Abstract
It is evident that a tumor is a dangerous lump of tissue developed through the uncontrollable division of cells, replacing healthy tissue with abnormal tissue. It is cancerous and spreads through the lymphatic system or blood within the body of a host individual while the human immune system, consisting of interrelated special cells, tissues, and organs, is employed for the protection of the body from microorganisms, foreign diseases, infections, and toxins. Thus, the conceptualization and understanding of the intersections of tumor–immune cells are valuable. In this article, the natural process of tumor–immune cell interactions is formulated through a mathematical framework. The intricate dynamics of tumor–immune interactions are then represented by means of operators of fractional calculus involving nonlocal and nonsingular kernels. The definitions and basic properties of non-integer derivatives are introduced for the investigation of the proposed system. In addition, a new numerical scheme is introduced for the dynamics, showing the chaos and oscillation of the tumor–immune system. The proposed dynamics of tumor–immune interaction are highlighted with the effect of different input factors. Our findings not only contribute to a thorough comprehension of the complex interactions between input parameters and tumor dynamics, but critical factors that have a major impact on the dynamics are also identified. These outcomes are pivotal for refining and optimizing the proposed system to enhance its predictive accuracy and efficacy in modeling tumor behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to immunologists, two main components of the human immune system interact to defend the organism of host body from pathogens, that are, innate mechanism and adaptive mechanism. The initial response involves a defensive mechanism with a faster and more general manner against pathogens, employing cells such as macrophages, natural killer cells, and neutrophil leukocytes. Neutrophils travel with the help of tissues and the bloodstream to eliminate pathogens, while natural killer cells regulate immune responses and combat cancerous cells. Monocytes, circulating in the blood, have the capacity to search for infected tissues. In contrast, the second response is tailored to a specific pathogen and typically takes longer than the innate response. The cells involved in this process include dendritic cells, B lymphocytes, and T lymphocytes, where dendritic cells initiate the adaptive process, activating naive T cells by pathogen exposure. B cells develop new antibodies that are unique to a given pathogen to fend off or handle infections, while T cells in the host’s body grow into specialized cells such as cytotoxic T lymphocytes, T helper cells and T memory cells. The strongest of these are the cytotoxic T lymphocytes, sometimes referred to as killer cells or CD8+ T cells, which are found in the body of the host. They have T-cell receptors developed on them, which kill off non-host cells. The immune system in humans actively strives to inhibit the proliferation of pathogens, such as bacteria and viruses, even when consistently confronted with foreign substances. This effort is undertaken to maintain optimal health and well-being.
Mathematical models play a crucial role in various areas of science and engineering, providing a quantitative framework for understanding, predicting, and optimizing complex phenomena [1,2,3,4]. On the other hand, different research investigated disease from different perspective for public health [5,6,7,8]. To illustrate the complexity of biological events associated with cancer, a multitude of mathematical models have been presented in the literature [9, 10]. These models use discrete, continuous, or hybrid assumptions. Discrete frameworks are better suited for understanding individual cells and their interactions, whereas continuous modeling is more effective for describing tissue-scale dynamics. The mathematical models are classified according to the scale that is of interest; these scales include population-level, patient-specific, organ/tissue, cellular, and molecular. Interestingly, tumor–immune interaction dynamics appear at multiple scales. In [11], the authors explored an optimal approach to chemotherapy within the context of a mathematical model depicting tumor–immune dynamics. The aim was to identify the most effective response strategies in the treatment of cancer. The utilization of the prey predictor concept is applied for modeling diverse biological phenomena, encompassing interactions among individual cells [12,13,14]. This approach proves particularly efficacious in elucidating the dynamics of interactions between natural killer cells and tumor cells. Diverse assumptions were considered by multiple researchers in the literature when presenting the complexities inherent in tumor cells, as documented in references [15, 16]. The role of tumor-associated macrophages in tumor vascularization offers insights into the complex interplay between macrophages and the vascular system investigated in the literature [17]. It explored the potential implications for understanding and targeting these interactions in the context of tumor development. In [18], the authors delineated that macrophages are recruited to hypoxic regions within tumors, where they undergo a pro-angiogenic M2-polarized transformation. This transition is facilitated by hypoxic cancer cell-derived cytokines, specifically Oncostatin M and Eotaxin. In [19], asymptotic analysis and numerical simulations were discussed to investigate a prostate tumor growth model. The study utilized the generalized moving least squares approximation with semi-implicit time integration to explore the dynamics of the model for an enhanced understanding of prostate tumor growth. Exploring tumor–immune interactions contributes to reduce the incidence and mortality rates of cancer. Moreover, understanding tumor–immune interactions can lead to the development of more effective treatment approaches. The motivation behind this work is to conduct a rigorous investigation into the dynamics of tumor–immune interactions, aiming to provide valuable insights for public health considerations.
