Abstract
Ascorbic acid (AA) may contribute to restoring hemostatic balance after mental stress (MS) in overweight/obese adults. We aimed to determine the effects of AA administration on hemostatic responses to MS in overweight/obese men. Fourteen overweight/obesity men (27 ± 7 years; BMI: 29.7 ± 2.6 kg m−2) performed the Stroop color-word stress task for 5 min after non-simultaneous infusion of placebo (PL, 0.9% NaCl) and AA (3 g). Blood was collected at baseline, during MS, and 60 min after MS to measure: activated partial thromboplastin time, prothrombin time, and fibrinogen concentration, by coagulometer; platelet-derived microvesicles (PMV, mv/μL), by flow cytometry; nitrite (μM), by chemiluminescence. In PL session, MS led to decreases in PTs (stress, p = 0.03; 60 min, p < 0.001), PT-INR (stress, p < 0.001; 60 min, p < 0.01), aPTTs (60 min, p = 0.03), aPTT ratio (60 min, p = 0.04) and fibrinogen (60 min, p = 0.04), while increased PT activity (60 min, p = 0.01) when compared to baseline. Furthermore, AA increased PTs (60 min, p < 0.001), PT-INR (60 min, p = 0.03) and decreased PT activity (60 min, p < 0.001) and fibrinogen (stress, p = 0.04) when compared to PL. Nitrite was increased in response to stress during AA session (p < 0.001 vs PL). There was no difference in PMV. Ascorbic acid prevented the impaired hemostatic profile and improved nitrite response to stress in the overweight and obese adults.
Similar content being viewed by others
Introduction
Psychological or mental stress induces a transient endothelial dysfunction and prothrombotic phenotype1,2. The hypercoagulability response to stress protects a healthy organism from bleeding during fight-or-flight conditions. However, chronic stress exposure enhances the risk of acute myocardial infarction and cardiovascular mortality by two times in adults at increased cardiometabolic risk, such as overweight or obese ones3,4.
Besides its role as vasodilator, nitric oxide (NO) may reduce the risk of atherothrombotic events5,6. During vascular damage, platelets are activated, and hemostatic responses are amplified to restore hemostatic balance with an occlusion plug formation7,8. Also, activated platelets can release platelet-derived microvesicles (PMV), biomarkers of endothelium damage9,10 with intercellular signaling functions11. NO regulates platelet function in both autocrine and paracrine manners, increasing cyclic GMP and reducing platelets adhesion and aggregation6.
Studies have demonstrated that sympathetic drive during stressful conditions activates the renin–angiotensin–aldosterone system12,13, increasing reactive oxygen species (ROS) by NADPH complex, ROS scavenger NO and increase peroxynitrite (ONOO-), which stimulates coagulation cascade and a hypercoagulability condition.
Considering that stress exposure and overweight/obesity are independent cardiovascular risk factors and are present in a large portion of the world's population; it is important to understand the underlying mechanisms that explain the increased atherothrombotic events when both conditions are combined14. Hence, our purpose was to test the hypothesis whether the administration of ascorbic acid (AA), a potent antioxidant15, may contribute to reducing prothrombotic factors, even under stressful situations in overweight/obese individuals.
Materials and methods
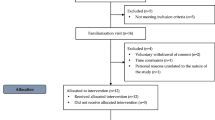
Fourteen overweight and obese men [Body mass index (BMI) between 25.0 and 34.9 kg m−216] were selected by print and electronic media based on the following inclusion criteria: absence of any chronic diseases (hypertension, diabetes, chronic kidney disease, heart failure, among others, except obesity), hemostatic impairments and musculoskeletal abnormalities; do not practice physical activity regularly (exercise lasting ≥ 30 min, 3 times a week); do not make regular use of medications and/or antioxidants. The present study’s protocol was approved by the Institution’s Ethics Committee (CAAE: 76594217.0.0000.5243) and followed the Declaration of Helsinki’s ethical standards. All participants were informed about the study’s protocol procedures and signed a consent form.
Three non-consecutive visits to the Laboratory of Exercise Sciences (LACE) at Fluminense Federal University were requested to carry out this study. On the first visit, anthropometric measures and body composition were analyzed by bioimpedance (RJL Systems Body Composition software—Clinton Township, MI, United States). Also, venous blood samples were collected after a 12-h fasting period, for the following biochemical tests: fasting glycemia, total cholesterol and triglycerides (dry chemistry; Vitros, Ortho-Clinical Diagnostics, New Jersey, United States).
The two following visits were randomized to experimental sessions with placebo (PL) or AA administration.
