolic memory. This section discusses the relationship between diabetic cardiovascular diseases and metabolic memory.
DCM and metabolic memory
DCM is a cardiovascular complication that arises from DM and causes alterations in cardiac structure and function, independent of hypertension, coronary atherosclerotic heart disease, or any other known cardiac risk factors (Jia et al. 2018). Previous studies have established that metabolic dysfunction in cardiomyocytes, myocardial interstitial fibrosis, abnormal calcium transients in cardiomyocytes, and cardiac autonomic neuropathy play pivotal roles in the pathogenesis of DCM (Palomer et al. 2018; Marwick et al. 2018). Roy et al. (1990) demonstrated that fibronectin mRNA expression increased even after blood glucose returned to normal in streptozotocin (STZ)-induced diabetic rats. Given the mechanisms and manifestations of DCM, metabolic memory may play a key role in its development and progression (Zhan et al. 2022). Additionally, previous studies have shown that miR-320 mediates apoptosis in DCM (Su et al. 2020). Moreover, multiple studies have suggested that cluster of differentiation 36 (CD36) regulates free fatty acid uptake in DCM, and CD36-deficient patients and CD36 knockout mice exhibit a significant reduction in the myocardial uptake of long-chain fatty acids (LCFAs) (Zhang et al. 2021). A recent study revealed a connection between these factors, revealing that miR-320 serves as a central ncRNA in metabolic memory and positively interacts with CD36 to alleviate diastolic dysfunction caused by hyperglycemic memory in cardiomyocytes (Zhan et al. 2023). This finding offers novel insights into the pathogenesis of DCM and its molecular functions.
Diabetic arterial vasculopathy and metabolic memory
Elevated blood glucose can inflict substantial harm on both the microvascular and macrovascular systems, ultimately leading to endothelial dysfunction, atherosclerosis, and various vascular complications (Li et al. 2023). Observations from studies such as the EDIC and UKPDS revealed that individuals in the intensive treatment group developed fewer microvascular and macrovascular diseases (C., I. 2003; Retnakaran et al. 2006). Jax et al. (2010) argued that structural alterations, including perivascular fibrosis of microvessels, can exert a direct impact on upstream arteries, gradually leading to endothelial dysfunction and, subsequently, the development of atherosclerosis. The endothelium, the largest organ of the body, plays a pivotal role in regulating the functionality of blood vessels. Persistent hyperglycemia leads to oxidative stress, inflammation, and abnormal mitochondrial metabolism, all of which contribute to endothelial dysfunction (Wang et al. 2022). Remarkably, even when transient hyperglycemic conditions revert to normal glycemic levels, oxidative stress and inflammatory factors persist within aortic endothelial cells (El-Osta et al. 2008). Damaged endothelial cells lose their functionality and undergo a process known as endothelial-to-mesenchymal transition (EndMT), during which they transform into mesenchymal cells or myofibroblasts, thereby contributing to pathological fibrosis (Xu and Kovacic 2023; Bischoff 2019). Previous research has shown that hyperglycemic memory can also trigger EndMT and fibrosis (Al-Dabet et al. 2022). In this context, miR-27a, a ncRNA closely associated with EndMT and fibrosis, has been further implicated in the NF-κB/miR-27a-3p/NRF2/ROS/TGF-β/EndMT feedback loop, which regulates metabolic memory in endothelial cells (Liu et al. 2019b; Yao et al. 2022). Reddy et al. (2016) demonstrated that the expression of miR-504 remains persistently high in diabetic VSMCs even after several passages of in vitro culture, enhancing ERK1/2 activation and VSMC dysfunction in atherosclerosis and restenosis.
In summary, metabolic memory is intricately linked to oxidative stress, inflammation, and fibrosis and plays a pivotal role in the pathogenesis of DCM and diabetic arterial vasculopathy. The involvement of ncRNAs, such as miR-320 and miR-27a, points to complex regulatory mechanisms underlying these processes. Nevertheless, other miRNAs, such as miR-423, miR-499, and miR-199a, have been implicated in metabolic memory and the diabetic heart, but further investigation is needed to fully elucidate their roles (Costantino et al. 2016).
DKD and metabolic memory
DKD is one of the most common and severe complications of DM and is also the leading cause of end-stage kidney disease (ESKD) in the general population (Novak et al. 2016; Collins, et al. 2011). The minimal functional unit of the kidney is the nephron, which consists of the glomerulus and renal tubule. Hyperglycemia can cause or exacerbate injuries in both the glomerulus and renal tubule to induce renal dysfunction.
Glomerular injury and metabolic memory
The glomeruli are composed of glomerular endothelial cells (GECs), mesangial cells, podocytes and parietal epithelial cells. As GECs serve as the primary barrier to exposure to high glucose conditions, they can initiate crosstalk between mesangial cells and podocytes. Hyperglycemia can increase the permeability of GECs, alter the glycocalyx and induce GEC apoptosis (Dou and Jourde-Chiche 2019). On the one hand, damaged GECs regulate the expression and secretion of endothelin-1 (ET-1), nitric oxide (NO), endothelial nitric oxide synthase (eNOS) and VEGF family members, thereby aggravating the dysfunction of other cell types, including mesangial cells and podocytes (Thomas and Ford Versypt 2022; Mahtal et al. 2021; Zou et al. 2019). Conversely, dysfunction in mesangial cells and podocytes can also deleteriously affect GECs through the regulation of VEGF expression (Fu, et al. 2022; Bartlett et al. 2016). This intricate crosstalk among glomerular cells plays a pivotal role in the pathogenesis and progression of glomerular injury. Notably, even after the restoration of normoglycemia, the damage to these cells persists. Li et al. (2022c) demonstrated that Sirt7 cooperates with ELK1 to participate in metabolic memory and DKD through the modulation of DAPK3 expression and endothelial inflammation both in vitro and in vivo. Similarly, for podocytes, the expression of SHP-1 remains elevated despite the reduction in blood glucose levels achieved by insulin treatment for the last two months in diabetic mice (Lizotte et al. 2016). Additionally, free fatty acids, such as palmitate, contribute significantly to the development of insulin resistance. Thus, Novak et al. (2016) further demonstrated that a high-fat diet or palmitate can alter H3K36me2 and H3K27me3 on the promoter region of the FOXO1 gene, thereby regulating metabolic memory in podocytes. This comprehensive understanding of the interactions and responses among glomerular cells highlights the complexity and persistence of glomerular injury in patients with diabetes.