Fractional derivatives hold significant relevance in addressing real-world problems across various domains [20, 21]. They offer a more nuanced and accurate representation of non-local and memory-dependent processes, enhancing the modeling and analysis of complex phenomena [22, 23]. This increased precision contributes to the development of more effective solutions in fields ranging from physics and engineering to biology and finance [24, 25]. Numerous fractional operators, as documented in the literature and exemplified by references [26], have demonstrated success across various scientific and engineering disciplines. The significance of tumor–immune research through fractional derivatives lies in its ability to offer a novel mathematical framework for understanding the complex dynamics of tumor–immune interactions. By incorporating fractional calculus, researchers can model the non-local and non-singular behavior inherent in these interactions more accurately. This approach allows for a more nuanced analysis of the intricate relationships between tumors and the immune system, potentially revealing new insights into cancer progression and treatment response. Moreover, employing fractional derivatives in tumor–immune research can facilitate the development of advanced computational models and simulation techniques, enabling researchers to explore the effects of various factors on tumor growth, immune response, and treatment efficacy in a more comprehensive manner. Therefore, we opt to present the dynamics of tumor–immune interaction through fractional framework.
Further work is organized as: In Sect. 2, we construct a mathematical model for tumor–immune interactions with the help of fractional operator. The basic theory and concepts of the fractional calculus are presented in Sect. 3 of the article. In Sect. 4, we introduce a novel numerical technique to highlight the dynamics of the proposed fractional model. We revealed the chaotic nature and oscillation behavior of the model with the variation of the different factors. Concluding remarks of our work has been illustrated in final section of the article.
2 Evaluation of tumor–immune cell dynamics
It is well-known that Cytotoxic T Lymphocytes (CTLs) stand out for their inherent characteristics and performance, making them a focal point in onco-immunology research. In this context, we aim to develop a mathematical model elucidating the dynamics among effector cells (E), tumor cells (T), and the concentration of IL-2. In our formulation, the impact of an external source on effector cells is represented by \(s_1\), while the natural death rate is symbolized by \(\mu _1\). The tumor growth is expressed by \(r_2(T)\) with the immune response strength denoted by parameter a. Furthermore, the external contribution to IL-2 is captured by \(s_2\), and its degradation is assumed to follow a rate of \(\mu _2\). Thus, the dynamics of tumor–immune can be represented as
with the following conditions
in which the tumor’s antigenicity is shown by the variable c, while the immune response’s resilience and efficacy are indicated by the constant term a, which represents the tumor cells’ rate of elimination through contacts with effector cells. In addition, the effector cells are recruited at a rate \(p_1\frac{E I_L}{g_1+I_L}\) in which \(p_1\) and \(g_1\) are constant associated with \(E I_L\) and \(I_L\), respectively. Similarly, the \(I_L\) cells are recruited at a rate \(p_2 \frac{ET}{g_3+T}\), where \(p_2\) and \(g_3\) are constant associate with E and T cells, respectively. The elimination of tumor cells through effector cells is indicated by \(a \frac{ET}{g_2+T}\) where \(g_2\) is constant associated with T. The unit of all terms in the model are in days\(^{-1}\) while the unit of \(g_1,\) \(g_2\) and \(g_3\) are volume. In this formulation, the growth term for tumor is assumed as
Fractional order models improve the way we model tumor–immune systems by including memory effects, capturing complex behaviors more accurately, and providing more detailed representations [28, 29]. They also offer stronger stability analysis and create a more unified and flexible framework [30]. These benefits make fractional calculus a powerful tool for studying and treating cancer, leading to more precise and dependable models of how tumors and the immune system interact. The anticipation of anomalous diffusion in tumor dynamics aligns closely with the application of fractional calculus. Anticipating anomalous diffusion in tumor dynamics suggests the potential utility of fractional calculus in capturing the intricate and non-local dependencies that characterize the movement of substances within tumors. Consequently, this improves our comprehension of tumor behavior and facilitates the creation of more precise models and therapeutic approaches. In particular, the ABC derivative makes it possible to represent these complex dynamics more precisely, which enhances modeling accuracy and predictive power. The above system (1) can be expressed in fractional framework as
where \({}^{{ABC}}_{0}D^{\ell }_{t}\) indicates the fractional Atangana–Baleanu operator of order \(\ell\). Numerical results derived from fractional calculus provide valuable insights into the behavior and dynamics of systems with non-integer order derivatives [27, 31]. The numerical outcomes contribute to a deeper understanding of the role of fractional calculus in modeling and analyzing diverse processes, guiding researchers and practitioners in making informed decisions and advancements in their respective fields. The ABC fractional derivative might exhibit improved properties, such as better capturing non-local dependencies, enhanced accuracy in modeling complex systems, or providing more efficient solutions for particular types of problems. However, the claim of superiority is highly context dependent and relies on the specific requirements and characteristics of the systems being analyzed. It is important to understand the specifics of each fractional operator and to test how well they work in the particular scientific or engineering context to decide which is best.