Experimental sessions
Visits to the laboratory took place in the morning, in an environment with controlled temperature (22–24 °C). All participants were instructed not to ingest alcohol, caffeine and food with high concentration of antioxidants, as well as not to perform physical exercises in the 48 h prior to the experimental sessions. Subjects ingested a light meal three hours before the experimental sessions. Prior to experimental protocol, electric bioimpedance was performed in order to measure fat mass of participants.
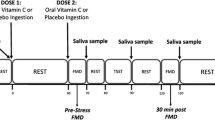
It is a randomized, single-blinded, placebo-controlled and crossover study. The experimental protocol was characterized by two sessions with either an intravenous administration of ascorbic acid (AA) or placebo (PL) in non-consecutive days, with an interval of at least seven days among them. All participants were subjected to both AA and PL administrations. In the beginning of each session, baseline hemodynamic and anthropometric variables were measured. Then, participants were instrumentalized at supine position, a catheter was placed in antecubital vein for infusion and blood sampling. During AA session, 3 g of AA diluted in 500 mL of saline solution was venous infused during 30 min; whereas in the PL session, only 500 mL saline venous infusion was used. Hemodynamic variables were measured throughout the experimental sessions. Venous blood samples were collected for hemostatic biomarkers and nitrite determination at baseline, during the last minute of the stress task, and 60 min after stress protocol. This study’s experimental design is represented in Fig. 1.
Hemodynamic variables
Hemodynamic variables were evaluated throughout the experimental protocol. Blood pressure was continuously checked, beat by beat, by means of a non-invasive system (Finometer, Finapres Measurement Systems, Arnhem, Netherlands). Heart rate was obtained by electrocardiographic recording. All signals were obtained at a frequency of 1000 Hz, integrated, and digitized through the PowerLab data acquisition system (ADInstruments, Sydney, Australia) and analyzed by LabChart 8 software (ADInstruments, Sydney, Australia).
Stress task
Stress was induced by a modified version of the Stroop color-word test17, in which the participants received three color information in a slideshow that changed every two seconds and were instructed to read quickly and aloud, the font color of the written word, successively. At the same time, the participants experienced a hearing conflict with voices that repeat, in a headset, colors different from those observed by the participant. Stress task was divided into two minutes of baseline measurements, five minutes of task induced by Stroop color-word test and three minutes of recovery, in which the participants rested in silence after the completion of the test. The level of perceptible/subjective stress was evaluated after the task with a scale from zero to four, in which: 0 = non-stressful, 1 = slightly stressful, 2 = stressful, 3 = very stressful and 4 = extremely stressful. Stress task protocol experimental design is represented in Fig. 2.
Hemostatic variables
Prothrombin time
Prothrombin time (PT) analysis was performed using HumaClot Jr. coagulometer (Human GmbH Max; Wiesbaden, Germany). The test was used to determine the activity of the extrinsic and common coagulation pathways by adding a source of factor (thromboplastin), which activates factor VII, and calcium. Plasma recalcification in the presence of thromboplastin generates activated factor Xa and, consequently, thrombin formation and, subsequently, an insoluble fibrin clot. In the present study, 200 μL of thromboplastin was added to the plasma collected in a citrate tube. The result was expressed in seconds, percentage activity in relation to normal (%act) and international normalized ratio (INR)18.
Activated partial thromboplastin time
Activated partial thromboplastin time (aPTT) analysis was also done by using HumaClot Jr. coagulometer (Human GmbH Max; Wiesbaden, Germany), and consists of determining the clotting time of the treated plasma, after the addition of reagents containing a plasma activator (ellagic acid) and phospholipids, which will act as platelet substitutes. The mixture is incubated for activation, and then recalcified with calcium chloride (CaCl2) triggering the intrinsic pathway coagulation mechanism. This step is timed until the formation of the clot18, and the results were expressed in seconds and ratio (test result divided by a control plasma’s result).
Fibrinogen concentration
The fibrinogen levels were measured through citrated plasma using the HumaClot Jr. coagulometer (Human GmbH Max; Wiesbaden, GER). Briefly, 50μL of human thrombin were added to 100 μL of plasma previously diluted with a buffered saline solution of Imidazole (0.05 mol/L), and incubated at 37 °C. The clotting time is inversely proportional to the concentration of fibrinogen in the sample.
