Abstract
Background
Supplementation with a phosphatidylserine and phosphatidylserine/ phosphatidic acid complex (PAS) has been observed to normalize stress induced dysregulations of the hypothalamus-pituitary-adrenal axis (HPAA). Prolonged stress first induces a hyper-activation of the HPAA, which then can be followed by a state of hypo-activation.
The aim of this study was to examine effects of an oral supplementation with 400 mg PS & 400 mg PA (PAS 400) per day on the endocrine stress response (ACTH, saliva and serum cortisol) to a psychosocial stressor. A special focus was to analyze subgroups of low versus high chronically stressed subjects as well as to test efficacy of 200 mg PS & 200 mg PA (PAS 200).
Methods
75 healthy male volunteers were enrolled for this double-blind, placebo-controlled study, stratified by chronic stress level, and randomly allocated to one of three study arms (placebo, PAS 200 and PAS 400 per day, respectively). Study supplementation was administered for 42 days for each participant. Chronic stress was measured with the Trier Inventory for Chronic Stress (TICS), and subgroups of high and low chronic stress were differentiated by median values as provided by the TICS authors. A six week period of supplementation was followed by an acute stress test (Trier Social Stress Test - TSST).
Results
Chronic stress levels and other baseline measures did not differ between treatment groups (all p > 0.05). Acute stress was successfully induced by the TSST and resulted in a hyper-responsivity of the HPAA in chronically stressed subjects. Compared to placebo, a supplementation with a daily dose of PAS 400 was effective in normalizing the ACTH (p = 0.010), salivary (p = 0.043) and serum cortisol responses (p = 0.035) to the TSST in chronically high but not in low stressed subjects (all p > 0.05). Compared to placebo, supplementation with PAS 200 did not result in any significant differences in these variables (all p > 0.05). There were no significant effects of supplementation with PAS on heart rate, pulse transit time, or psychological stress response (all p > 0.05).
Conclusion
In chronically stressed subjects, a supplementation with PAS 400 (MemreePlus™) can normalize the hyper-responsivity of the HPAA to an acute stressor.
Trial registration
Trial registration: DRKS-ID: DRKS00005125
Similar content being viewed by others
Background
Stress is an individual response to external and internal challenges ranging from behavioral to molecular adaptions, affecting the stress response network in the central nervous system and its crosstalk with peripheral organs via the endocrine and the autonomic nervous systems [1]. The hypothalamic-pituitary-adrenal axis (HPAA) plays a central role in such adaptation processes of the organism [2]. In response to acute stressors, the hypothalamus secretes corticotropin–releasing factor, which triggers the release of adrenocorticotropic hormone (ACTH) from the pituitary gland. ACTH then promotes the release of cortisol from the adrenal cortex. Cortisol affects numerous physiological functions, such as carbohydrate, lipid and protein metabolism, the immune system, and enhances the energy supply of the central nervous system. Chronic psychological stress has been shown to induce dysregulations of the HPAA, which are associated with impaired psychological and somatic well-being. Under such conditions, a biphasic response of the HPAA has been observed: prolonged psychological stress may first induce a hyper-activation of the HPAA, which may then be followed by a state of hypo-activation [3, 4]. While a hyper-activation of the HPAA has been shown to be associated with stress related disorders such as melancholic depression, anxiety disorders, and metabolic syndrome, a hypo-activation is rather linked to fatigue, pain, irritability and associated disorders such as fibromyalgia, arthritis, and chronic fatigue syndrome [5–7]. Thus, a hyper-activation of the HPAA seems to be associated with a hyper-reactivity to acute stress, while a reduced synthesis of cortisol may mainly account for a hypoactive state [6, 8–12].
Recent research showed that the intake of certain nutraceuticals reversed or normalized such dysregulations and improved stress symptomatology. For example, the intake of fertilized egg powder for the duration of four weeks normalized the psychological and physiological stress response in chronically stressed but otherwise healthy males [13]. Moreover, studies on different substances enriched with phospholipids have shown beneficial effects in chronically stressed subjects. It has been suggested that milk phospholipids may increase cortisol availability in subjects with high chronic stress [13–15].
Phosphatidic acid (PA) is one of the most important glycerophospholipids found in bio-membranes. PA has different roles in the cell: it is a precursor for other lipids such as phosphatidylserine (PS) or phosphatidylcholine via the conversion of PA to diacylglycerol [16]. Moreover, PA influences membrane curvature [17, 18] and acts as signaling lipid [19, 20]. Animal research suggests that PA may prevent or restore gastrointestinal disorders [21, 22] and may increase skeletal muscle mass [23]. PS plays a key role in neuronal cell structure and functioning and may improve memory, learning, mood and stress management [24–29]. PS has been shown to decrease ACTH and cortisol responses to acute physical and mental stress [30–35]. Additionally, the intake of PS has been associated with an improvement of psychiatric disorders, such as bipolar and major depressive disorders reviewed in [26, 36], as well as with the prevention of inflammatory neurodegenerative events [37].
