Abstract
Background
Apart from its application in human diagnostics, magnetic resonance imaging (MRI) can also be used to study the internal anatomy of zoological specimens. As a non-invasive imaging technique, MRI has several advantages, such as rapid data acquisition, output of true three-dimensional imagery, and provision of digital data right from the onset of a study. Of particular importance for comparative zoological studies is the capacity of MRI to conduct high-throughput analyses of multiple specimens. In this study, MRI was applied to systematically document the internal anatomy of 98 representative species of sea urchins (Echinodermata: Echinoidea).
Findings
The dataset includes raw and derived image data from 141 MRI scans. Most of the whole sea urchin specimens analyzed were obtained from museum collections. The attained scan resolutions permit differentiation of various internal organs, including the digestive tract, reproductive system, coelomic compartments, and lantern musculature. All data deposited in the Giga DB repository can be accessed using open source software. Potential uses of the dataset include interactive exploration of sea urchin anatomy, morphometric and volumetric analyses of internal organs observed in their natural context, as well as correlation of hard and soft tissue structures.
Conclusions
The dataset covers a broad taxonomical and morphological spectrum of the Echinoidea, focusing on ‘regular’ sea urchin taxa. The deposited files significantly expand the amount of morphological data on echinoids that are electronically available. The approach chosen here can be extended to various other vertebrate and invertebrate taxa. We argue that publicly available digital anatomical and morphological data gathered during experiments involving non-invasive imaging techniques constitute one of the prerequisites for future large-scale genotype—phenotype correlations.
Similar content being viewed by others
Data description
Purpose of data acquisition
Despite the fact that sea urchins (Echinodermata: Echinoidea) have served as model organisms in various biological disciplines for over a century and a half [1], the internal anatomy of this taxon had never been systematically analyzed on a large scale. Until recently, such broad inferences would invariably have required the undesirable dissection of valuable material, including museum type specimens. However, non-invasive scanning techniques such as magnetic resonance imaging (MRI) now permit studying echinoid internal anatomy without the need for dissection [2]. In addition to its suitability for studies on sea urchins, MRI can also be used to visualize the soft tissue anatomy of other invertebrate as well as vertebrate taxa [3]. Most importantly, MRI experiments result in digital data suitable for rapid online dissemination [4].
In recent years, morphology has fallen behind data gathering, deposition and transparency practices considered as standard in other biological disciplines, such as proteomics or genomics [5]. Apart from its multiple potential applications, the dataset presented here is therefore also intended to serve as a catalyst for new approaches aimed at the large-scale deposition of digital morphological and anatomical information [6].
Scanned specimens
The deposited dataset comprises 141 MRI scans from 98 representative extant sea urchin species. The scanned specimens were whole sub-adult or adult individuals ranging in diameter or length from 5–43 mm. Most of the specimens were obtained from museum collections, where they are preserved in ethanol for long-term storage. Some of the specimens were collected and fixed more than 135 years ago, while others were collected a few months prior to scanning. Additional file 1 gives a taxonomical list of the species analyzed and provides specimen data (Table S1).
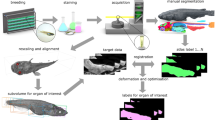
Data acquisition and processing
Basic information on the protocols used for specimen preparation, contrasting, and scanning are provided in Additional file 1, complemented by a description of each file type produced during the MRI experiments (Table S2). More specific information on sample preparation and equipment or the application of contrast agents and different scanning protocols has been published elsewhere [7]. The detailed acquisition and reconstruction parameters for each scan can be found in the MRI metadata files deposited online together with the raw and derived image data [8].
Forty-four two-dimensional (2D) and 97 three-dimensional (3D) scans were obtained using various high-field MRI scanners. Table 1 provides information on the scanning systems employed in this study. 2D MRI scans have a reduced voxel resolution in the third dimension (e.g., 50 × 50 × 200 μm), while 3D MRI scans are characterized by an isotropic voxel resolution (e.g., 40 × 40 × 40 μm). In various instances, specimens were scanned twice using the same scanning protocol, but once before and once after the application of a contrast agent (Magnevist, Bayer HealthCare, Leverkusen, Germany). The folders containing scans of contrasted specimens have been specifically labeled using the word 'Magnevist'.