Platelet-derived microvesicles
Venous blood samples were collected in sodium citrate tubes and centrifuged at 2000 g for 15 min at room temperature. This centrifugation procedure was repeated twice to obtain platelet-poor plasma. Then, plasma was collected and stored at − 80 °C. For analysis, 25 μL of plasma was incubated with 5 μL of CD41-APC monoclonal antibody for 30 min, at 4 °C, in the dark. Samples were diluted in 225 μL of phosphate-saline buffer (PBS) before flow cytometry analysis. TruCount tubes (BD Biosciences, Franklin Lakes, NJ, United States) were used for absolute microparticle count per μL of plasma, using the formula provided by manufacturer: (number of events acquired/absolute number of TruCount beads) X (total number of TruCount beads per test/total sample volume). All samples were analyzed using a flow cytometer (BD FACSVerse; BD Biosciences; Franklin Lakes, NJ, United States)19.
Nitrite concentration
Plasma nitric oxide levels were indirectly evaluated from nitrite concentration in the participants’ samples using NO Analyzer (NOA, Nitric Oxide Analyzer Sievers), model 280i (GE Analytical Instruments, Colorado, United States). The method is based on measurement of nitrite resulting from the oxidation of NO. Initially, a reducing agent (1% wt/vol of NaI or KI in acetic acid) is used for conversion of nitrite into NO. Later on, 20μL of deproteinized human serum in 5 mL of acidified tri-iodide solution is injected into the sealed glass chamber of the analyzer. NO gas reaction with ozone is detected by chemiluminescence. Concentrations were determined by interpolation of a nitrite standard curve (Sigma-Aldrich, Missouri, United States)20.
Statistical analysis
Sample size calculation was previously performed considering a statistical power of 0.8 and an alpha error of 0.05. A minimum sample size required for PT INR and nitrite responses to stress (main outcomes) were 11 and 10 individuals, respectively. The Shapiro–Wilk test was used to verify the normality in the distribution of the variables and homoscedasticity was performed by the Levene test. Data are represented as the mean ± standard error of the mean. Repeated measures analysis of variance was used to compare hemostatic variables before, during and 60 min after stress between PL and AA sessions. Aside from hemodynamic variables, values were expressed as fold changes from baseline. Student's t-test or its respective non-parametric test was used to compare the hemostatic variables' response to stress (Δ, 60 min minus baseline). A p-value less than or equal to 0.05 was considered statistically significant. GraphPad Prism software (version 8.0, San Diego, California, USA) was used for analysis.
Results
Fourteen participants aged 27 ± 2 years old and body mass index (BMI) averaged 29.7 ± 2.6 kg m−2 (fat mass: 31.8 ± 0.94%; glucose: 88 ± 2.34 mg/dL; total cholesterol: 178 ± 12.42 mg/dL; triglycerides: 119 ± 17.34 mg/dL) were considered in the sample. Hemodynamic variables at baseline, during and after stress task in PL and AA sessions are presented in Table 1. Stress task increased heart rate, systolic, diastolic, and mean blood pressures similarly in all sessions (p < 0.05 vs baseline) and returned to baseline levels during recovery (p < 0.05 vs stress). The level of perceived stress was assessed in both visits by showing the subject a table containing the aforementioned perceived stress scale. The average level of perceived stress for both sessions was 2 (stressful).
Hemostatic measurements
Stress induced a reduction in PT seconds (baseline, 1.00 ± 0.02 s; stress, 0.95 ± 0.01 s, p = 0.03; 60 min after stress, 0.92 ± 0.02 s, p < 0.001) and INR (baseline, 1.00 ± 0.02; stress, 0.97 ± 0.02, p < 0.001; 60 min after stress,0.90 ± 0.02, p < 0.01) as well as an increase of PT activity (baseline, 1.00 ± 0.03% vs 60 min after stress, 1.14 ± 0.04%, p < 0.001) at PL session. During AA session, PT seconds was higher during stress and 60 min after stress in comparison to placebo session (PL stress, 0.95 ± 0.01 s vs AA stress, 1.00 ± 0.02 s, p = 0.04; PL 60 min after stress, 0.92 ± 0.02 s vs AA 60 min after stress, 1.03 ± 0.03 s, p < 0.001). PT INR increased during AA session at 60 min after stress (stress, 0.97 ± 0.02 vs 60 min after stress, 1.03 ± 0.03, p = 0.03), which was higher than the same moment in the PL session (PL, 0.90 ± 0.02 vs 60 min after stress, 1.03 ± 0.03, p < 0.01). During AA session, PT activity was reduced in comparison to the placebo session at 60 min after stress (PL, 1.14 ± 0.04% vs AA, 0.96 ± 0.03%, p < 0.0001). In response to stress, both PT seconds (PL, Δ − 0.69 ± 2.02 s vs AA, Δ 0.66 ± 1.18 s, p = 0.003) and INR were increased during AA session (PL, Δ − 0.10 ± 0.14 vs AA, Δ 0.06 ± 0.10, p < 0.001) while PT activity presented a significant reduction (PL, Δ7.00 ± 13.62% vs AA, Δ − 4.81 ± 7.53%, p = 0.02) (Fig. 3).