Moreover, in combination with PS, PA has been shown to reduce cortisol levels and enhance well-being under acute social stress. In a previous study, we could show that the administration of PAS 400 per day over a period of 21 days resulted in both a significant decrease of the ACTH- and cortisol-response, and, in addition, a reduction of anxiety to the TSST [27]. The exact mechanisms on how PA and PS contribute to an adaptation of the stress response network are still unknown. Several stress-related disorders such as depression and mood disorders have been associated with fatty acid deficiencies [36, 38–41]. The intake of dietary phospholipids such as PA and PS may cause alterations and optimize the composition of cell membranes which in turn may modulate cellular function and activity of membrane [42].
The aim of the current study was to confirm the HPAA normalizing effects of a dose of PAS 400 found in a previous study. Here, we were especially interested whether there was a difference in effects between chronically stressed or non-stressed people. Chronic stress was assessed by using the screening scale for chronic stress of the Trier Inventory for Chronic Stress (TICS) [43]. Cut-off for low versus high chronic stress was defined as provided by a norm population of the TICS authors. In addition, we wanted to investigate whether these effects can also be observed with a lower dosage of PAS 200. In order to investigate PAS effects in a homogeneous study population, we included only male subjects and enlarged group size. We investigated the effects of two different doses of PAS (200 mg PA & 200 mg PS and 400 mg PA & 400 mg PS per day) over 42 days compared to placebo on psychological and physiological stress reactivity in subjects stratified by low and high chronic stress levels.
Results
Baseline measures
Groups did not differ with respect to age, height, body mass, BMI (body mass index), WHR (waist to hip ratio), systolic blood pressure, diastolic blood pressure and heart rate (Table 1). Furthermore, no baseline differences were observed for measures of chronic stress (Trier Inventory of Chronic Stress; TICS; all p > 0.05; data not shown) between all three treatment groups.
Compliance
Mean overall compliance was about 89.65% (SE = 1.30%) as assessed by a medication event monitoring system (MEMS®) and about 95.31% (SE = 0.62%) as assessed by counting of returned capsules. Analyses for covariance were performed to explore the potential influence of compliance on outcome measures. Neither MEMS® data nor counting the returned capsules showed an effect of compliance on ACTH, serum cortisol or saliva cortisol response to the TSST (all p > 0.05, data not shown).
Stress responsivity
Acute stress was induced by the Trier Social Stress Test (TSST). Induction of acute stress was confirmed by significant time effects in repeated measures analyses of variance for relevant variables. As expected, the TSST induced a pronounced increase in salivary cortisol, serum cortisol, and ACTH levels as well as heart rate and a decrease of pulse transit time (PTT) (see Table 2). The PTT is determined by the R wave of the heart beat to the pulse wave arrival at the finger. PTT is used as an index for arterial blood pressure [44–46] and as a biomarker for stress reactivity. Acute stress is associated with an increased blood pressure and a reduced PTT [47, 48].
Furthermore, subjects showed an increase of state anxiety (STAI: State-trait-anxiety inventory; VAS: visual analogue scales), perceived stress, insecurity (VAS), and negative mood (MDBF: Multidimensional mood state questionnaire) (Table 3).
Treatment effects
We compared differences between treatment groups (placebo, PAS 200 and PAS 400) and between subjects scoring either high or low on a chronic stress inventory (TICS). To compare general cortisol levels and cortisol increases, we calculated the area under the curve with respect to ground (AUCG) and its increase (AUCI) as described by Pruessner and colleagues [49]. The AUCG provides information on general cortisol levels whereas the AUCI on cortisol increase and reactivity.
Treatment group
Neither supplementation of PAS 200 nor of PAS 400 resulted in significant effects on saliva or serum cortisol levels on the overall study population (saliva cortisol: AUCG: χ2 2 = 0.44, p = 0.801; AUCI: χ2 2 = 2.01, p = 0.366; serum cortisol: AUCG: F2, 61 = 0.56, p = 0.576; AUCI: F2, 59 = 2.19, p = 0.121). For ACTH responses there was a significant main effect of treatment group (F2, 59 = 3.66, p = 0.032). Tukey post hoc tests revealed significant lower ACTH levels in the PAS 400 group compared to the PAS 200 group (p = 0.025).
Chronic stress level
Furthermore, no significant main effect of chronic stress was observed for ACTH increases (F1, 59 = 1.820, p = 0.182), AUCG saliva cortisol (Z = −0.28, p = 0.783), and AUCG serum cortisol (F1, 61 = 0.14, p = 0.721). However, high chronically stressed subjects (HCS) showed a tendency towards higher saliva cortisol AUCI (Z = −1.70, p = 0.089) and significantly higher serum cortisol AUCI (F1, 59 = 13.05, p = 0.001) than low chronically stressed subjects (LCS).