For each scan, one image stack in tagged image file format (TIFF, .tif) was created based on the standard Bruker MRI 2dseq raw image file using the software ImageJ [9]. To facilitate a rapid recognition of internal structures, TIFF stacks based on 3D scans were rotated to a standardized orientation along the oral-aboral axis of the animal using the tool TransformJ Rotate, which is part of the ImageJ plugin TransformJ [10]. In addition, some of the TIFF stacks were reduced in their pixel dimensions by removing uninformative parts using the ‘Image:Crop’ command in ImageJ.
Data quality
The suitability of a given specimen for MRI was ascertained through visual inspection of the MRI scout images and the low-resolution scans performed prior to scanning at high resolutions [7]. The achieved 2D and 3D scan resolutions constitute the current state-of-the-art in high-field MRI at the given fields of view and are largely comparable to results derived from dissections carried out under direct observation through a stereomicroscope [2]. The quality of a given scan depended on various factors, some of which were outside our control, such as specimen health prior to fixation, the fixation itself, or the quality of the long-term storage. Although the 141 deposited scans constitute a selection of those with the best quality, several scans still show a significant presence of artifacts. These artifacts are primarily related to the biology of the animal, in particular the presence of para- or ferromagnetic substances contained within the digestive tract [7]. Additional file 1 provides brief information on artifacts encountered in each scan.
Potential uses
The methodological approach employed here allows conducting high-throughput analyses of hundreds or even thousands of zoological specimens [7]. Potential uses of the present dataset include morphometric or volumetric analyses of internal organs [11] and interactive exploration of sea urchin anatomy using digital 2D and 3D visualization tools [12]. For example, MRI scans with an isotropic voxel resolution are particularly suitable for 3D modeling [13]. In addition, MRI stacks can be aligned with image data derived from complementary non-invasive imaging techniques that permit visualizing mineralized structures, in particular micro-computed tomography [14]. Furthermore, the relatively quick 2D MRI scanning protocols used for some of the deposited scans could find application in the in vivo study of sea urchins whose gonads are intended for human consumption.
Relevance of the dataset
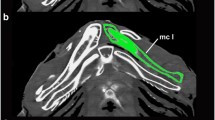
The dataset presented here constitutes a representative sample of sea urchin structural diversity. No significant differences in scanning results were observed when employing freshly fixed or museum specimens [2], while the application of a contrast agent resulted in an improved signal-to-noise ratio, as well as a reduction of the negative effects of artifacts [7]. The MRI data allow identification of numerous internal organs, including lantern muscles [15], axial complex [16], gastric caecum [17], or intestinal caecum [18]. These studies demonstrate that initial difficulties with regard to the interpretation of MRI data do not render the scanning approach itself unsuitable [19].
Due to the digital nature of data based on non-invasive imaging techniques, the rapid online dissemination of morphological and anatomical information has finally become possible. This development is bound to lead to an unprecedented level of data availability and transparency in zoomorphology and paleontology, ultimately resulting in more widespread data mining in these two scientific fields [20]. Furthermore, the enforced deposition of digital morphological data as a prerequisite for publication will pave the way for the correlation of geno- and phenotype on a large scale [6]. We believe that digital datasets and enforced data deposition constitute essential components for the success of the expanding field of morphomics, which aims to complement the already established ‘omics’ disciplines [21].
Availability of supporting data
The dataset supporting the results of this study is available in the GigaScience repository, Giga DB [8]. Additional file 1 provides specimen data, detailed information on data availability and requirements, as well as information on the preparation, contrasting and scanning of sea urchin specimens. The authors ask that any publication arising from the use of the deposited data acknowledges the source of the dataset. See [22] for a discussion of copyright licenses and waiver agreements used in open access research.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- MRI:
-
Magnetic resonance imaging
- TIFF:
-
Tagged image file format.