Prothrombin time (PT) in seconds, percentual activity and international normalized ratio (A, C, E) and their respective delta responses to stress (B, D, F) after placebo (PL) and ascorbic acid (AA) administration in overweight/obese men. Δ represents 60-min minus baseline. *p < 0.05 versus baseline; †p < 0.05 versus placebo; ‡p < 0.05 versus stress; §p < 0.05 versus placebo.
There was a significant reduction in aPTT seconds (baseline, 1.00 ± 0.05 s vs 60 min after stress, 0.90 ± 0.05 s; p = 0.04) and a PTT ratio (baseline, 1.00 ± 0.05 s vs 60 min after stress, 0.87 ± 0.03 s, p = 0.03; stress, 0.99 ± 0.0 vs 60 min after stress, 0.87 ± 0.03 s, p = 0.04) in PL session. However, no differences were observed at AA session for neither aPTT seconds (baseline, 1.00 ± 0.07 s; stress, 1.01 ± 0.06 s; 60 min after stress, 0.90 ± 0.04 s) nor ratio (baseline, 0.93 ± 0.04 s; stress, 1.02 ± 0.05 s; 60 min after stress, 1.00 ± 0.06 s). Also, there were no differences in stress response for aPTT seconds (PL, Δ − 2.00 ± 11.36 s vs AA, Δ − 1.88 ± 4.42 s) or ratio (PL, Δ − 0.042 ± 0.18 s vs AA, Δ − 0.06 ± 0.12 s) (Fig. 4).
There was a significant reduction in fibrinogen concentration (baseline, 1.00 ± 0.04 mg/dL vs 60 min after stress, 0.84 ± 0.08 mg/dL, p = 0.04; stress, 1.07 ± 0.09 mg/dL vs 60 min after stress, 0.84 ± 0.08 mg/dL, p < 0.01) in the placebo session. However, no differences were observed at AA session (baseline, 1.00 ± 0.10 mg/dL; stress, 0.97 ± 0.08 mg/dL; 60 min after stress, 0.88 ± 0.04 mg/dL). During stress, fibrinogen presented significantly lower levels in the AA session when compared to placebo (PL, 1.07 ± 0.09 mg/dL vs AA, 0.97 ± 0.08 mg/dL; p = 0.04). Also, there were no differences in stress response (PL, Δ − 16.91 ± 40.46 mg/dL vs AA, Δ − 28.44 ± 97.17 mg/dL) (Fig. 5).
No differences were observed in PMV response to stress between sessions [PL (baseline, 1.00 ± 0.17 MV/µL; stress, 0.95 ± 0.16 MV/µL; 60 min after stress, 0.93 ± 0.16 MV/µL); AA (baseline, 1.00 ± 0.16; stress, 0.89 ± 0.15 MV/µL; 60 min after stress, 0.95 ± 0.13 MV/µL)] or in response to stress (PL, Δ 4.91 ± 237.97 MV/µL vs AA, Δ − 54.72 ± 298.68 MV/µL; p < 0.01) (Fig. 6).
Nitrite measurement
Even though there were no differences in nitrite bioavailability between sessions [PL (baseline, 1.00 ± 0.08 µM; stress, 0.96 ± 0.08 µM; 60 min after stress, 0.95 ± 0.07 µM); AA (baseline, 1.00 ± 0.07 µM; stress, 1.03 ± 0.07 µM; 60 min after stress, 0.99 ± 0.11 µM)], the nitrite response to stress was higher in AA session when compared to PL (PL, Δ − 0.02 ± 0.08 µM vs AA, Δ 0.09 ± 0.08 µM; p = 0.003) (Fig. 7).
Discussion
Novel findings from the present study are: (1) acute stress induces a procoagulant state, through reducing prothrombin time; (2) ascorbic acid prevented the procoagulant response to stress, through increasing the prothrombin time and reducing the fibrinogen concentration, especially 60 min after MS; (3) ascorbic acid increased the nitrite concentration in response to stress in overweight/obese men.