Tubular injury and metabolic memory
The injury of tubular epithelial cells (TECs), which account for the largest proportion of all cell types in the kidney, is an essential link in the pathogenesis of DKD (Vallon and Thomson 2020). On the one hand, hyperglycemia can cause structural alterations in renal tubules, including renal tubule atrophy, tubular cell hypertrophy, thickening of the tubular basement membrane and tubulointerstitial fibrosis (Slyne et al. 2015; Pourghasem et al. 2015). On the other hand, high glucose conditions can also lead to inflammation, programmed cell death, senescence and mitochondrial dysfunction in TECs (Zhou et al. 2023; Shen et al. 2022; Chang et al. 2021). Among them, cellular senescence in TECs is related to epigenetic modifications, which are the core mechanism of metabolic memory (Shen et al. 2022; Tonna et al. 2010). Recent research identified p21 as a key hyperglycemic memory-related gene that regulates TEC senescence in DKD, and activated protein C (aPC), an enzyme that epigenetically inhibits redox p66Shc, could inhibit p21 methylation to ameliorate metabolic memory and senescence (Al-Dabet et al. 2022).
In conclusion, metabolic memory is an emerging mechanism in glomerular and tubular injury. Regrettably, studies on the role of metabolic memory in DKD are rare, especially studies on mesangial cells and the crosstalk between different cell types in the kidney. A recent study on the multimodal integration of single nucleus RNA (snRNA-seq) and an assay for transposase-accessible chromatin sequencing (snATAC-seq) in DKD may provide more information on the epigenetic regulation of chromatin accessibility, which could contribute to the long-term expression of DKD and metabolic memory-related genes (Wilson et al. 2022). However, further studies are still needed.
DR and metabolic memory
DR, characterized as a neurodegenerative and microangiopathic disease, is the major cause of visual impairment in patients with diabetes, accounting for approximately 30 to 40% of cases (Ting et al. 2016; Altmann and Schmidt 2018). Hyperglycemia remains the major factor that contributes to the development and progression of DR (Cheung et al. 2010). The pathophysiological mechanisms underlying DR are complex and include oxidative stress, inflammation, autophagy, cellular dysfunction and cell death. The inflammatory cascades are primarily triggered by oxidative stress. Both inflammation and oxidative stress stimulate retinal autophagy, which leads to cellular dysfunction and cell death in nerve cells, endothelial cells and pericytes. All these factors may interact with each other, ultimately contributing to the development of DR (Wei et al. 2022; Madsen-Bouterse and Kowluru 2008).
Coincidentally, multiple studies have shown that the mechanisms mentioned above regulate hyperglycemic memory to affect DR pathogenesis (Liu et al. 2023). Metabolic memory-induced retinopathy was initially observed in diabetic dogs, which indicated that DR was not improved by good glycemic control (Engerman and Kern 1987). Tewari et al. (2012) reported that despite the restoration of normoglycemia in retinal endothelial cells, hypermethylation of POLG1 promoters did not change, which resulted in mtDNA replication dysfunction. Liu et al. (2019a) demonstrated that miR-195 remained upregulated in human retinal pigment epithelial cells (RPEs) following three days of culture under high glucose conditions and subsequent normalization to normal glucose levels for another three days, leading to mitochondrial dysfunction-induced apoptosis. Furthermore, Astragalus polysaccharide (APS) attenuated the expression of miR-195 in a dose-dependent manner. Recent studies have demonstrated that the pathogenesis of metabolic memory-induced microvascular dysfunction in DR is regulated by mitochondrial dysfunction, which can be ameliorated by dopamine, mdivi-1 and leflunomide (Lee et al. 2022; Kowluru and Alka 2023; Mohammad and Kowluru 2022). Therefore, mitochondrial dysfunction may be the core mechanism of metabolic memory in DR. Both DKD and DR are microvascular complications of diabetes, and the kidneys and eyes are mitochondria-rich organs. However, studies on the relationship between mitochondrial dysfunction and hyperglycemia in DKD are limited and may be worthy of further research.
Epigenetic modifications also play a vital role in the progression of DR. The high glucose-induced histone 3 lysine 4 (H3K4) hypomethylation status of retinal Sod2 remains persistent even after reversing hyperglycemia (Zhong and Kowluru 2013). Mishra et al. (2014) also proved that as termination of hyperglycemia injury cannot change H3K4 methylation, the binding activity of the transcription factor Nrf2 remains compromised, which leads to oxidative stress. Furthermore, numerous miRNAs also participate in metabolic memory in DR. Apart from miR-195 mentioned above, miR-23b-3p regulates the miR-23b-3p/SIRT1/NF-κB feedback loop to maintain metabolic memory in DR (Zhao et al. 2016). Nevertheless, the mechanisms of DNA methylation, lncRNA or other epigenetic modifications are still relatively unknown.