Theorem 1
The solutions of the proposed fractional model (2) are positive and bounded for proper initial values of state variables.
Proof
To prove that the solutions of proposed fractional model (2) are positive and bounded for proper initial values of state variables, we proceed as follows:
this implies that the solutions of the model are non-negative. Thus, the solution of the recommended model are positive.
To ensure boundedness of the solutions, we analyze the behavior of each equation to find upper bounds. From the first equation of model (2), we have
further simplification implies \(E \bigg ( \mu _1 - \frac{p_1 I_L}{g_1+I_L} \bigg )=s_1+cT.\) Now, assume E reaches a maximum \(E_\textrm{max}\), then we have \(E_{max} \le \frac{s_1+c T}{\mu _1-\frac{p_1 I_L}{g_1+I_L}}\). Thus, E remains bounded. From the second equation of the model (2), we have
this implies that \(r_2 T(1-bT) = a \frac{ET}{g_2+T}.\) Assume T reaches a maximum \(T_{max},\) \(1-bT_{max} \ge 0,\) this implies \(T_\textrm{max} \le \frac{1}{b}.\) Since E is bounded, T is also bounded with an upper bound determined by b. From the third equation of our model, we have
this implies that \(I_L= \frac{s_2+p2 \frac{ET}{g_3+T}}{\mu _2}.\) Here, assume that \(I_L\) reaches a maximum \(I_{L_\textrm{max}}\), then we have \(I_{L_\textrm{max}} \le \frac{s_2+p2 \frac{E_\textrm{max}T_{max}}{g_3+T_\textrm{max}}}{\mu _2}\). This implies that \(I_{L}\) remains bounded. Thus, the required proof is completed. \(\hfill \square\)
3 Theory of fractional calculus
It is well known that fractional calculus is rich in applications and is applied to many problems in engineering, physics, economics, biology, and other fields of science and technology. Recent investigations, as documented by references [32,33,34,35], have affirmed the capability of fractional calculus to yield outcomes characterized by heightened accuracy, precision, and reliability. In this context, we shall introduce the foundational concept of fractional calculus for scrutinizing our tumor cancer model. Here, we present various fundamental outcomes and concepts of fractional calculus concerning the Atangana–Baleanu derivative in the Caputo sense:
Definition 1
Consider a function k such that k belongs to the function space \(H^1(p, q)\), with \(q>p\), and let \(\ell\) be in the interval [0, 1]. In this context, the Atangana–Baleanu operator in the Caputo sense is indicated by ABC and is given by:
Definition 2
Let \({}^{{ABC}}_{p}I^{\ell }_{t}k(t)\) be the integral of AB derivative which is defined by
where \(B(\ell )\) is a normalization function satisfying \(B(0)=B(1)=1\).