Several studies have been using stress tasks to better understand the cardiovascular pathophysiological processes21. A greater hemodynamic responsivity to acute stress, even when it is developed in a controlled environment (i.e., research laboratory), represents an increased risk of cardiovascular events22. Then, the laboratory stress tasks may help us to determine underlying mechanisms involved in cardiovascular responses or to identify preventive strategies to manage it. The modified Stroop color word test is a well-established protocol that induces psychological hemodynamic and vascular responses20,23,24,25 through increasing sympathetic drive26 and activating the renin angiotensin II system27. The present study reinforced those findings, demonstrating that stress tasks were able to similarly increase blood pressure and heart rate of overweight / obese men, even in the presence of ascorbic acid.
Previous studies have demonstrated an association between psychological stress and hypercoagulability28. In the present study, the stress task was able to reduce PT and aPTT in overweight and obese men, immediately after stress and 60 min after MS, providing evidence of a possible long-lasting effect of stress on hemostasis. These findings suggest a possible underlying mechanism for increased risk for acute myocardial infarction and sudden cardiac death days after stressful events29,30,31,32. Increased plasma levels of catecholamines and glucocorticoids seem to induce platelet activation and a higher concentration of procoagulant factors, such as tissue factor (TF), von Willebrand factor, factors VII, VIII and XII besides the fibrinogen33,34. Vascular injury, remodeling, or endothelial dysfunction triggers TF exposure in the subendothelial cell membrane, stimulating extrinsic and intrinsic coagulation pathways35.
Although stress exerts faster effects on the extrinsic coagulation pathway, it also seems to reduce fibrinogen concentration one hour after the task. Hepatic fibrinogen plays an important role in hemostasis as it is the thrombin' substrate to form fibrin polymers and blood clot36. High plasmatic fibrinogen levels were already demonstrated in several inflammatory and oxidant pathological conditions36. Also, the norepinephrine administration, usually present in acute stressful conditions, increased fibrinogen levels, d-dimer and FVIII clotting activity37. Then, its reduction after a stress task could mean an increased thrombin activity or even distinct plasmatic fibrinogen responses to physiological (i.e., Stroop color word task) or pharmacological (i.e., norepinephrine infusion) protocols for inducing stress.
Strategies to reduce reactive oxygen or nitrogen species (ROS-RNS) could be good alternatives to diminish the impact of daily life stressful situations on cardiovascular function, especially in populations at increased cardiometabolic risk. Ascorbic acid is a water-soluble vitamin known to neutralize ROS-RNS, and its venous administration was able to prolong the PT and reduce fibrinogen response to stress task in overweight and obese men. Previous studies published by our group38,39 and others40 reinforce that finding, demonstrating that ascorbic acid prevented redox imbalance and reduced vascular remodeling in overweight and obese men38,39. Also, it was already demonstrated that fibrinogen is a vulnerable target for oxidant attack41. Increased ROS-RNS seem to alter its primary and three-dimensional protein structure or posttranslational oxidative modifications that result in partial or complete loss of fibrinogen function and/or alternative assembly of fibrin42. It is believed that increased nitrite concentration, a representative of NO bioavailability, induced by ascorbic acid administration would restore the hemostatic function.
Indeed, the present study demonstrated that ascorbic acid increased the nitrite concentration despite the stress. This result was observed when the magnitude of response was analyzed, suggesting that the effects of ascorbic acid on nitrite concentration were not immediate to MS but rather delayed, possibly by the pharmacokinetics of ascorbic acid infusion43,44,45. Reductions in NO bioavailability induce hypercoagulability state in overweight or obese adults1. NO acts by increasing cyclic GMP levels and reducing intracellular calcium in platelets, avoiding platelet aggregation and clot formation46. Enhanced ROS production reduces intraplatelet NO bioavailability and its receptor activity (sGC)47, contributing to platelet hyperaggregability and impaired vascular function48,49.
No differences were observed in PMV concentration among moments and sessions. Results considering PMVs measurements are controversial. One hand, studies have demonstrated a positive correlation between PMVs release and metabolic, inflammatory, and pro-thrombotic parameters in obese subjects50. On the other hand, some studies demonstrated no significant difference in platelet-activation biomarkers’ expression between obese and non-obese individuals51. It is likely that the use of distinct superficial biomarkers for PMV identification might explain the unequal results among studies with overweight/obese adults.
Limitations
Some limitations should be taken into consideration for our results’ interpretation. The present study did not measure redox homeostasis parameters nor catecholamines levels. However, studies from our group and others38,40 have shown the effects of ascorbic acid on oxidative stress after exposure to a mental stress task. Considering those aspects already shown, the present study focuses on studying whether the ascorbic acid, a well-known antioxidant, is able to blunt hemostatic responses to stress. Also, present results cannot be extrapolated to other populations, such as women. It is recognized that the risk of atherothrombotic diseases is higher in middle-aged men than women52. However, further studies are important to better investigate and understand the hemostatic responses to stress in age-matched women. Moreover, all the participants were young healthy overweight/obese adults, whereby the magnitude of obesity-mediated disorders is, presumably, much less aggravated than in older subjects.