DF and metabolic memory
DF, a common and severe complication of DM, is a major cause of extremity amputation, and the worldwide prevalence of DF is 6.3% (Zhang et al. 2017; Afonso, et al. 2021). The risk factors involved in the progression of DF are diabetic neuropathy, vascular insufficiency and immunological dysfunction (Noor et al. 2015). As there were no obvious improvements in wound healing even when glycemic control was achieved in patients with DM, metabolic memory may participate in DF (Zhao et al. 2021; Berlanga-Acosta et al. 2023). Del Cuore et al. (2023) used single nucleotide polymorphism (SNP) analysis in a population with diabetic foot disease. Their results indicated that patients with DF showed predominant expression of the VEGF C2578A CC polymorphism and reduced expression of the AC allele. They also found that miR-217-5p and miR-503-5p may be involved in regulating hyperglycemic memory in DF. Inflammation and DNA methylation are involved in metabolic memory, which is also a key mechanism in DR (Acosta et al. 2008; Deng et al. 2023). The genome-wide DNA methylation profiles of foot fibroblasts indicated that the change in DNA methylation was associated with metabolic memory, especially in patients with poor wound healing outcomes of diabetic foot ulceration (Park et al. 2014). Zhao et al. (2021) further demonstrated that transient hyperglycemia upregulated DNA methyltransferase 1 (DNMT1) expression, leading to the persistent hypermethylation of Ang-1 during subsequent normoglycemia, which induced inflammation and endothelial dysfunction in vitro and in vivo. These findings implied that epigenetic modifications are a hub contributor to metabolic memory in DR, although the present research is still limited.
Other diabetic complications and metabolic memory
As high blood glucose can injure multiple tissues and organs, hyperglycemic memory is also associated with other chronic complications of DM. Erectile dysfunction (ED) is a common complication of DM, with an approximate prevalence of 35–90% (Malavige and Levy 2009). A retrospective case‒control study showed that early hyperglycemia exposure could have long-term effects on erectile function in patients with DM, which could be sustained even after good glycemic control (Hui et al. 2021). A previous study showed that hyperglycemia could induce endothelial cell injury to cause microvascular leakage in the lung, which can further lead to pulmonary fibrosis (Lee et al. 2022). Jeon et al. (2023) further illustrated that high glucose-induced microvascular leakage and fibrosis in the lung could not be alleviated even after good blood glucose control. Furthermore, the pathophysiological mechanisms of diabetic neuropathy (DN) are epigenetic modifications, inflammation, oxidative stress and mitochondrial dysfunction, which are similar to the mechanisms of metabolic memory (Jankovic, et al. 2021). Thus, a review suggested that metabolic memory may also take part in the development of DN (Jankovic, et al. 2021). Regrettably, directly relevant research is rare, so more solid evidence is needed.
In summary, numerous studies have demonstrated that metabolic memory plays an important role in the progression of multiple chronic complications in patients with DM.
Potential therapeutic drugs for metabolic memory
Numerous molecular compounds have been proven to act on the key mechanisms of hyperglycemic memory, such as epigenetic modifications, inflammation, and senescence (Table 1). In addition, some molecular compounds may also regulate metabolism, but there is a lack of clear evidence supporting this possibility. Thus, in this section, we summarize the progress of current studies on metabolic memory-related potential drugs for treating diabetes and its complications.
SGI-1027, a highly lipophilic small-molecule inhibitor of DNMT1 based on its quinoline structure, potently inhibits DNA methylation, thereby suppressing senescence, apoptosis, and fibrosis (Sun et al. 2018; Gao et al. 2022; Wang et al. 2019). In DKD, DNMT1 regulates senescence and fibrosis by modulating the DNA methylation status of p21 (Al-Dabet et al. 2022). Given these findings, we hypothesize that SGI-1027 may hold promise for mitigating hyperglycemia memory and hyperglycemic memory-induced senescence, apoptosis, and fibrosis.
Chaetocin, a small-molecule natural product isolated from Chaetomium fungi, can regulate several mechanisms of metabolic memory, such as apoptosis, oxidative stress, autophagy and immune function (Jiang et al. 2021). SUV39H1 regulated sustained inflammation in vascular cells that were transiently cultured in high glucose through the modification of H3K9me3 (Villeneuve et al. 2008). Moreover, chaetocin can also decrease histone H3K9me3 levels at the promoter of the p21 WAF1 gene, which has also been proven to be a hyperglycemic memory-related gene in DKD (Al-Dabet et al. 2022; Lin et al. 2016). Interestingly, miR-125b, a key ncRNA that regulates hyperglycemic memory, plays an upstream role in the regulation of inflammatory genes in diabetic mice by downregulating SUV39H1 (Villeneuve et al. 2010; Wang and Chang 2011). These results further support the notion that chaetocin or a miR-125b inhibitor may be effective inhibitors of metabolic memory.
Research on these drugs is currently only at the experimental stage due to safety and other reasons, so their clinical use is still limited. Targeting the site of metabolic processes without interfering with regular metabolic processes is still a challenge. However, understanding the mechanisms of metabolic memory in diabetic complications is benefit in exploring new therapeutic approaches.
Models of metabolic memory
The concept of metabolic memory was proposed in 2003, with studies involving insulin treatment groups and an average follow-up duration of 6.5 years (Pop-Busui et al. 2009). More recently, Al-Dabet et al. (2022) used SGLT2i for 7.2 ± 0.8 months to manage hyperglycemia and evaluated urinary P21 expression as a marker of persistent tubular damage in DKD. Li et al. (2022c) tested DAPK3 in kidney tissue from DKD patients with poor HbA1c (10.2 ± 3.9) and those with good glycemic control (HbA1c 5.4 ± 0.5). However, crucial details such as the hypoglycemic medications used and the duration of glycemic control were omitted from their study. Hui et al. (2021) divided participants into three groups: a glycemic control group (regular treatment with normal glycemic levels in the past 5 years), a glycemic non-control group (non-regular treatment with poor glycemic control in the past 5 years), and a metabolic memory group (regular treatment and normal glycemic levels in the past year but non-regular treatment with poor glycemic control a year ago). Nevertheless, they also did not describe the hypoglycemic medications used in detail. Given the inherent challenges in controlling variables in clinical research, the majority of studies have resorted to animal and cell models to investigate the mechanisms underlying metabolic memory. Regrettably, there is a lack of consistency in the models of metabolic memory, both in vitro and in vivo. Thus, Table 2 lists different models used in different studies.
