Abstract
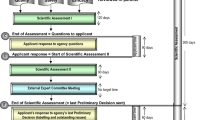
The aim of the study was to evaluate the Gulf Cooperation Council (GCC) centralized regulatory review process. Regulatory review times-including submission and application dates for new active substances (NASs) and existing active substances (EASs) using a standardized template for the period of 2006 to 2010—were collected directly from the GCC office located in Riyadh, Saudi Arabia. A total of 413 products (96 NASs and 317 EASs) were approved during the period, with an overall significant increase in the EASs (P <.001). The median approval times increased from 107 calendar days in 2006 to 265 in 2010 (P <.001). The lowest approval time was for EASs submitted by the Gulf companies (134 days) and the longest for NASs submitted by international companies (346 days) (P <.001). These data were also analyzed according to therapeutic classes and dosage forms. The results also showed that the lowest number (n = 16) approved during the period was in 2010, and this was due to a major regulatory change implementing the International Conference on Harmonisation product stability guideline for the region. The findings indicate that the delay and the wide range in approval times could be reduced by utilizing a standard assessment template for product review and the implementation of a clock stop system for company responses to questions from the GCC central registration committee. Furthermore, using information technology tools would speed up the registration process rather than the manual exchange of product registration files between the executive office and the member states.
Similar content being viewed by others
References
Al-Essa R. An Evaluation of the Regulatory Review Processes, the Quality of Decision-Making and Strategic Planning in the Gulf Cooperation Council (GCC) States [PhD thesis]. Cardiff, UK: Cardiff University; 2011.
Anderson C. An Evaluation of Harmonization in the Regulatory Environment and Its Impact on Patients’ Access to New Medicines [PhD thesis]. Cardiff, UK: Cardiff University; 2004.
Rawson NSB. Time required for approval of new drugs in Canada, Australia, Sweden, the United Kingdom and the United States in 1996–1998. CMAJ. 2000;162:501–504.
Al-Essa R, Salek S, Walker S. An appraisal of good regulatory review practices in the Gulf Cooperation Council States. Drug Information J. 2012;46:57–64.
Al-Essa R, Salek S, Walker S. Regulatory review process in the Gulf Cooperation Council States: similarities and differences. Drug Information J. 2012;46:65–72.
ICH Harmonised Tripartite Guideline. The Common Technical Document (Module 2 and 3). ICH Publication; 2000.
Centre for Innovation in Regulatory Science. New Drug Approvals in ICH Countries 2003–2012: Focus on 2012. London, UK: Centre for Innovation in Regulatory Science; 2013. R&D briefing 52.
Mallia-Milanes A. An Evaluation of Quality Measures Applied to the Regulatory Review Process of Major Regulatory Authorities [PhD thesis]. Cardiff, UK: Cardiff University; 2006.
Hill S, Johnson K. Emerging Challenges and Opportunities in Drug Registration and Regulation in Developing Countries. London, England: DFID Health Systems Resources Centre; 2004.
Lambert G. Delays in the Registration of Pharmaceuticals in the Middle East: How Can These Be Resolved? London, England: CMR International; 2000.
Centre for Innovation in Regulatory Science. Evolving the Regulatory Review Process: What Are the Features That Enable a Transparent, Timely, Predictable and Good-Quality Review? Workshop Report, 6–7 December 2011, Kuala Lumpur, Malaysia. London, UK: Centre for Innovation in Regulatory Science; 2012.
Yauba Saidu Y, De Angelis D, Silvia Aiolli S, Stefano G, Georges AM. Product registration in developing countries: a proposal for an integrated regional licensing system among countries in regional economic blocs [published online March 7, 2013]. Therapeutic Innovation & Regulatory Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Rubaie, M.H., Walker, S.R. & Salek, S.S. Evaluation of the Gulf Cooperation Council Centralized Procedure: The Way Forward. Ther Innov Regul Sci 48, 709–716 (2014). https://doi.org/10.1177/2168479014529572
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479014529572