Theorem 2
[36]. Take a continuous function k such that \(k \in C[p,q]\), then we have
Moreover, AB operator satisfies the Lipschitz condition as
Theorem 3
[36]. Assume the fractional system
This fractional system has a unique solution of the form
4 Numerical scheme for model
Here, we present a numerical technique to demonstrate the oscillatory behavior and chaos inherent in our fractional model of interactions between tumor and immune cells. Numerous numerical techniques have been introduced for systems involving fractional orders. In the context of the dynamics pertaining to the tumor–immune system, we employ the innovative scheme presented by [37] to elucidate the solution trajectory of the model (2). To derive the required numerical scheme for our fractional model of tumor–immune interactions, consider the following system:
this implies that
Put \(t=t_n\), then we have
for \(t_{n+1}\), one have
the above implies that
Further, we have
in which
Then, through approximation, we have
in which \(h=t_m-t_{m-1}\). After that, one get
we get that
Taking the same steps, we have
consequently, we have
From the above, we obtain
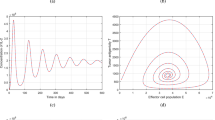
The presented scheme delineates a two-step Adams–Bashforth scheme specifically tailored for the ABC operator. Here, different simulations were conducted to elucidate the influence of varying parameters on the dynamics characterizing the interactions between tumor cells and the immune system. The values of the system’s input parameters are assumed for numerical purposes. In Fig. 1, 2, 3 and 4, the blue color represents the solution pathways of the model in integer case while the red color represents the solution pathways in non-integer case. In this section, the parameter values are supposed for simulations proposes to highlight the chaotic and oscillatory behavior of the system (2). In Figs. 1, 3, we have depicted the dynamic behavior of our proposed non-integer order model (2) using various values of the fractional parameter \(\ell\), specifically \(\ell =0.9\), \(\ell =0.5\), and \(\ell =0.95\), while in the other figures, we assumed the value of \(\ell =0.85\), which is also mentioned there in the figures. Our simulations revealed a noteworthy influence of the parameter \(\ell\) on the system dynamics, positioning it as a viable control parameter.
Figures 3, 4 and 5 depict the oscillatory and chaotic patterns exhibited by our system, subject to varying input parameters, specifically \(c=100\), \(a=0.1\), and \(a=1\). These simulations underscore the chaotic attributes inherent in the system, indicative of the potential significance of the parameter a in influencing and modulating the observed chaos within the system. The findings suggest that the manipulation of the parameter a may play a pivotal role in mitigating or suppressing the observed chaotic behavior.
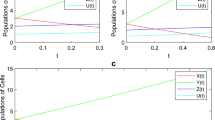
In the next step, we have shown the dynamics of the system with the variation of several input parameter to detect the effect of these input parameter on the system of tumor–immune cell interactions. We mainly focus on the variation of the parameters \(r_2, a,p_1, c\) and \(g_3\) to highlight the propose dynamics in Fig. 5, 6, 7, 8, 9 and 10. In Fig. 5, the system is plotted with different values of the input parameter \(r_2\). The graphical representation shows that this parameter is critical which increase the level of tumor antigenicity in the system. In Fig. 9, we assume different values of the parameter c and observed through simulations that the grater value of c can reduce the tumor antigenicity. Here, the role of the parameter c is highly appreciable in the system. In the simulations presented in Figs. 8, 9 and 10, it is interrogated that the input parameter a and \(g_3\) slightly disturb the dynamics of the propose system while the parameter \(p_1\) offer an interesting phenomenon during simulations. We have demonstrated in Fig. 8 that an increased value of the parameter \(p_1\) leads the system to exhibit both lower and upper peaks. On one hand, it decreases the level of tumor antigenicity, while on the other hand, it increases the level.