Subgroup analysis
To further explore the impact of chronic stress levels on treatment effects, we compared subgroups of HCS and LCS.
HCS of the PAS 400 group showed significantly lower increases of ACTH, serum cortisol, and saliva cortisol as compared to the placebo and the PAS 200 group. These effects could not be shown in LCS of the PAS 400 group. Also, no differences were observed for HCS and LCS of the PAS 200 group as compared to placebo. This suggests that a supplementation with PAS 400 normalized the enhanced ACTH- and cortisol-responses to an acute stressor in subjects reporting a chronic stress load (Figures 1, 2 and 3). The statistical information on effects of PAS on the endocrine stress response is provided in Table 4 for both HCS and LCS. Comparable to our previous study [27], no effects of supplementation were found for autonomic (heart rate; pulse transit time) and for psychological response measures (mood, insecurity, perceived stress) in both low and high chronically stressed subjects (data not shown). While we observed significant dampening effects on anxiety in the first study, this effect was only marginal in the present study (p < 0.07).
Discussion
In the present study, we investigated the effects of a 42-day dose-dependent intake of PAS on biological and psychological responses to the TSST in both HCS and LCS. As expected, the TSST reliably induced robust biological and psychological stress responses. Perceived stress responses were similar in all treatment groups. The placebo group showed a slight tendency for higher anxiety (VAS) during the TSST as compared to the PAS groups. Compliance assessed by MEMS® (about 90%) and by counting remaining capsules (about 95%) was rather high. Differences between both might have been caused by subjects taking their daily dose along to work with only one opening of the track cap which then results in a lower number of total openings. In that case the number of openings as assessed by MEMS® does not reflect the number of intake occasions.
A pronounced stress dampening effect of PAS on HPAA responses could only be observed for the 400 mg treatment group in HCS. These data confirm our previous findings, and are in line with other studies showing that acute and chronic administration of PS and PAS can normalize cortisol responses to acute physical and mental stress [27, 30–32, 34, 35].
As addressed in the introduction, chronic stress can first induce a hyper- and then a subsequent hypo-cortisolemic state. Phospholipid effects on the HPAA can best be described in terms of a normalization of stress responsivity. If chronic stress results in a hyper-responsivity of the HPAA, as observed in this and other studies [15, 27], phospholipids can be expected to buffer the HPAA response to stress. On the other hand, phospholipids may restore normal ACTH- and cortisol responses to stress in subjects with a reduced HPAA response [13, 28]. Notably, relaxation and stress management techniques show similar effects [50–55]. These observations support the assumption that both phospholipids and relaxation can normalize the HPAA-responsivity to stress.
As in our previous PAS study, effects of PAS 400 could only be observed for endocrine but not for autonomic stress responses. If PAS would primarily affect the stress response network in the brain, one would expect common secondary effects on psychological, endocrine, and autonomic measures. This is unlikely, since in both studies psychological effects were inconsistent and autonomic effects could not be observed. This points to the possibility, that PAS primarily targets peripheral components of the HPAA. Chronic stress has been shown to reduce cortisol binding globulin (CBG) [56–58]. CBG is a glycoprotein synthesized in the liver and secreted in the blood where it binds with a high affinity but low capacity to glucocorticoid hormones, such as cortisol in humans and corticosterone in laboratory rodents. In mammals, 95% of circulating glucocorticoids are bound to either CBG (80%) or albumin (15%), and only the 5% free fraction is able to enter the brain [59]. During stress, the concentration of glucocorticoids rises significantly, and the free fraction increases even more, once CBG becomes saturated. However, glucocorticoids unbound to CBG are cleared from the blood more quickly. It is assumed that CBG plays an important role in the fast actions of glucocorticoids on behavior by maintaining a blood glucocorticoid pool that will be able to access the brain for the fast effects of glucocorticoids [60, 61].
The observed pronounced increase of ACTH and cortisol in the TSST can possibly be explained by a drop of CBG in chronically stressed subjects. If so, one may hypothesize that PAS 400 first causes a normalization of CBG levels under such conditions, which then would result in a normalization of the activity and reactivity of the HPAA.
Conclusions
In chronically stressed subjects, PAS 400 can be expected to buffer a hyper-responsivity of the HPAA to acute stressors by normalizing cortisol responses. In contrast, PAS 400 does not affect endocrine stress response in LCS who do not have elevated cortisol levels. Although sample sizes for subgroup analyses of chronic stress were rather small, the results of this study are intriguing.