For in vitro models of metabolic memory, most researchers used high glucose conditions in cultured cells and then changed the glucose concentration to a normal level to simulate intensive treatment in DCCT/EDIC studies. In the majority of studies exploring diabetes and its chronic complications, a hyperglycemic exposure period of 48–72 h is typically considered representative of chronic hyperglycemia with long-term deleterious effects. This holds true for conditions such as DKD, DF, and diabetic cardiomyopathy (Hu et al. 2024; Wang et al. 2024; Li, et al. 2024; Song et al. 2023; Feng et al. 2023). However, it is worth noting that the duration of exposure to high glucose, as well as the periods of exposure to normal glucose, vary significantly across studies examining metabolic memory. In DKD, Al-Dabet et al. (2022) Mouse primary tubular cells were cultured under high-glucose conditions for 24 h and then at normal levels for another 24 h. However, Li et al. (2022c) Human glomerular endothelial cells were exposed to high levels of glucose for 3 days, followed by 3 days under normal conditions. Although these studies cultured cells for different durations, they equally distributed the time to high and normal glucose levels. Zhong et al. (2021) compared different in vitro models of metabolic memory in DF. Interestingly, different models performed similarly, and they used cultured human aortic endothelial cells for 1 day under high glucose and 6 days under normal glucose conditions. Another uncommon method involves deriving human proximal tubular epithelial cells from people with type 2 diabetes and culturing them under normal glucose conditions for 4 passages to establish a metabolic memory model (Bansal et al. 2020). Regrettably, there are no accepted standards for metabolic memory models in vitro, and most studies have chosen these models directly. However, for different cell lines, comparing different culture times might be more accurate.
For in vivo models of metabolic memory, the majority of studies have utilized insulin to lower blood glucose levels in diabetic mice or rats. Notably, in 2022, Al-Dabet et al. (2022) reported a novel attempt to employ SGLT2i in the construction of a metabolic memory model. Although they presumably considered the renal benefits of SGLT2i, they did not compare its effectiveness with that of insulin in model establishment. The duration of glycemic control in these studies varied significantly, ranging from a minimum of 3 weeks in rats with DKD treated with insulin to a maximum of 4 months in rats with DR also treated with insulin (Li et al. 2022c; Mohammad and Kowluru 2022). This wide range raises the following question: what is the optimal duration for the construction of in vivo models of metabolic memory? Furthermore, does this duration differ across various diabetic complications?
Outlook
In this review, we explored the regulatory mechanism of metabolic memory, including inflammation and immunity, oxidative stress and mitochondrial dysfunction, senescence and various kinds of cell death. Then, we discuss the function of metabolic memory in diabetic complications. In addition, we analyzed confirmed and potential metabolic memory-related inhibitors. Finally, we also summarized the in vitro and in vivo models of metabolic memory. In conclusion, metabolic memory might be a vital mechanism in the occurrence and development of various diabetic complications and is a promising therapeutic target for preventing the progression of complications.
There have been extensive studies on the essential mechanisms and regulators of metabolic memory, oxidative stress, mitochondrial dysfunction and apoptosis, which are the most studied and are mainly focused on DR. On the one hand, these traditional mechanisms may be the core mechanisms of metabolic memory and need to be validated more widely for the treatment of different diabetic complications. However, emerging mechanisms, such as senescence, ferroptosis, and pyroptosis, also deserve further exploration. In addition, animal and cellular models of metabolic memory are still controversial. Thus, we speculated that for different cell lines and methods for generating diabetic models, preexperiments may be necessary. Furthermore, numerous key molecules, including miR-320, p21 and Sod2, have been identified. However, there are currently no well-recognized markers that can represent metabolic memory in vitro or in vivo, such as ColI and fibronectin for fibrosis, let alone in vitro diagnostic markers used in clinical practice. In addition, small molecule drugs, such as polysaccharide, dopamine and aPC, have also been found to alleviate hyperglycemic memory to mitigate diabetic complications. We also speculated that SGI-1027 and chaetocin may be potential molecular compounds against metabolic memory. Regrettably, these drugs have been limited to animal and cellular models.
In the future, further studies of metabolic memory may start from the following perspectives: (1) develop explicit animal and cellular models of metabolic memory; (2) elucidate the mechanisms of metabolic memory and further uncover more hub molecules that regulate metabolic memory to obtain representative markers; (3) transform small molecule compounds that can be used to regulate metabolic memory in clinical practice; and (4) extensively study the crosstalk between lncRNAs and miRNAs; however, regrettably, pertinent research on the intricate network of lncRNAs and other ncRNAs remains to be conducted.
Although there have been certain studies that have summarized the relationship between metabolic memory and DKD, DR or epigenetic modification, studies on the connection between metabolic memory and chronic complications of DM, along with potential therapeutic drugs, remain scarce. Notably, our review is the first to comprehensively summarize various models of metabolic memory, given that the exact duration and severity of high-glucose toxicity are still elusive. Despite our efforts to comprehensively review the pathogenesis of metabolic memory in diverse chronic complications of DM, we acknowledge certain limitations, including the constraints of our research perspectives. Nevertheless, with further in-depth exploration of metabolic memory in chronic DM complications and elucidation of its underlying mechanisms, we are confident that this study will pave the way for reliable and innovative therapeutic targets that can retard or even arrest the progression of DM and its associated complications. Additionally, we intend to conduct regular systematic summaries in this field of research.