Illustration of the solution pathways and chaotic behavior of the model (2) of tumor–immune cell interactions with fractional order \(\ell =0.9\)
Graphical view analysis of the solution pathways and chaotic behavior of the model (2) of tumor–immune cell interactions with fractional order \(\ell =0.5\)
Time series analysis and chaotic behavior of the model (2) of tumor–immune cell interactions with input parameter \(c=100\) and \(\ell =0.95\)
Illustration of a phase portrait of tumor antigenicity verses concentration of IL-2, b phase portrait of effector cell population verses concentration of IL-2, c phase portrait of effector cells verses tumor antigenicity, and d chaotic behavior of the fractional tumor–immune cell interactions model (2) with the input parameter \(a=0.1\) and \(\ell =0.85\)
Phase portrait of a tumor antigenicity verses concentration of IL-2, b the effector cell population verses concentration of IL-2, c the effector cells verses tumor antigenicity, and d chaotic behavior of the fractional tumor–immune cell interactions model (2) with the input parameter \(a=1.0\) and \(\ell =0.85\)
Illustration of dynamical behavior of the proposed fractional system (2) of tumor–immune cell interactions with input parameter \(r_2=0.10,0.12,0.14\) and \(\ell =0.85\)
Illustration of dynamical behavior of the recommended system (2) of tumor with fractional parameter \(\ell =0.85\) and different values of a, i.e., \(a=0.4,0.5,0.6\)
Graphical view analysis of the dynamical behavior of the proposed system (2) of tumor with fractional parameter \(\ell =0.85\) and different values of input parameter \(p_1\), i.e., \(p_1=0.12,0.15,0.18\)
Time series analysis of the proposed model (2) of tumor with fractional parameter \(\ell =0.85\) and the effect of c, i.e., \(c=0.5,1.0,1.5\)
Time series analysis of the recommended system (2) of tumor–immune with fractional parameter \(\ell =0.85\) and the effect of \(g_1\), i.e., \(g=1.0\times 10^3, 1.5 \times 10^3, 2.0 \times 10^3\)
Moving forward, we will delve into the convergence and stability outcomes of our devised numerical scheme. The subsequent section outlines the convergence result attained through our method:
Theorem 4
Take a continuous and bounded function k, then the fractional system
has a solution of the form \(x(\xi )\) and is given by
where \(||H_{\ell }||_{\infty } < N.\)
Proof
To establish the desired outcome, we follow the below steps:
this implies that
in which, we have
and
In the coming step, we proceed as
which is the required result. \(\hfill\square\)
Theorem 5
Suppose that the function k satisfies the Lipschitz condition. In this case, the stability criterion for the aforementioned numerical scheme in the Atangana–Baleanu fractional framework in the Caputo sense is expressed as:
as n approaches to \(\infty .\)
Proof
For the demanded outcomes, take the following
taking norm on both sides, we have
From the above, we have
where
and
Consequently, one have
which give that \(| k(\xi _n, z_n)-k(\xi _{n-1},z_{n-1})|\) approaches to 0 as n approaches to \(\infty\). In addition to this, \(\frac{Mn!h^n}{4\ell }\) approaches to 0 as h approaches to 0, in which \(M=\max _{\xi \in [0,\xi _{n+1}]} | k(\xi ,z(\xi ))|\). \(\hfill\square\)
Stability ensures that the numerical solution remains bounded and behaves correctly over time, even for long simulations. An unstable scheme can produce wildly incorrect results, rendering simulations unreliable and useless. They provide confidence that the numerical results are a true representation of the underlying mathematical model, which is essential for scientific studies and any application where model predictions influence decisions. To be more specific, the stability analysis of a numerical scheme are fundamental to ensure that the numerical solutions are accurate, reliable, and useful for practical applications.
5 Conclusion
Tumors refer to the anomalous proliferation and division of cells, leading to the formation of abnormal tissue masses. On the other hand, the immune system plays a crucial role in combating infections and is equally significant in the context of tumor immunology. Two pivotal concepts within this field are immune surveillance and tumor immune escape. The interplay between the immune system and tumor cells is essential in regulating tumor progression and outgrowth. Thus, understanding the interactions between tumor and immune cells, along with the key factors involved, is of paramount importance. In this article, we conceptualized the natural process of tumor–immune cell interactions through a mathematical framework. The complex dynamics of these interactions were presented using a non-local and non-singular kernel. We explored the fundamental properties of fractional calculus to analyze our proposed system. A novel numerical scheme was developed to examine the dynamical behavior of the fractional tumor–immune cell interactions model. We have shown the stability criterion for the proposed numerical scheme to ensure the accuracy of the results in the long term. Our findings emphasized the significance of key parameters within tumor–immune interactions. The analysis identified specific parameters that are critical for control interventions. Visualizing parameter importance enhanced the understanding of the underlying dynamics and facilitated the identification of critical factors for targeted intervention strategies.
Availability of data and materials
Not applicable.