The CBG-hypothesis, derived from this study, can now be tested to elucidate a possible relevant mechanism of the action of PAS. If this hypothesis holds true, an indication of PAS for stress related disorders is evident, particularly for disorders which are considered to occur in consequence of stress-elevated cortisol levels, such as cardiovascular disease, central obesity, and the metabolic syndrome [5, 62]. In sum, we conclude that PAS 400 (MemreePlus™) is a safe and effective supplement for the reduction of both physical and mental stress. Based on the previous study, it can be speculated that women also benefit from MemreePlus™ supplementation. This needs to be confirmed in further studies.
Participants and methods
Participant selection
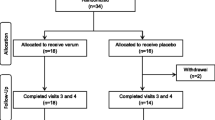
Healthy, non-smoking male volunteers between age 20 and 45 years were recruited by newspaper advertisement and flyer distribution in the area of Trier, Germany. Subjects’ health was assessed in a structured medical interview by a study physician and by assessing clinical blood chemistry. Individuals were not eligible if any of the following criteria applied: known allergies to ingredients of the test substance, addiction to nicotine, drugs or alcohol, recent changes in nutritional habits (e.g. start of a weight loss diet), any serious general illness within the last 12 months, any febrile illness (longer than 24 hours) within seven days prior to assessment, any intake of antibiotics during the four weeks prior to study inclusion, diabetes mellitus, heart disease, hypertension, kidney disease, significant respiratory disease, or epilepsy, immunologic or infectious disease (e.g. hepatitis, tuberculosis, HIV or AIDS, lupus, rheumatoid arthritis) which could place the subject at risk or interfere with the accuracy of the study results, current or past participation in a TSST study, employee of the sponsor or CRO, medications that are likely to affect treatment response, or any conditions that may affect the ability of the individual to complete the study or the interpretation of the study results.A total number of 124 subjects were screened for study inclusion and exclusion criteria of which 75 male subjects met the eligibility criteria. These subjects were randomly assigned and stratified by chronic stress level to one of the three treatment groups (25 subjects per group: placebo, PAS 200, PAS 400). After study inclusion, one subject of the placebo group and two subjects of the PAS 200 group were lost to follow up, leaving 24 subjects of the placebo group, 23 subjects of the PAS 200 group, and 25 subjects of the PAS 400 group for analysis (total sample size of 72 subjects) (see Figure 4). The study protocol was approved by the ethical review board of the Medical Association of Rhineland-Palatinate (Mainz, Germany), and written informed consent was obtained from all participants. The study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Conference on Harmonization/Good Clinical Practice (2008).
Study design and procedures
This was a randomized, double-blind, placebo-controlled, single-center study with three treatment arms (placebo, PAS 200, PAS 400 daily for six weeks). The study included four visits at the study site and had a total duration of 44 days for each subject.
Before subjects were invited to the study site, a first screening took place by telephone interview. Here, subjects received some general information on study procedures as well as a first check of inclusion and exclusion criteria. At visit one (V1), subjects received an extended written information on the aim of this study and all detailed study procedures. After clarifying possible questions, the signed written informed consent was obtained. The study physician then assessed vital signs and took a blood sample for determining safety parameters (i.e. blood profile for hemoglobin, hematocrit, platelet count, leukocytes, monocytes, neutrophils, eosinophils and basophils). Thereafter, subjects completed the Trier Inventory for Chronic Stress (TICS [43]). Chronic stress was determined using the Screening Scale for Chronic Stress, a subscale of the TICS. For classification of low and high chronic stress, the median value of 13 reported by Schulz and colleagues [43] was used as a cut off value. Thirty-five HCS and 35 LCS were randomly assigned to one of the three treatment groups. Randomization sequence was stratified with a 1:1 allocation using random blocks of variable sizes with the computer software SPSS 20.0 (IBM Corporation, Somer NY; USA).
At visit two (V2) those subjects meeting eligibility criteria received a random number. According to the randomization sequence, subjects received their daily supplementation of 4 capsules for the following 21 days. Capsules were packed in a container with a Medication Event Monitoring System (MEMS®) track cap (Aardex Ltd, Zug, Switzerland) to monitor container openings and indicating protocol compliance. MEMS® recorded time and date of each opening electronically. At visit three (V3), MEMS® track caps were read out and subjects received their supplementation dose for the following three weeks. At the final study visit (V4), a post supplementation blood sample for safety parameters was taken and the Trier Social Stress Test (TSST); [63] was performed to induce an acute psycho-social stressful situation. At V2, V3 and V4 subjects were carefully checked for adverse events and concomitant medication.
V4 had an overall duration of 180 minutes. Subjects received their last supplement dosage 90 minutes prior to the stress test. Pre- and post-stress psychometrics tests were performed. For HPAA activity measures saliva (free cortisol) and blood (total cortisol) samples were taken at 2 min. prior to the TSST as well as 1 min., 10 min., 20 min., 30 min. and 60 min. after the TSST. Blood samples, taken at 2 min. prior and 1 min. after the TSST, were also used for ACTH analyses.