Availability of data and materials
The data of this study are included within the paper.
Abbreviations
- DM:
-
Diabetes mellitus
- DR:
-
Diabetic retinopathy
- DKD:
-
Diabetic kidney disease
- IDF:
-
International Diabetes Federation
- AGE:
-
Advanced glycation end product
- mtDNA:
-
Mitochondrial DNA
- SGLT2i:
-
Sodium glucose co-transporter 2 inhibitor
- DPP4i:
-
Dipeptidyl peptidase 4 inhibitors
- GLP-1RAs:
-
Glucagon-like peptide 1 receptor agonists
- DCCT:
-
Diabetes Control and Complications Trial
- EDIC:
-
Epidemiology of Diabetes Interventions and Complications
- UKPDS:
-
United Kingdom Prospective Diabetes Study
- ncRNAs:
-
Noncoding RNAs
- TXNIP:
-
Thioredoxin-interacting protein
- 3′ UTR:
-
3’ Untranslated region
- HPTMs:
-
Histone posttranslational modifications
- T2DM:
-
Type 2 diabetes mellitus
- SUV39H1:
-
Suppressor of variegation 3–9 homolog 1
- H3K4me1:
-
Histone H3 lysine 4 monomethylation
- lncRNAs:
-
Long noncoding RNAs
- miRNAs:
-
MicroRNAs
- DF:
-
Diabetic foot
- DCM:
-
Diabetic cardiomyopathy
- STZ:
-
Streptozotocin
- CD36:
-
Cluster of differentiation 36
- EndMT:
-
Endothelial-to-mesenchymal transition
- VSMC:
-
Vascular smooth muscle cells
- ESKD:
-
End-stage kidney disease
- GECs:
-
Glomerular endothelial cells
- ET-1:
-
Endothelin-1
- TECs:
-
Tubular epithelial cells
- aPC:
-
Activated protein C
- snRNA-seq:
-
Single-nucleus RNA
- snATAC-seq:
-
Assay for transposase-accessible chromatin sequencing
- RPEs:
-
Retinal pigment epithelial cells
- H3K4:
-
Histone 3 lysine 4
- DNMT1:
-
DNA methyltransferase 1
- DN:
-
Diabetic neuropathy
- ER:
-
Endoplasmic reticulum
- ROS:
-
Reactive oxygen species
- TGase:
-
Transglutaminase
- HEK-293:
-
Human embryonic kidney cells
- BUMPT:
-
Boston University mouse proximal tubular cells
- NOD:
-
Nonobese diabetic
- tBHQ:
-
Tert-butylhydroquinone
- GSH:
-
Glutathione
- AAV:
-
Adeno-associated virus
- 5-Aza:
-
5-Aza-deoxycytidine
- PTCs:
-
Proximal tubular cells
- HRECs:
-
Human retinal endothelial cells
- BRECs:
-
Bovine retinal endothelial cells
References
Aboouf MA, et al. Genotype screening of APLN rs3115757 variant in Egyptian women population reveals an association with obesity and insulin resistance. Diabetes Res Clin Pract. 2015;109(1):40–7.
Acosta JB, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5(4):530–9.
Afonso AC, et al. Biofilms in diabetic foot ulcers: impact, risk factors and control strategies. Int J Mol Sci 2021;22(15).
Aiello LP, D.E.R. Group (2014) Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 37(1): 17–23
Al-Dabet MM, et al. Reversal of the renal hyperglycemic memory in diabetic kidney disease by targeting sustained tubular p21 expression. Nat Commun. 2022;13(1):5062.
Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci 2018;19(1).
Bansal A, et al. Integrative omics analyses reveal epigenetic memory in diabetic renal cells regulating genes associated with kidney dysfunction. Diabetes. 2020;69(11):2490–502.
Bartlett CS, Jeansson M, Quaggin SE. Vascular growth factors and glomerular disease. Annu Rev Physiol. 2016;78:437–61.
Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr. 2016;10(2 Suppl 1):S176–83.
Berlanga-Acosta J, et al. Endogenous biological drivers in diabetic lower limb wounds recurrence: hypothetical reflections. Int J Mol Sci 2023;24(12).
Bhatti JS, et al. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022;184:114–34.
Bischoff J. Endothelial-to-mesenchymal transition. Circ Res. 2019;124(8):1163–5.
Bleriot C, et al. Inflammatory and immune etiology of type 2 diabetes. Trends Immunol. 2023;44(2):101–9.
Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7(7):369–75.
Chang J, et al. Update on the mechanisms of tubular cell injury in diabetic kidney disease. Front Med (lausanne). 2021;8: 661076.
Chen Z, Natarajan R. Epigenetic modifications in metabolic memory: what are the memories, and can we erase them? Am J Physiol Cell Physiol. 2022;323(2):C570–82.
Chen Z, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci U S A. 2016;113(21):E3002–11.
Cheng Y, et al. Central role of cardiac fibroblasts in myocardial fibrosis of diabetic cardiomyopathy. Front Endocrinol (lausanne). 2023;14:1162754.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36.
Choi EH, Park SJ. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp Mol Med. 2023;55(7):1348–56.
Cojocaru KA, et al. Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants (Basel), 2023;12(3).
Collins AJ, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl):A7, e1-420.
Costantino S, et al. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37(6):572–6.
Del Cuore A, et al. Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms (SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction. Cardiovasc Diabetol. 2023;22(1):148.
Deng JY, et al. Targeting DNA methylation and demethylation in diabetic foot ulcers. J Adv Res 2023.
Ding Q, et al. Inflammation-related epigenetic modification: the bridge between immune and metabolism in type 2 diabetes. Front Immunol. 2022;13: 883410.