References
Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, Angerer LM, Arnone MI, Burgess DR, Burke RD, Coffman JA, Dean M, Elphick MR, Ettensohn CA, Foltz KR, Hamdoun A, Hynes RO, Klein WH, Marzluff W, McClay DR, Morris RL, Mushegian A, Rast JP, Smith LC, Thorndyke MC, Vacquier VD, Wessel GM, Wray G, Zhang L, Elsik CG: The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006, 314: 941-952. doi:10.1126/science.1133609
Ziegler A, Faber C, Mueller S, Bartolomaeus T: Systematic comparison and reconstruction of sea urchin (Echinoidea) internal anatomy: a novel approach using magnetic resonance imaging. BMC Biol. 2008, 6: 33-10.1186/1741-7007-6-33. doi:10.1186/1741-7007-6-33
Ziegler A, Kunth M, Mueller S, Bock C, Pohmann R, Schröder L, Faber C, Giribet G: Application of magnetic resonance imaging in zoology. Zoomorphology. 2011, 130: 227-254. 10.1007/s00435-011-0138-8. doi:10.1007/s00435-011-0138-8
Berquist RM, Gledhill KM, Peterson MW, Doan AH, Baxter GT, Yopak KE, Kang N, Walker HJ, Hastings PA, Frank LR: The Digital Fish Library: using MRI to digitize, database, and document the morphological diversity of fish. PLoS One. 2012, 7: e34499-10.1371/journal.pone.0034499. doi:10.1371/journal.pone.0034499
Giribet G: A new dimension in combining data? The use of morphology and phylogenomic data in metazoan systematics. Acta Zool. 2010, 91: 11-19. 10.1111/j.1463-6395.2009.00420.x. doi:10.1111/j.1463-6395.2009.00420.x
Ziegler A, Ogurreck M, Steinke T, Beckmann F, Prohaska S, Ziegler A: Opportunities and challenges for digital morphology. Biol Direct. 2010, 5: 45-10.1186/1745-6150-5-45. doi:10.1186/1745-6150-5-45
Ziegler A, Mueller S: Analysis of freshly fixed and museum invertebrate specimens using high-resolution, high-throughput MRI. Meth Mol Biol. 2011, 771: 633-651. 10.1007/978-1-61779-219-9_32. doi:10.1007/978-1-61779-219-9_32
Ziegler A, Faber C, Mueller S, Nagelmann N, Schröder L: MRI scans of whole sea urchin specimens. Giga Science Database. 2014,http://dx.doi.org/10.5524/100124,
NIH: ImageJ - image processing and analysis in Java.http://imagej.nih.gov/ij/,
Meijering E: TransformJ - a Java package for geometrical image transformation.http://www.imagescience.org/meijering/software/transformj/,
Sigl R, Imhof H, Settles M, Laforsch C: A novel, non-invasive and in vivo approach to determine morphometric data in starfish. J Exp Mar Biol Ecol. 2013, 449: 1-9. doi:10.1016/j.jembe.2013.08.002
Ziegler A, Menze BH: Accelerated Acquisition, Visualization, and Analysis of zoo-Anatomical Data. Computation for Humanity: Information Technology to Advance Society. Edited by: Zander J, Mostermann PJ. 2013, Boca Raton: CRC Press, 233-260.
Ziegler A, Faber C, Mueller S: 3D visualization of sea urchin anatomy.http://www.nhm.ac.uk/research-curation/research/projects/echinoid-directory/models/,
Ziegler A: Broad application of non-invasive imaging techniques to echinoids and other echinoderm taxa. Zoosymposia. 2012, 7: 53-70.