Saliva samples were collected using Salivettes® (Sarstedt AG & Co., Nümbrecht, Germany). Blood samples were collected using a vein catheter and 2.7 ml EDTA monovettes® (Sarstedt AG & Co., Nümbrecht, Germany). Saliva samples were stored at −20°C, blood samples were centrifuged and stored at −20°C and −80°C until all participants completed the study.
Saliva cortisol levels were analyzed at the laboratory of the University of Trier, Germany using a competitive solid phase time-resolved fluorescence immunoassay with flouromeric end point detection (DELFIA). The intra- and interassay variability was below 10%.
ACTH was determined using a two-step immunoassay with streptavidin microparticles and electrochemiluminescence detection. Serum cortisol levels were analyzed using an electrochemiluminescence immunoassay (ECLIA). The assay is based on luminescence produced during photochemical reactions in solutions. Both assays used Elecsys® (Roche Diagnostics GmbH, Mannheim, Germany) and their intra- and interassay variability was below 10%.
For measures of the autonomic nervous system, heart rate and pulse transit time (PTT) were measured continuously from 20 min. prior to the TSST to 20 min. after the TSST using an electrocardiogram device (SOMNOscreen™ plus, SOMNOmedics GmbH, Randersacker, Germany). PTT is determined by measuring the electrocardiography and the pulse wave at a finger. Studies indicate that PTT is an appropriate parameter for stress measurement [48, 64].
Psychometric assessment included a mood questionnaire MDBF [65], before and after the TSST. State anxiety was assessed with the STAI-X1 [66] before and after the TSST. Furthermore, levels of perceived anxiety, stress and insecurity were measured before, during and after the TSST, as described by Hellhammer and Schubert [67].
Study treatment
The study supplement PAS (produced by Lipogen Ltd, Israel) and sold as “MemreePlus™” by Lonza Ltd, Switzerland was administered in capsules. Lipogen holds a patent (US 6,410,522 & EP 1201244) and the supplement has an US FDA GRAS (Generally Recognized As Safe) status. In this study, 4 capsules were administered per day: subjects were asked to take two capsules in the morning, one at noon and one in the evening with a glass of water after meals. Single capsules of the PAS 400 group (400 mg PA & 400 mg PS per day) consisted of 100 mg phosphatidylserine (PS) and lysophosphatidylserine, 100 mg phosphatidic acid (PA) and lysophosphatidic acid, 235 mg other phospholipids and glycerides, 5 mg silicone dioxide. Single capsules of the PAS 200 group (200 mg PA & 200 mg PS per day) consisted of 50 mg phosphatidylserine (PS) and lysophosphatidylserine, 50 mg phosphatidic acid (PA) and lysophosphatidic acid, 335 mg other phospholipids and glycerides, 5 mg silicone dioxide. Placebo capsules contained 435 maize starch and 5 mg silicone dioxide. All capsules were identical in size, shape and colour.
Stress test
The Trier Social Stress Test TSST; [63] was established in 1993 combining key elements of social stress with novelty, unpredictability, uncontrollability and ego-involvement in a standardized laboratory protocol for approximately15 minutes. Since then, it has been used in many clinical studies and in various areas of research. A meta-analysis of stress test protocols of Dickerson and Kemeny [68] showed that the protocol of the TSST is most effective in provoking HPAA responses. Furthermore, the TSST has been shown to provoke a strong increase in an individual’s perception of stress, insecurity and anxiety [67].
The TSST protocol compromises an introduction by the study manager: the subject is introduced to a panel and is asked to prepare for a job interview in a new company. After this introduction, the study manager leaves the TSST-room and the subject had a 3-min. period to prepare for the job interview. The 5-min. interview is followed by a 5 min. mental arithmetic task, stepwise subtracting 17 from 2023 as quickly and correctly as possible. The TSST is terminated by the study manager leading the participant back to every participant’s individual room. All TSSTs were conducted in the afternoon to control for diurnal cycles.
Statistical analysis
For statistical analyses, we used the software SPSS 21 (IBM Corporation, Somer NY, USA). For statistical inference, different statistical tests were performed. Only two-sided tests were used and data were interpreted on an α-level below 5% (α < .05). Baseline characteristics and demographics as well as data violating normality distribution were analyzed using Kruskal Wallis H test.