Domingueti CP, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–45.
Dou L, Jourde-Chiche N. Endothelial toxicity of high glucose and its by-products in diabetic kidney disease. Toxins (Basel), 2019;11(10).
Drzewoski J, Kasznicki J, Trojanowski Z. The role of “metabolic memory” in the natural history of diabetes mellitus. Pol Arch Med Wewn. 2009;119(7–8):493–500.
El Mouhayyar C, et al. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol. 2020;2020:1762164.
El-Mesallamy HO, et al. Adiponectin and E-selectin concentrations in relation to inflammation in obese type 2 diabetic patients with coronary heart disease(s). Minerva Endocrinol. 2011;36(3):163–70.
El-Mesallamy HO, Hamdy NM, Sallam AA. Effect of obesity and glycemic control on serum lipocalins and insulin-like growth factor axis in type 2 diabetic patients. Acta Diabetol. 2013;50(5):679–85.
El-Osta A, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–17.
Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–12.
Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–67.
Feng Q, et al. Quercetin ameliorates diabetic kidney injury by inhibiting ferroptosis via activating Nrf2/HO-1 signaling pathway. Am J Chin Med. 2023;51(4):997–1018.
Filgueiras LR, et al. Imbalance between HDAC and HAT activities drives aberrant STAT1/MyD88 expression in macrophages from type 1 diabetic mice. J Diabetes Complications. 2017;31(2):334–9.
Fu J, et al. Regeneration of glomerular metabolism and function by podocyte pyruvate kinase M2 in diabetic nephropathy. JCI Insight, 2022;7(5).
Galicia-Garcia U. et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci, 2020. 21(17).
Galluzzi L, Myint M. Cell death and senescence. J Transl Med. 2023;21(1):425.
Gao Q, et al. Inhibition of DNA methyltransferase aberrations reinstates antioxidant aging suppressors and ameliorates renal aging. Aging Cell. 2022;21(1): e13526.
Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–8.
Gong ZG, et al. Epigenetic regulator BRD4 is involved in cadmium-induced acute kidney injury via contributing to lysosomal dysfunction, autophagy blockade and oxidative stress. J Hazard Mater. 2022;423(Pt A): 127110.
Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–22.
Hamdy NM, Suwailem SM, El-Mesallamy HO. Influence of vitamin E supplementation on endothelial complications in type 2 diabetes mellitus patients who underwent coronary artery bypass graft. J Diabetes Complications. 2009;23(3):167–73.
Hammes HP, et al. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol vis Sci. 1993;34(6):2092–6.
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–53.
Holman RR, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Hu K, et al. MicroRNA-221-3p inhibits the inflammatory response of keratinocytes by regulating the DYRK1A/STAT3 signaling pathway to promote wound healing in diabetes. Commun Biol. 2024;7(1):300.
Hui J, et al. Effects of “metabolic memory” on erectile function in diabetic men: a retrospective case-control study. Andrology. 2021;9(1):288–96.
Jankovic M, et al, Genetic and epigenomic modifiers of diabetic neuropathy. Int J Mol Sci 2021;22(9).
Jax TW. Metabolic memory: a vascular perspective. Cardiovasc Diabetol. 2010;9:51.
Jeon HY, et al. Simultaneous attenuation of hyperglycemic memory-induced retinal, pulmonary, and glomerular dysfunctions by proinsulin C-peptide in diabetes. BMC Med. 2023;21(1):49.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Jiang Z, et al. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic Biol Med. 2011;50(8):907–17.
Jiang H, et al. Chaetocin: a review of its anticancer potentials and mechanisms. Eur J Pharmacol. 2021;910: 174459.
Jin ML, Jeong KW. Histone modifications in drug-resistant cancers: from a cancer stem cell and immune evasion perspective. Exp Mol Med. 2023;55(7):1333–47.
Johnson J, et al. Oxidative stress in neutrophils: implications for diabetic cardiovascular complications. Antioxid Redox Signal. 2022;36(10–12):652–66.
Joslin EP. Diabetes mellitus. N Engl J Med. 1946;234:476.
Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15(6):327–45.
Khella MS, et al. The (FTO) gene polymorphism is associated with metabolic syndrome risk in Egyptian females: a case-control study. BMC Med Genet. 2017;18(1):101.
Kowluru RA, Alka K. Mitochondrial quality control and metabolic memory phenomenon associated with continued progression of diabetic retinopathy. Int J Mol Sci 2023;24(9).
Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610.
Kumari N, et al. The potential role of m6A RNA methylation in diabetic retinopathy. Exp Eye Res. 2021;208: 108616.
Kung HC, et al. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson's disease. Biomedicines 2021;9(8).
Lee YJ, et al. Dopamine ameliorates hyperglycemic memory-induced microvascular dysfunction in diabetic retinopathy. FASEB J. 2022;36(12): e22643.
Li C, et al. Non-coding RNAs in diabetes mellitus and diabetic cardiovascular disease. Front Endocrinol (lausanne). 2022a;13: 961802.
Li Q, et al. Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1alpha-mediated oxidative stress and mitochondrial dysfunction. Front Pharmacol. 2022b;13: 859723.
Li X, et al. Sirt7 associates with ELK1 to participate in hyperglycemia memory and diabetic nephropathy via modulation of DAPK3 expression and endothelial inflammation. Transl Res. 2022c;247:99–116.
Li Y, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8(1):152.
Li SS, et al. Diabetes promotes myocardial fibrosis via AMPK/EZH2/PPAR-gamma signaling pathway. Diabetes Metab J 2024.
Lin SH, et al. Histone methyltransferase Suv39h1 attenuates high glucose-induced fibronectin and p21(WAF1) in mesangial cells. Int J Biochem Cell Biol. 2016;78:96–105.