Conclusion
Our results suggest that stress exposure induces a pro-thrombotic response in overweight and obese individuals once it led to reductions in prothrombin and activated thromboplastin times. Also, ascorbic acid seems to have an anticoagulant role, increasing the prothrombin time and the nitrite concentration, whereas reducing fibrinogen concentration in response to stress.
Preventive and therapeutic strategies to reduce the impact of stress on vascular health are necessary to minimize the risk of cardiovascular morbimortality, especially in adults with overweight/obesity.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bentur, O. S., Sarig, G., Brenner, B. & Jacob, G. Effects of acute stress on thrombosis. Semin. Thromb. Hemost. 44, 662–668. https://doi.org/10.1055/s-0038-1660853 (2018).
Ghiadoni, L. et al. Mental stress induces transient endothelial dysfunction in humans. Circulation 102, 2473–2478. https://doi.org/10.1161/01.cir.102.20.2473 (2000).
Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364, 937–952. https://doi.org/10.1016/S0140-6736(04)17018-9 (2004).
Braekkan, S. K., van der Graaf, Y., Visseren, F. L. & Algra, A. Obesity and risk of bleeding: The SMART study. J. Thromb. Haemost. 14, 65–72. https://doi.org/10.1111/jth.13184 (2016).
Theofilis, P. et al. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines https://doi.org/10.3390/biomedicines9070781 (2021).
Forstermann, U., Xia, N. & Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120, 713–735. https://doi.org/10.1161/CIRCRESAHA.116.309326 (2017).
Hoffman, M. A cell-based model of coagulation and the role of factor VIIa. Blood Rev. 17(Suppl 1), S1-5. https://doi.org/10.1016/s0268-960x(03)90000-2 (2003).
Al-Koussa, H., AlZaim, I. & El-Sabban, M. E. Pathophysiology of coagulation and emerging roles for extracellular vesicles in coagulation cascades and disorders. J. Clin. Med. https://doi.org/10.3390/jcm11164932 (2022).
Nomura, S., Inami, N., Shouzu, A., Urase, F. & Maeda, Y. Correlation and association between plasma platelet-, monocyte- and endothelial cell-derived microparticles in hypertensive patients with type 2 diabetes mellitus. Platelets 20, 406–414. https://doi.org/10.1080/09537100903114545 (2009).
Koganti, S. et al. Persistent circulating platelet and endothelial derived microparticle signature may explain on-going pro-thrombogenicity after acute coronary syndrome. Thromb. Res. 206, 60–65. https://doi.org/10.1016/j.thromres.2021.07.018 (2021).
Rajagopalan, S. et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 97, 1916–1923. https://doi.org/10.1172/JCI118623 (1996).
Alvarez, G. E., Beske, S. D., Ballard, T. P. & Davy, K. P. Sympathetic neural activation in visceral obesity. Circulation 106, 2533–2536. https://doi.org/10.1161/01.cir.0000041244.79165.25 (2002).
Sarkozy, M. et al. Mechanisms and modulation of oxidative/nitrative stress in type 4 cardio-renal syndrome and renal sarcopenia. Front. Physiol. 9, 1648. https://doi.org/10.3389/fphys.2018.01648 (2018).
Fowler, A. A. 3rd. et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 12, 32. https://doi.org/10.1186/1479-5876-12-32 (2014).
Okamoto, K. et al. Antioxidant effect of ascorbic acid against cisplatin-induced nephrotoxicity and P-glycoprotein expression in rats. Eur. J. Pharmacol. 909, 174395. https://doi.org/10.1016/j.ejphar.2021.174395 (2021).
Amani-Beni, R., Darouei, B., Zefreh, H., Sheikhbahaei, E. & Sadeghi, M. Effect of obesity duration and BMI trajectories on cardiovascular disease: A narrative review. Cardiol. Ther. 12, 307–326. https://doi.org/10.1007/s40119-023-00317-6 (2023).
Scarpina, F. & Tagini, S. The stroop color and word test. Front. Psychol. 8, 557. https://doi.org/10.3389/fpsyg.2017.00557 (2017).
Clauss, A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 17, 237–246. https://doi.org/10.1159/000205234 (1957).
Storch, A. S. et al. Oscillatory shear stress induces hemostatic imbalance in healthy men. Thromb. Res. 170, 119–125. https://doi.org/10.1016/j.thromres.2018.08.019 (2018).