References
R. Jan, N.N.A. Razak, S. Boulaaras, K. Rajagopal, Z. Khan, Y. Almalki, Fractional perspective evaluation of Chikungunya infection with saturated incidence functions. Alex. Eng. J. 83, 35–42 (2023)
F.A. Rihan, Delay Differential Equations and Applications to Biology (Springer, Singapore, 2021), pp.123–141
F.A. Rihan, U. Kandasamy, H.J. Alsakaji, N. Sottocornola, Dynamics of a fractional-order delayed model of Covid-19 with vaccination efficacy. Vaccines 11(4), 758 (2023)
I. Ahmad, I. Ali, R. Jan, S.A. Idris, M. Mousa, Solutions of a three-dimensional multi-term fractional anomalous solute transport model for contamination in groundwater. PLoS One 18(12), e0294348 (2023)
A. Hasegan, M. Totan, E. Antonescu, A.G. Bumbu, C. Pantis, C. Furau, C.B. Urducea, N. Grigore, Prevalence of urinary tract infections in children and changes in sensitivity to antibiotics of E. coli Strains. Rev. Chim. 70, 3788–3792 (2019)
A. Boicean, D. Bratu, C. Bacila, C. Tanasescu, R.S. Fleaca, C.I. Mohor, A. Comaniciu, T. Baluţa, M.D. Roman, R. Chicea, A.N. Cristian, Therapeutic perspectives for microbiota transplantation in digestive diseases and neoplasia-a literature review. Pathogens 12(6), 766 (2023)
N. Grigore, M. Totan, V. Pirvut, S.I.C. Mitariu, R. Chicea, M. Sava, A. Hasegan, A risk assessment of clostridium difficile infection after antibiotherapy for urinary tract infections in the urology department for hospitalized patients. Rev. Chim. 68(7), 1453–1456 (2017)
N.I.C.O.L.A.E. Grigore, V. Pirvut, M. Totan, D. Bratu, S.I.C. Mitariu, M.C. Mitariu, R. Chicea, M. Sava, A. Hasegan, The evaluation of biochemical and microbiological parameters in the diagnosis of emphysematous pyelonephritis. Rev. Chim. 68, 1285–1288 (2017)
H. Enderling, M. AJ Chaplain, Mathematical modeling of tumor growth and treatment. Curr. Pharm. Des. 20(30), 4934–4940 (2014)
P. Bi, S. Ruan, X. Zhang, Periodic and chaotic oscillations in a tumor and immune system interaction model with three delays. Chaos Interdiscipl. J. Nonlinear Sci. 24(2), 023101 (2014)
U. Ledzewicz, M. Naghnaeian, H. Schättler, Optimal response to chemotherapy for a mathematical model of tumor-immune dynamics. J. Math. Biol. 64, 557–577 (2012)
C. Letellier, F. Denis, L.A. Aguirre, What can be learned from a chaotic cancer model? J. Theor. Biol. 322, 7–16 (2013)
A.M.A. Rocha, M.F.P. Costa, E.M. Fernandes, On a multiobjective optimal control of a tumor growth model with immune response and drug therapies. Int. Trans. Oper. Res. 25(1), 269–294 (2018)
S. Singh, P. Sharma, P. Singh, Stability of tumor growth under immunotherapy: a computational study. Biophys. Rev. Lett. 12(02), 69–85 (2017)
S.R. Nielsen, M.C. Schmid, Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm. 2017, 9624760 (2017)
T. Chanmee, P. Ontong, K. Konno, N. Itano, Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 6(3), 1670–1690 (2014)
C. Guo, A. Buranych, D. Sarkar, P.B. Fisher, X.Y. Wang, The role of tumor-associated macrophages in tumor vascularization. Vasc. Cell 5(1), 1–12 (2013)
C. Tripathi, B.N. Tewari, R.K. Kanchan, K.S. Baghel, N. Nautiyal, R. Shrivastava, H. Kaur, M.L.B. Bhatt, S. Bhadauria, Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget 5(14), 5350 (2014)
V. Mohammadi, M. Dehghan, A. Khodadadian, N. Noii, T. Wick, An asymptotic analysis and numerical simulation of a prostate tumor growth model via the generalized moving least squares approximation combined with semi-implicit time integration. Appl. Math. Model. 104, 826–849 (2022)
R. Jan, S. Boulaaras, S. Alyobi, M. Jawad, Transmission dynamics of Hand-Foot-Mouth Disease with partial immunity through non-integer derivative. Int. J. Biomath. 16(06), 2250115 (2023)