Liu P, et al. Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol Med. 2019a;25(1):21.
Liu T, et al. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019b;227:64–73.
Liu DD, et al. Epigenetic modifications and metabolic memory in diabetic retinopathy: beyond the surface. Neural Regen Res. 2023;18(7):1441–9.
Lizotte F, et al. Persistent insulin resistance in podocytes caused by epigenetic changes of SHP-1 in diabetes. Diabetes. 2016;65(12):3705–17.
Maamoun H, et al. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Front Physiol. 2019;10:70.
Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9(4):315–27.
Mahmoud MM, Sanad EF, Hamdy NM. MicroRNAs’ role in the environment-related non-communicable diseases and link to multidrug resistance, regulation, or alteration. Environ Sci Pollut Res Int. 2021;28(28):36984–7000.
Mahtal N, Lenoir O, Tharaux PL. Glomerular endothelial cell crosstalk with podocytes in diabetic kidney disease. Front Med (lausanne). 2021;8: 659013.
Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–47.
Mansour MA, et al. P21 overexpression promotes cell death and induces senescence in human glioblastoma. Cancers (Basel), 2023;15(4).
Mao R, et al. Enhancer RNAs: a missing regulatory layer in gene transcription. Sci China Life Sci. 2019;62(7):905–12.
Marwick TH, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51.
Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic Biol Med. 2014;75:129–39.
Mohammad G, Kowluru RA. Mitochondrial dynamics in the metabolic memory of diabetic retinopathy. J Diabetes Res. 2022;2022:3555889.
Mossel DM, et al. Epigenetic regulation of S100A9 and S100A12 expression in monocyte-macrophage system in hyperglycemic conditions. Front Immunol. 2020;11:1071.
Mostafa AM, et al. Glucagon-like peptide 1 (GLP-1)-based therapy upregulates LXR-ABCA1/ABCG1 cascade in adipocytes. Biochem Biophys Res Commun. 2015;468(4):900–5.
Mostafa AM, et al. Effect of vildagliptin and pravastatin combination on cholesterol efflux in adipocytes. IUBMB Life. 2016;68(7):535–43.
Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029–44.
Nathan DM, DER Group, The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37(1):9-16.
Nathan DM, D.E.R. Group (2014) The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37(1): 9–16
Nedosugova LV, et al. Inflammatory mechanisms of diabetes and its vascular complications. Biomedicines 2022;10(5).
Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192–9.
Novak M, et al. Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in patients with diabetes with comorbid depression. Diabetes Care. 2016;39(11):1940–7.
Okabe J, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res. 2012;110(8):1067–76.
Palomer X, Pizarro-Delgado J, Vazquez-Carrera M. Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci. 2018;39(5):452–67.
Park LK, et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014;9(10):1339–49.
Peng QH, et al. Astragalus polysaccharide attenuates metabolic memory-triggered ER stress and apoptosis via regulation of miR-204/SIRT1 axis in retinal pigment epithelial cells. Biosci Rep 2020;40(1).
Phoenix A, Chandran R, Ergul A. Cerebral microvascular senescence and inflammation in diabetes. Front Physiol. 2022;13: 864758.
Pop-Busui R, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119(22):2886–93.
Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med. 2015;6(3):120–7.
Ranjit Unnikrishnan I, Anjana RM, Mohan V (2011) Importance of controlling diabetes early—the concept of metabolic memory, legacy effect and the case for early insulinisation. J Assoc Physicians India. 59(Suppl): 8–12
Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–55.
Reddy MA, et al. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36(5):864–73.
Retnakaran R et al (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55(6): 1832–9
Roy S, et al. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990;87(1):404–8.
Shen S, Ji C, Wei K. Cellular senescence and regulated cell death of tubular epithelial cells in diabetic kidney disease. Front Endocrinol (lausanne). 2022;13: 924299.
Slyne J, et al. New developments concerning the proximal tubule in diabetic nephropathy: in vitro models and mechanisms. Nephrol Dial Transplant. 2015;30(Suppl 4):60–7.
Song Y, et al. Novel lncRNA-prader willi/angelman region RNA, SNRPN neighbour (PWARSN) aggravates tubular epithelial cell pyroptosis by regulating TXNIP via dual way in diabetic kidney disease. Cell Prolif. 2023;56(2): e13349.
Su D, et al. Tcf3-activated lncRNA Gas5 regulates newborn mouse cardiomyocyte apoptosis in diabetic cardiomyopathy. J Cell Biochem. 2020;121(11):4337–46.
Sun N, et al. DNMTs inhibitor SGI-1027 induces apoptosis in Huh7 human hepatocellular carcinoma cells. Oncol Lett. 2018;16(5):5799–806.
Sun H, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119.
Sun Y, et al. Epigenetic regulation of mesenchymal stem cell aging through histone modifications. Genes Dis. 2023;10(6):2443–56.
Taft RJ, et al. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–39.
Takahashi P, et al. Transcript expression profiles and microRNA regulation indicate an upregulation of processes linked to oxidative stress, DNA repair, cell death, and inflammation in type 1 diabetes mellitus patients. J Diabetes Res. 2022;2022:3511329.
Teodoro JS, et al. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol. 2018;9:1857.
Tewari S, et al. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest Ophthalmol vis Sci. 2012;53(8):4881–8.
Thielen L, Shalev A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr Opin Endocrinol Diabetes Obes. 2018;25(2):75–80.
Thiem K, et al. Hyperglycemic memory of innate immune cells promotes in vitro proinflammatory responses of human monocytes and murine macrophages. J Immunol. 2021;206(4):807–13.
Thomas HY, Ford Versypt AN. Pathophysiology of mesangial expansion in diabetic nephropathy: mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J Biol Eng. 2022;16(1):19.
Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–77.