Rocha, H. N. M. et al. AT1R blocker prevents mental stress induced retrograde blood flow in overweight/obese men. Physiol. Rep. 11, e15566. https://doi.org/10.14814/phy2.15566 (2023).
Steptoe, A. & Vogele, C. Methodology of mental stress testing in cardiovascular research. Circulation 83, II14-24 (1991).
Chida, Y. & Steptoe, A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension 55, 1026–1032. https://doi.org/10.1161/HYPERTENSIONAHA.109.146621 (2010).
Sales, A. R. et al. Aerobic exercise acutely prevents the endothelial dysfunction induced by mental stress among subjects with metabolic syndrome: The role of shear rate. Am. J. Physiol. Heart Circ. Physiol. 306, H963-971. https://doi.org/10.1152/ajpheart.00811.2013 (2014).
Rocha, N. G. et al. Aerobic exercise modulation of mental stress-induced responses in cultured endothelial progenitor cells from healthy and metabolic syndrome subjects. Life Sci. 123, 93–99. https://doi.org/10.1016/j.lfs.2014.12.026 (2015).
Mason, S. A. et al. Association between carotid atherosclerosis and brain activation patterns during the Stroop task in older adults: An fNIRS investigation. Neuroimage 257, 119302. https://doi.org/10.1016/j.neuroimage.2022.119302 (2022).
Formolo, N. P. S. et al. Heart rate reactivity to acute mental stress is associated with adiposity, carotid distensibility, sleep efficiency, and autonomic modulation in young men. Physiol. Behav. 254, 113908. https://doi.org/10.1016/j.physbeh.2022.113908 (2022).
Fyhrquist, F. & Saijonmaa, O. Renin-angiotensin system revisited. J. Intern. Med. 264, 224–236. https://doi.org/10.1111/j.1365-2796.2008.01981.x (2008).
Yin, H. et al. High perceived stress may shorten activated partial thromboplastin time and lead to worse clinical outcomes in patients with coronary heart disease. Front. Cardiovasc. Med. 8, 769857. https://doi.org/10.3389/fcvm.2021.769857 (2021).
Levine, G. N. Psychological stress and heart disease: Fact or folklore?. Am. J. Med. 135, 688–696. https://doi.org/10.1016/j.amjmed.2022.01.053 (2022).
Meisel, S. R. et al. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet 338, 660–661. https://doi.org/10.1016/0140-6736(91)91234-l (1991).
Moller, J., Theorell, T., de Faire, U., Ahlbom, A. & Hallqvist, J. Work related stressful life events and the risk of myocardial infarction. Case-control and case-crossover analyses within the Stockholm heart epidemiology programme (SHEEP). J. Epidemiol. Commun. Health 59, 23–30. https://doi.org/10.1136/jech.2003.019349 (2005).
Trichopoulos, D., Katsouyanni, K., Zavitsanos, X., Tzonou, A. & Dalla-Vorgia, P. Psychological stress and fatal heart attack: The Athens (1981) earthquake natural experiment. Lancet 1, 441–444. https://doi.org/10.1016/s0140-6736(83)91439-3 (1983).
Lazzarino, A. I. et al. The association between fibrinogen reactivity to mental stress and high-sensitivity cardiac troponin T in healthy adults. Psychoneuroendocrinology 59, 37–48. https://doi.org/10.1016/j.psyneuen.2015.05.002 (2015).
von Kanel, R. Acute mental stress and hemostasis: When physiology becomes vascular harm. Thromb. Res. 135(Suppl 1), S52-55. https://doi.org/10.1016/S0049-3848(15)50444-1 (2015).
Maly, M. A. et al. The role of tissue factor in thrombosis and hemostasis. Physiol. Res. 56, 685–695. https://doi.org/10.33549/physiolres.931054 (2007).
Kaufmanova, J. et al. Fibrin clot formation under oxidative stress conditions. Antioxidants (Basel) https://doi.org/10.3390/antiox10060923 (2021).
von Kanel, R. et al. Prothrombotic response to norepinephrine infusion, mimicking norepinephrine stress-reactivity effects, is partly mediated by alpha-adrenergic mechanisms. Psychoneuroendocrinology 105, 44–50. https://doi.org/10.1016/j.psyneuen.2018.09.018 (2019).
Batista, G. M. S. et al. Ascorbic acid inhibits vascular remodeling induced by mental stress in overweight/obese men. Life Sci. 250, 117554. https://doi.org/10.1016/j.lfs.2020.117554 (2020).