For cortisol the area under the curve with respect to ground (AUCG) and its increase (AUCI) were calculated using the formula described by Pruessner and colleagues [49]. The AUCG holds information about general cortisol levels, as it is an integrated value of all measurements. The AUCI holds information about the increase of cortisol levels, as it is an integrated value of all measurements adjusted for the cortisol level under resting conditions. For salivary cortisol AUCG and AUCI we conducted Kruskal-Wallis-H-tests for effects of treatment groups and Mann–Whitney-U-tests for treatment group to treatment group comparison and for effects of chronic stress level. For serum cortisol AUCG and AUCI we conducted univariate analyses of variance with treatment group and chronic stress level as fixed factors. Post hoc comparison was done with Tukey post hoc testing. For ACTH we conducted repeated measures analyses of variance with treatment group and chronic stress level as factors. Post hoc comparison of treatment groups was done with Tukey post hoc testing. Post hoc comparison of increase differences was done by repeated measures analyses of variance, with analysis-filters for relevant groups.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- AUCG:
-
Area under the curve with respect to ground
- AUCI:
-
Area under the curve with respect to its increase
- BMI:
-
Body mass index
- CBG:
-
Corticosteroid-binding globulin
- CRO:
-
Clinical research organization
- DELFIA:
-
Dissociation-enhanced lanthanide fluorescence immunoassay
- ECLIA:
-
Electrochemiluminescence immunoassay
- EDTA:
-
Ethylendiamintetraacetat
- HCS:
-
High chronically stressed subjects
- HPAA:
-
Hypothalamic-pituitary-adrenal axis
- LCS:
-
Low chronically stressed subjects
- MDBF:
-
Multidimensional mood state questionnaire (German: Mehrdimensionaler Befindlichkeitsfragebogen)
- MEMS:
-
Medication event monitoring system
- PA:
-
Phosphatidic acid
- PS:
-
Phosphatidylserine
- PAS:
-
Phosphatidic acid and phosphatidylserine
- PTT:
-
Pulse transit time
- SE:
-
Standard error
- SPSS:
-
Statistical package for the social sciences
- STAI:
-
State trait anxiety inventory
- TICS:
-
Trier inventory for chronic stress
- TSST:
-
Trier social stress test
- V:
-
Visit
- VAS:
-
Visual analogue scale
- WHR:
-
Waist to hip ratio.
References
Finan PH, Zautra AJ, Wershba R: The dynamics of emotions in adaptation to stress. The Handbook of Stress Science: Biology, Psychology, and Health. Edited by: Contrada RJ, Baum A. 2011, 209-220. New York: Springer Publishing Company,
Dallman MF, Hellhammer DH: Regulation of the Hypothalamic-Pituitary-Adrenal Axis, Chronic Stress, and Energy: The Role of Brain Networks. 2010, New York: Springer,
Hellhammer DH, Wade S: Endocrine correlates of stress vulnerability. Psychother Psychosom. 1993, 60: 8-17.
Nyuyki KD, Beiderbeck DI, Lukas M, Neumann ID, Reber SO: Chronic subordinate colony housing (CSC) as a model of chronic psychosocial stress in male rats. PLoS One. 2012, 7: e52371-
Chrousos GP: Stress and disorders of the stress system. Nat Rev Endocrinol. 2009, 5: 374-381.
Heim C, Ehlert U, Hellhammer DH: The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000, 25: 1-35.
Fries E, Hesse J, Hellhammer J, Hellhammer DH: A new view on hypocortisolism. Psychoneuroendocrinology. 2005, 30: 1010-1016.
Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schürmeyer TH, Kirschbaum C: The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999, 64: 1653-1660.
Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ: Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med. 2003, 65: 92-99.
Buske-Kirschbaum A, Von Auer K, Krieger S, Weis S, Rauh W, Hellhammer DH: Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease. Psychosom Med. 2003, 65: 806-810.
Kakiashvili T, Leszek J, Rutkowski K: The medical perspective on burnout. International journal of occupational medicine and environmental health. Int J Occup Med Environ Health. 2013, 26: 401-412.
Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID: Adrenal insufficiency and colonic inflammation after a novel chronic psycho-socoial stress paradigm in mice: implications and mechanisms. Endocrinology. 2007, 148: 670-682.
Schult J, Hero T, Hellhammer J: Effects of powdered fertilized eggs on the stress response. Clin Nutr. 2010, 29: 255-260.
Schubert M, Contreras C, Franz N, Hellhammer J: Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutr Res. 2011, 31: 413-420.
Hellhammer J, Hero T, Franz N, Contreras C, Schubert M: Omega-3 fatty acids administered in phosphatidylserine improved certain aspects of high chronic stress in men. Nutr Res. 2012, 32: 241-250.
Athenstaedt K, Daum G: Phosphatidic acid, a key intermediate in lipid metabolism. Eur J Biochem. 1999, 266: 1-16.
Kooijman EE, Chupin V, De Kruijff B, Burger KN: Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic. 2003, 4: 162-174.
McMahon HT, Gallop JL: Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005, 438: 590-596.
Foster DA: Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim Biophys Acta. 2009, 1791: 949-955.
Sergeant S, Waite KA, Heravi J, McPhail LC: Phosphatidic acid regulates tyrosine phosphorylating activity in human neutrophils: enhancement of Fgr activity. J Biol Chem. 2000, 276: 4737-4746.