R. Jan, S. Boulaaras, S. Alyobi, K. Rajagopal, M. Jawad, Fractional dynamics of the transmission phenomena of dengue infection with vaccination. Discrete Contin. Dyn. Syst.-S 16(8), 2096–2117 (2023)
A. Jan, S. Boulaaras, F.A. Abdullah, R. Jan, Dynamical analysis, infections in plants, and preventive policies utilizing the theory of fractional calculus. Eur. Phys. J. Spec. Top. 232, 1–16 (2023)
S. Alyobi, R. Jan, Qualitative and quantitative analysis of fractional dynamics of infectious diseases with control measures. Fractal Fract. 7(5), 400 (2023)
M. Parvizi, M.R. Eslahchi, M. Dehghan, Numerical solution of fractional advection-diffusion equation with a nonlinear source term. Numer. Algorithms 68, 601–629 (2015)
Z. Shah, E. Bonyah, E. Alzahrani, R. Jan, N. Aedh Alreshidi, Chaotic phenomena and oscillations in dynamical behaviour of financial system via fractional calculus. Complexity 2022, 8113760 (2022)
D. Valério, M.D. Ortigueira, A.M. Lopes, How many fractional derivatives are there? Mathematics 10(5), 737 (2022)
R. Jan, A. Khan, S. Boulaaras, S. Ahmed Zubair, Dynamical behaviour and chaotic phenomena of HIV infection through fractional calculus. Discrete Dyn. Nat. Soc. 2022, 5937420 (2022)
F.A. Rihan, K. Udhayakumar, Fractional order delay differential model of a tumor-immune system with vaccine efficacy: Stability, bifurcation and control. Chaos Solitons Fractals 173, 113670 (2023)
F.A. Rihan, G. Velmurugan, Dynamics of fractional-order delay differential model for tumor-immune system. Chaos Solitons Fractals 132, 109592 (2020)
F.A. Rihan, K. Udhayakumar, Fractional order delay differential model of a tumor-immune system with vaccine efficacy: Stability, bifurcation and control. Chaos Solitons Fractals 173, 113670 (2023)
R. Jan, S. Boulaaras, S.A.A. Shah, Fractional-calculus analysis of human immunodeficiency virus and CD4+ T-cells with control interventions. Commun. Theor. Phys. 74(10), 105001 (2022)
H. Khan, W. Chen, A. Khan, T.S. Khan, Q.M. Al-Madlal, Hyers-Ulam stability and existence criteria for coupled fractional differential equations involving p-Laplacian operator. Adv. Differ. Equ. 2018, 1–16 (2018)
A. Jan, H.M. Srivastava, A. Khan, P.O. Mohammed, R. Jan, Y.S. Hamed, In vivo HIV dynamics, modeling the interaction of HIV and immune system via non-integer derivatives. Fractal Fract. 7(5), 361 (2023)
H. Khan, F. Ahmad, O. Tunç, M. Idrees, On fractal-fractional Covid-19 mathematical model. Chaos Solitons Fractals 157, 111937 (2022)
C.M. Pinto, A.R. Carvalho, A latency fractional order model for HIV dynamics. J. Comput. Appl. Math. 312, 240–256 (2017)
A. Atangana, D. Baleanu, New fractional derivatives with nonlocal and non-singular kernel: theory and application to heat transfer model. arXiv preprint arXiv:1602.03408 (2016)
A. Atangana, K.M. Owolabi, New numerical approach for fractional differential equations. Math. Model. Nat. Phenom. 13(1), 3 (2018)
Acknowledgements
This research work was funded by Institutional Fund Projects under Grant no. (IFPIP: 1533-665-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
MS conceptualize the biological process of tumor–immune system. ZS checked and supervised the overall work. RJ formulated and analyzed the model. AGH investigated and find out the numerical results. NV conceptualized and validated the dynamics. MS and RJ wrote the first draft while ZS, AGH and NV revised the final draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
There is no conflict of interest regarding this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shutaywi, M., Shah, Z. & Jan, R. A robust study of the dynamics of tumor–immune interaction for public health via fractional framework. Eur. Phys. J. Spec. Top. (2024). https://doi.org/10.1140/epjs/s11734-024-01210-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjs/s11734-024-01210-6