Tonna S, et al. Metabolic memory and diabetic nephropathy: potential role for epigenetic mechanisms. Nat Rev Nephrol. 2010;6(6):332–41.
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16(6):317–36.
Vastolo V, et al. High-fat diet unveils an enhancer element at the Ped/Pea-15 gene responsible for epigenetic memory in skeletal muscle. Metabolism. 2018;87:70–9.
Villeneuve LM, et al. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105(26):9047–52.
Villeneuve LM, et al. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes. 2010;59(11):2904–15.
Voronova V, et al. Interpretation of metabolic memory phenomenon using a physiological systems model: what drives oxidative stress following glucose normalization? PLoS ONE. 2017;12(2): e0171781.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–14.
Wang Z, et al. Metabolic memory in mitochondrial oxidative damage triggers diabetic retinopathy. BMC Ophthalmol. 2018;18(1):258.
Wang JY, et al. Suppressing microRNA-29c promotes biliary atresia-related fibrosis by targeting DNMT3A and DNMT3B. Cell Mol Biol Lett. 2019;24:10.
Wang M, et al. Endothelial dysfunction and diabetic cardiomyopathy. Front Endocrinol (lausanne). 2022;13: 851941.
Wang H, et al. VDR activation attenuates renal tubular epithelial cell ferroptosis by regulating Nrf2/HO-1 signaling pathway in diabetic nephropathy. Adv Sci (weinh). 2024;11(10): e2305563.
Wei L, et al. The pathophysiological mechanisms underlying diabetic retinopathy. Front Cell Dev Biol. 2022;10: 963615.
Wilson PC, et al. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat Commun. 2022;13(1):5253.
Writing Team for the Diabetes C, I. Complications Trial/Epidemiology of Diabetes, and G. Complications Research (200) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290(16): 2159–67
Xian Y, et al. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(10):2009–17.
Xie L, et al. Emerging roles of sodium glucose cotransporter 2 (SGLT-2) inhibitors in diabetic cardiovascular diseases: focusing on immunity inflammation and metabolism. Front Pharmacol. 2022;13: 836849.
Xu Y, Kovacic JC. Endothelial to mesenchymal transition in health and disease. Annu Rev Physiol. 2023;85:245–67.
Yamagishi SI, Nakamura N, Matsui T. Glycation and cardiovascular disease in diabetes: a perspective on the concept of metabolic memory. J Diabetes. 2017;9(2):141–8.
Yao Y, et al. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc Res. 2022;118(1):196–211.
Yu XY, et al. High levels of glucose induce “metabolic memory” in cardiomyocyte via epigenetic histone H3 lysine 9 methylation. Mol Biol Rep. 2012;39(9):8891–8.
Zapata RC, et al. Adipocytes control food intake and weight regain via Vacuolar-type H(+) ATPase. Nat Commun. 2022;13(1):5092.
Zhan J, et al. Hyperglycemic memory in diabetic cardiomyopathy. Front Med. 2022;16(1):25–38.
Zhan J, et al. Positive feedback loop of miR-320 and CD36 regulates the hyperglycemic memory-induced diabetic diastolic cardiac dysfunction. Mol Ther Nucleic Acids. 2023;31:122–38.
Zhang E, Wu Y. Metabolic memory: mechanisms and implications for diabetic vasculopathies. Sci China Life Sci. 2014;57(8):845–51.
Zhang P, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med. 2017;49(2):106–16.
Zhang P, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583–600.
Zhang X, et al. CD36 signaling in diabetic cardiomyopathy. Aging Dis. 2021;12(3):826–40.
Zhao S, et al. miR-23b-3p induces the cellular metabolic memory of high glucose in diabetic retinopathy through a SIRT1-dependent signalling pathway. Diabetologia. 2016;59(3):644–54.
Zhao J, et al. Transient high glucose causes persistent vascular dysfunction and delayed wound healing by the DNMT1-mediated Ang-1/NF-kappaB pathway. J Invest Dermatol. 2021;141(6):1573–84.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Zhong Q, Kowluru RA. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest Ophthalmol vis Sci. 2013;54(1):244–50.
Zhong X, et al. The microRNAs in the pathogenesis of metabolic memory. Endocrinology. 2015;156(9):3157–68.
Zhong Y, et al. Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: a narrative review. Int J Biol Macromol. 2023;259(Pt 1): 128182.
Zhou X, et al. Role of renal tubular programed cell death in diabetic kidney disease. Diabetes Metab Res Rev. 2023;39(2): e3596.
Zou HH, et al. Endothelial cells secreted endothelin-1 augments diabetic nephropathy via inducing extracellular matrix accumulation of mesangial cells in ETBR(-/-) mice. Aging (albany NY). 2019;11(6):1804–20.
Acknowledgements
We thank the Translational Medicine Center, the First Affiliated Hospital of Zhengzhou University, for their support.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (U200410394), The Research and Innovation Team Project of the First Affiliated Hospital of Zhengzhou University (QNCXTD2023015) and 2021 Henan Province Health Young and Middle-aged Discipline Leader Training Project.
Author information
Authors and Affiliations
Contributions
TY, YZ and QF conceptualized and wrote the manuscript. TY, FG, MS, YS, GR, LZ and YZ contributed to manuscript writing. QF, GQ and YZ reviewed and revised the manuscript. All authors have read and approved the final version of the manuscript being submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Authors, hereby consent to the publication of my paper "An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory" in Molecular Medicine. I confirm that the content is accurate and that I am aware of any potential disclosures. I waive any rights to claim authorship and hold the publisher harmless from any liabilities arising from publication.
Competing interests
The authors declare no conflicts of interest that pertain to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, T., Qi, F., Guo, F. et al. An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory. Mol Med 30, 71 (2024). https://doi.org/10.1186/s10020-024-00824-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-024-00824-9