Adachi M, Horiuchi G, Ikematsu N, Tanaka T, Terao J, Satouchi K, Tokumura A: Intragastrically administered lysophosphatidic acids protect against gastric ulcer in rats under water-immersion restraint stress. Dig Dis Sci. 2011, 56: 2252-2261.
Tanaka T, Horiuchi G, Matsuoka M, Hirano K, Tokumura A, Koike T, Satouchi K: Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves. Biosci Biotechnol Biochem. 2009, 73: 1293-1300.
Pasiakos SM: Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients. 2012, 4: 740-758.
Benton D, Donohoe RT, Sillance B, Nabb S: The influence of phosphatidylserine supplementation on mood and heart rate when faced with an acute stressor. Nutr Neurosci. 2001, 4: 169-178.
Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD: Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord. 2010, 29: 467-474.
Kidd PM: Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007, 12: 207-227.
Hellhammer J, Fries E, Buss C, Engert V, Tuch A, Rutenberg D, Hellhammer DH: Effects of soy lecithin phosphatidic acid and phosphatidylserine complex (PAS) on the endocrine and psychological responses to mental stress. Stress. 2004, 7: 119-126.
Hellhammer J, Waladkhani A-R, Hero T, Buss C: Effects of milk phospholipid on memory and psychological stress response. Nutr Res. 2010, 112: 1124-1137.
McDaniel MA, Maier SF, Einstein GO: “Brain-specific” nutrients: a memory cure?. Nutrition. 2003, 19: 957-975.
Monteleone P, Beinat L, Tanzillo C, Maj M, Kemali D: Effects of phosphatidylserine on the neuroendocrine response to physical stress in humans. Neuroendocrinology. 1990, 52: 243-248.
Simopoulos AP: Omega-3 fatty acids in inflammation and autoimmune disease. J Am Coll Nutr. 2002, 21: 495-505.
Starks MA, Starks SL, Kingsley M, Purpura M, Jäger R: The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J Int Soc Sports Nutr. 2008, 5: 1-6.
Monteleone P, Maj M, Beinat L, Natale M, Kemali D: Blunting by chronic phosphatidylserine administration of the stress-induced activation of the hypothamamo-pituitary-adrenal axis in healthy men. Eur J Clin Pharmacol. 1992, 41: 385-388.
Kingsley M: Effects of phosphatidylserine supplementation on execising humans. Sports Med. 2006, 36: 657-669.
Jäger R, Purpura M, Kingsley M: Phospholipids and sports performance. J Int Soc Sports Nutr. 2007, 4: 1-8.
Appleton KM, Rogers PJ, Ness AR: Is there a role for n-3 long-chain polyunsaturated fatty acids in the regulation of mood and behaviour? A review of the evidence to date from epidemiological studies, clinical studies and intervention trials. Nutr Res Rev. 2008, 21: 13-41.
Pandey NR, Sultan K, Twomey E, Sparks DL: Phospholipids block nuclear factor-kappa B and tau phosphorylation and inhibit amyloid-beta secretion in human neuroblastoma cells. Neuroscience. 2009, 164: 1744-1753.
Peet M, Stokes C: Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005, 65: 1051-1059.
Hibbeln JR: Fish consumption and major depression. Lancet. 1998, 351: 1213-
Hibbeln JR: Depression, Suicide and Deficiencies of Omega-3 Essential Fatty Acids in Modern Diets. Omega-3 Fatty Acids, the Brain and Retina. Edited by: Simopoulos AP, Bazan NG. 2009, 17-30. Basel: Karger, Volume 99,
Parker G, Gibson N, Brotchie H, Heruc G, Rees A-M, Hadzi-Pavlovic D: Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006, 163: 969-978.
Küllenberg D, Taylor LA, Schneider M, Massing U: Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11: 1-16.
Schulz P, Schlotz W, Becker P: Das Trierer Inventar zur Erfassung von chronischem Stress-Version 2 (TICS 2)[Trier Inventory for the Assessment of Chronic Stress]. 2004, Gottingen, Germany: Hogrefe,
De Boer D, Ring C, Carroll D: Time course and mechanisms of hemoconcentration in response to mental stress. Biol Psychol. 2006, 72: 318-324.
Geddes L, Voelz M, Babbs C, Bourland J, Tacker W: Pulse transit time as an indicator of arterial blood pressure. Psychophysiology. 1981, 18: 71-74.
Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C: Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med. 2006, 68: 231-237.
Fechir M, Schlereth T, Purat T, Kritzmann S, Geber C, Eberle T, Gamer M, Birklein F: Patterns of sympathetic responses induced by different stress tasks. Open Neurol J. 2008, 2: 25-
Hey S, Gharbi A, Von Haaren B, Walter K, Konig N, Loffler S: Continuous noninvasive pulse transit time measurement for psycho-physiological stress monitoring. eHealth, Telemedicine, and Social Medicine, 2009 eTELEMED’09 International Conference on. IEEE. 2009, 113-116.
Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH: Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003, 28: 916-931.
Pawlow LA, Jones GE: The impact of abbreviated progressive muscle relaxation on salivary cortisol. Biol Psychol. 2002, 60: 1-16.
Matousek RH, Dobkin PL, Pruessner JC: Cortisol as a marker for improvement in mindfulness-based stress reduction-. Complement Ther Clin Pract. 2010, 16: 13-19.
MacLean CR, Walton KG, Wenneberg SR, Levitsky DK, Mandarino JV, Waziri R, Hillis SL, Schneider RH: Effects of the Transcendental Meditation program on adaptive mechanisms: changes in hormone levels and responses to stress after 4 months of practice. Psychoneuroendocrinology. 1997, 22: 277-295.
Olff M, De Vries GJ, Guzelcan Y, Assies J, Gersons BP: Changes in cortisol and DHEA plasma levels afer psychotherapy for PTSD. Psychoneuroendocrinology. 2007, 32: 619-626.
Bonifazi M, Suman AL, Cambiaggi C, Felici A, Grasso G, Lodi L, Mencarelli M, Muscettola M: Changes in salivary cortisol and corticosteroid receptor-alpha mRNA expression following a 3-week multidisciplinary treatment program in patients with fibromyalgia. Psychoneuroendocrinology. 2006, 31: 1076-1086.
Mommersteeg PM, Keijsers GP, Heijnen CJ, Verbraak MJ, van Doornen LJ: Cortisol deviations in people with burnout before and after psychotherapy: a pilot study. Health Psychol. 2006, 25: 243-248.
Stefanski V: Social stress in laboratory rats: behavior, immune function, and tumor metastasis. Physiol Behav. 2001, 73: 385-391.
Spencer RL, Miller AH, Moday H, McEwen BS, Blanchard RJ, Blanchard DC, Sakai RR: Chronic social stress produces reductions in available splenic type II corticosteroid receptor binding and plasma corticosteroid binding globulin levels. Psychoneuroendocrinology. 1996, 21: 95-109.
Stefanski V: Social stress in laboratory rats: hormonal responses and immune cell distribution. Psychoneuroendocrinology. 2000, 25: 389-406.
Perogamvros I, Ray DW, Trainer PJ: Regulation of cortisol bioavailability–effects on hormone measurement and action. Nat Rev Endocrinol. 2012, 8: 717-727.
Chan WL, Carrell RW, Zhou A, Read RJ: How changes in affinity of corticosteroid-binding globulin modulate free cortisol concentration. J Clin Endocrinol Metab. 2013, 98: 3315-3322.
Moisan M-P: CBG: a cortisol reservoir rather than a transporter. Nat Rev Endocrinol. 2013, 9: 78-
Mavrides N, Nemeroff C: Treatment of depression in cardiovascular disease. Depress Anxiety. 2013, 30: 328-341.
Kirschbaum C, Pirke KM, Hellhammer DH: The ‘Trier Social Stress Test’-a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993, 28: 76-81.
Hey S, Sghir H: Psycho-physiological stress monitoring using mobile and continous pulse transit time measurement. eTELEMED 2011, The Third International Conference on eHealth, Telemedicine, and Social Medicine. 2011, 126-129.
Steyer R, Schwenkmezger P, Notz P, Eid M: MDBF: Der Mehrdimensionale Befindlichkeitsfragebogen. 1997, Göttingen, Germany: Hogrefe,
Laux L, Glanzmann P, Schaffner P, Spielberger CD: STAI: Das State-Trait-Angstinventar. 1981, Göttingen, Germany: Hogrefe,
Hellhammer J, Schubert M: The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology. 2012, 37: 119-124.
Dickerson SS, Kemeny ME: Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004, 130: 355-391.
Acknowledgements
We are grateful to all participants of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
This study was financed by Lipogen Ltd (DR) and Lonza Ltd (UF) and performed by Daacro GmbH & Co.KG (JH, DV, NF), a clinical research organization. DR, UF disclose a commercial interest. JH, DV, NF declare that they have no competing interest.
Authors’ contributions
JH, UF, DR designed the clinical study protocol. NF managed the study. DV performed the statistical analysis. JH, DV, NF drafted the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hellhammer, J., Vogt, D., Franz, N. et al. A soy-based phosphatidylserine/ phosphatidic acid complex (PAS) normalizes the stress reactivity of hypothalamus-pituitary-adrenal-axis in chronically stressed male subjects: a randomized, placebo-controlled study. Lipids Health Dis 13, 121 (2014). https://doi.org/10.1186/1476-511X-13-121
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-13-121