Abstract
Background
Mycophenolate mofetil (MMF) is recommended as a first-line immunosuppressant to treat lupus nephritis (LN). Prognosis and therapeutic response in LN are known to vary depending on race. We investigated the benefits of MMF and therapeutic drug monitoring (TDM) in the treatment of Japanese LN patients.
Methods
In this retrospective cohort study, a total of 20 patients with LN who started MMF treatment were included. Clinical data were collected regularly after MMF administration. We evaluated complete remission (CR) rate as the primary outcome. Predictors of CR were identified using univariate and multivariate analyses. In the research of TDM, the correlation with the area under the curve (AUC) was analyzed at MMF dose, single-point value, treatment response, and adverse events.
Results
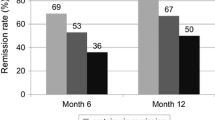
Overall, 70% of cases showed CR; both flare-ups and refractory cases had favorable results. Cases of LN with nephrotic syndrome (NS) or class III/IV + V showed a significantly lower CR rate (p < 0.005). The ratio of maintaining CR after MMF therapy was as high as 85.7%. In multivariate analysis, NS was an independent negative predictor of CR (HR 0.09, 95% confidence interval 0.01–0.81; p = 0.03). The relationship between AUC and MMF dose was low, and AUC correlated with trough level (r = 0.73). AUC tended to be high in the treatment responder (p = 0.09), but did not correlate with adverse events of infection (p = 0.92).
Conclusion
MMF is a beneficial treatment option for Japanese LN patients, and further investigation on TDM-based therapy is needed.







Similar content being viewed by others
References
Austin HA 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:6149.
Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64:797–808.
Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. The Joint European League against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–82.
Korbet SM, Schwartz MM, Evans J, Lewis EJ; Collaborative Study Group. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18:244–54.
Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496–503.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revised. J Am Soc Nephrol. 2004;15:241–50.
KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:221–32.
Johnson AG, Rigby RJ, Taylor PJ, Jones CE, Allen J, Franzen K, et al. The kinetics of mycophenolic acid and its glucuronide metabolite in adult kidney transplant recipients. Clin Pharmacol Ther. 1999;66:492–500.
Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–12.
Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–28.
Mok CC. Prognostic factors in lupus nephritis. Lupus. 2005;14:39–44.
Najafi CC, Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J. Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney Int. 2001;59:2156–63.
Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V + IV lupus nephritis with multitarget therapy. J Am Soc Nephrol. 2008;19:2001–10.
Parikh SV, Nagaraja HN, Hebert L, Rovin BH. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin J Am Soc Nephrol. 2014;9:279–84.
Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365:1886–95.
Houssiau FA, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al; MAINTAIN Nephritis Trial Group. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010; 69:2083–9.
Shaw LM, Korecka M, Venkataramanan R, Goldberg L, Bloom R, Brayman KL. Mycophenolic acid pharmacodynamics and pharmacokinetics provide a basis for rational monitoring strategies. Am J Transplant. 2003;3:534–42.
Zahr N, Arnaud L, Marquet P, Haroche J, Costedoat-Chalumeau N, Hulot JS, et al. Mycophenolic acid area under the curve correlates with disease activity in lupus patients treated with mycophenolate mofetil. Arthritis Rheum. 2010;62:2047–54.
van Gelder T, Berden JH, Berger SP. To TDM or not to TDM in lupus nephritis patients treated with MMF? Nephrol Dial Transplant. 2015;30:560–4.
Lertdumrongluk P, Somparn P, Kittanamongkolchai W, Traitanon O, Vadcharavivad S, Avihingsanon Y. Pharmacokinetics of mycophenolic acid in severe lupus nephritis. Kidney Int. 2010;78:389–95.
Okamoto M, Wakabayashi Y, Higuchi A, Kadotani Y, Ogino S, Ushigome H, et al. Therapeutic drug monitoring of mycophenolic acid in renal transplant recipients. Transplant Proc. 2005;37:859–60.
Acknowledgements
This study was supported partly by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan. The authors also acknowledge Editage for providing editorial and publication support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Department of Nephrology, Nagoya University Graduate School of Medicine received research promotion grants from Otsuka Pharmaceutical Co, Kissei Pharmaceutical Co, Novartis Pharma K.K, Kowa Pharmaceutical Co, Chugai Pharmaceutical Co, Nippon Boehringer Ingelheim Co ., Ltd, Pfizer Japan Inc, Kyowa Hakko Kirin Co ., Ltd, Torii Pharmaceutical Co.,Ltd, Astellas Pharma Inc, MSD K.K, Daiichi Sankyo Company Limited, Takeda Pharmaceutical Company Limited, Bristol-Myers Squibb, Mitsubishi Tanabe Pharma Corporation, Sumitomo Dainippon Pharma Co., Ltd, Teijin Pharma Limited, and Mochida Pharmaceutical Co ., Ltd.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB Approval Number 2017-0086) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The ethical committee approved this retrospective cohort study without written informed consent, but informed consent was obtained from most patients at the time of renal biopsy.
About this article
Cite this article
Katsuno, T., Ozaki, T., Ozeki, T. et al. Investigation on the benefits of mycophenolate mofetil and therapeutic drug monitoring in the treatment of Japanese patients with lupus nephritis. Clin Exp Nephrol 22, 1341–1350 (2018). https://doi.org/10.1007/s10157-018-1590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1590-2