Ziegler A, Schröder L, Ogurreck M, Faber C, Stach T: Evolution of a novel muscle design in sea urchins (Echinodermata: Echinoidea). PLoS One. 2012, 7: e37520-10.1371/journal.pone.0037520. doi:10.1371/journal.pone.0037520
Ziegler A, Faber C, Bartolomaeus T: Comparative morphology of the axial complex and interdependence of internal organ systems in sea urchins (Echinodermata: Echinoidea). Front Zool. 2009, 6: 10-10.1186/1742-9994-6-10. doi:10.1186/1742-9994-6-10
Ziegler A, Mooi R, Rolet G, De Ridder C: Origin and evolutionary plasticity of the gastric caecum in sea urchins (Echinodermata: Echinoidea). BMC Evol Biol. 2010, 10: 313-10.1186/1471-2148-10-313. doi:10.1186/1471-2148-10-313
Ziegler A: Rediscovery of an internal organ in heart urchins (Echinoidea: Spatangoida): morphology and evolution of the intestinal caecum. Org Div Evol. 2014, doi:10.1007/s13127-014-0178-2
Holland ND, Ghiselin MT: Magnetic resonance imaging (MRI) has failed to distinguish between smaller gut regions and larger haemal sinuses in sea urchins (Echinodermata: Echinoidea). BMC Biol. 2009, 7: 39-10.1186/1741-7007-7-39. doi:10.1186/1741-7007-7-39
Rowe T, Frank LR: The disappearing third dimension. Science. 2010, 331: 712-714. doi:10.1126/science.1202828
Altenberg L: Modularity in Evolution: Some low-Level Questions. Modularity: Understanding the Development and Evolution of Complex Natural Systems. Edited by: Callebaut W, Rasskin-Gutman D. 2005, Cambridge: MIT Press, 99-128.
Hrynaszkiewicz I, Cockerill MJ: Open by default: a proposed copyright license and waiver agreement for open access research and data in peer-reviewed journals. BMC Res Notes. 2012, 5: 494-10.1186/1756-0500-5-494. doi:10.1186/1756-0500-5-494
Acknowledgements
We are indebted to the following for providing access to sea urchin specimens and for granting permission to make the MRI scans publicly available: Nadia Améziane (Muséum national d’Histoire naturelle, Paris), Owen Anderson (National Institute of Water and Atmospheric Research, Auckland), Andrew Cabrinovic (British Museum of Natural History, London), Andreas Kroh (Naturhistorisches Museum Wien, Vienna), Carsten Lüter (Museum für Naturkunde, Berlin), Rich Mooi (California Academy of Sciences, San Franciso), Jørgen Olesen (Zoologisk Museum København, Copenhagen), David L. Pawson (National Museum of Natural History, Washington DC), Bernhard Ruthensteiner (Zoologische Staatssammlung München, Munich), and Andreas Schmidt-Rhaesa (Zoologisches Institut und Museum, Hamburg). We are particularly grateful to Mariko Kondo (Misaki Marine Biological Station, Misaki) and Kirill Minin (P. P. Shirshov Institute of Oceanology, Moscow) for donating specimens. Christopher Hunter provided professional assistance with data upload and management. Comments by Maria Byrne, Scott C. Edmunds, Sarah Faulwetter, and Daniel Mietchen helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AZ designed the study, drafted the manuscript, and gathered, analyzed and curated data. CF, SM, NN, and LS designed the experiments and gathered data. All authors read and approved the final manuscript.
Electronic supplementary material
13742_2014_50_MOESM1_ESM.pdf
Additional file 1: Supplementary specimen information and tables. Table S1. Overview of the dataset comprising 141 MRI scans of 98 extant sea urchin species. Table S2. Overview of Bruker MRI file types. (PDF 235 KB)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ziegler, A., Faber, C., Mueller, S. et al. A dataset comprising 141 magnetic resonance imaging scans of 98 extant sea urchin species. GigaSci 3, 21 (2014). https://doi.org/10.1186/2047-217X-3-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2047-217X-3-21