Rocha, H. N. M. et al. Mental stress induces endothelial dysfunction by AT1R-mediated redox imbalance in overweight/obese men. Braz. J. Med. Biol. Res. 56, e12547. https://doi.org/10.1590/1414-431X2023e12547 (2023).
Van Guilder, G. P., Hoetzer, G. L., Greiner, J. J., Stauffer, B. L. & DeSouza, C. A. Acute and chronic effects of vitamin C on endothelial fibrinolytic function in overweight and obese adult humans. J. Physiol. 586, 3525–3535. https://doi.org/10.1113/jphysiol.2008.151555 (2008).
Nowak, P., Zbikowska, H. M., Ponczek, M., Kolodziejczyk, J. & Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: Functional consequences. Thromb. Res. 121, 163–174. https://doi.org/10.1016/j.thromres.2007.03.017 (2007).
Bychkova, A. V. et al. Oxidation-induced modification of the fibrinogen polypeptide chains. Dokl. Biochem. Biophys. 474, 173–177. https://doi.org/10.1134/S1607672917030115 (2017).
Nielsen, T. K. et al. Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: A pharmacokinetic evaluation. Basic Clin. Pharmacol. Toxicol. 116, 343–348. https://doi.org/10.1111/bcpt.12323 (2015).
Ou, J. et al. The safety and pharmacokinetics of high dose intravenous ascorbic acid synergy with modulated electrohyperthermia in Chinese patients with stage III-IV non-small cell lung cancer. Eur. J. Pharm. Sci. 109, 412–418. https://doi.org/10.1016/j.ejps.2017.08.011 (2017).
Stephenson, C. M., Levin, R. D., Spector, T. & Lis, C. G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 72, 139–146. https://doi.org/10.1007/s00280-013-2179-9 (2013).
Whelan, C., Burnley-Hall, N., Morris, K., Rees, D. A. & James, P. E. The procoagulant effects of extracellular vesicles derived from hypoxic endothelial cells can be selectively inhibited by inorganic nitrite. Nitric Oxide 122–123, 6–18. https://doi.org/10.1016/j.niox.2022.02.002 (2022).
Friebe, A. & Koesling, D. The function of NO-sensitive guanylyl cyclase: What we can learn from genetic mouse models. Nitric Oxide 21, 149–156. https://doi.org/10.1016/j.niox.2009.07.004 (2009).
DeSouza, C. A. et al. Basal endothelial nitric oxide release is preserved in overweight and obese adults. Obes. Res. 13, 1303–1306. https://doi.org/10.1038/oby.2005.157 (2005).
Van Guilder, G. P., Stauffer, B. L., Greiner, J. J. & Desouza, C. A. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am. J. Physiol. Heart Circ. Physiol. 294, H1685-1692. https://doi.org/10.1152/ajpheart.01281.2007 (2008).
Csongradi, E. et al. Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb. Haemost. 106, 683–692. https://doi.org/10.1160/TH11-01-0030 (2011).
Samocha-Bonet, D. et al. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm. 2008, 834153. https://doi.org/10.1155/2008/834153 (2008).
Yerly, A. et al. Sex-specific and hormone-related differences in vascular remodelling in atherosclerosis. Eur. J. Clin. Invest. 53, e13885. https://doi.org/10.1111/eci.13885 (2023).
Acknowledgements
The authors of the present study thank and appreciate all the time and efforts put into making this paper’s idealization, writing and publication possible. We also wish to thank the staff and students that are part of the Laboratory of Exercise Sciences at Fluminense Federal University for the partnership provided as well as funding from the Foundation for Research Support of Rio de Janeiro (FAPERJ), the Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq).
Funding
The present study was supported by grants from the Foundation for Research Support of Rio de Janeiro (FAPERJ; E-26/202.770/2019 and E-26/211.148/2019) and the Coordination for the Improvement of Higher Education Personnel (CAPES, scholarship).
Author information
Authors and Affiliations
Contributions
Conception and design for the work were conducted by H.N.M.R., L.L.V., A.C.L.N. e N.G. R. Material preparation, data collection and analysis were performed by H.N.M.R., L.L.V., G.M.S.B., A.S.S., V.P.G., G.F.T., J.M., AC.L.N., N.G.R. The first draft of the manuscript was written by H.N.M.R. and L.L.V., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rocha, H.N.M., Velasco, L.L., Batista, G.M.S. et al. Ascorbic acid prevents stress-induced hypercoagulability in overweight and obese individuals. Sci Rep 14, 3122 (2024). https://doi.org/10.1038/s41598-024-53794-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53794-7
- Springer Nature Limited