Abstract
The intensive experimental and theoretical research into the nerve signalling, which lasts for more than 230 years, has provided many valuable pieces of knowledge but no definite, really satisfying solution. Such an unfavourable state is due to the extraordinary complexity of this phenomenon and enormous technical difficulties encountered by experiments. Therefore, the problem till now persists as a challenging subject of research, being opened to various approaches. In the present contribution we are thus trying to summarize the accessible experimental findings and compare them critically with existing alternative theories. Finally, we attempt to compile a minimal model of the signal transmission in nerves, intentionally based only on well turned-out physically transparent arguments. The model combines two types of diffusion processes, microscopic and macroscopic ones, which act simultaneously and ensure nerve signalling. The full-time evolution of the corresponding action potential, from its emergence, increase, decrease and recovery phase, is controlled by the two types of membrane channels: by dissipative protein-based channels of Hodgkin–Huxley type and randomly created non-dissipative fissures in membranes. This approach could be useful for the efforts aiming to the improvement of the current models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
From the end of eighteenth century, when the astonishing results of experiments made by Galvani and Galeazzi were published [1], the conviction gradually prevailed in scientific and medical community that just the electrical, i.e., physical, and not some exclusively biological, unspecified phenomena, are behind the life processes in living creatures. In the following century the science of nerve and muscle stimulation was almost identified with some special branch of theory of electricity and the research into animal electricity became one of the recognized foundations of physiology. In the following decades, the idea, that biological objects should be investigated by physical methods and not in compliance with the paradigm of vitalism, appeared to be very useful and fertile. Leading personality representing these trends (physicalism) was E. du Bois-Raymond [2] who devoted his whole active life to the research into bioelectricity. His most significant achievement was the introduction of reliable physical experimental methods into electrophysiology. For solving a particular problem experimentally, the rule was first to look for the most advanced methods, improve them or construct a completely new measuring device [3]. Such a philosophy and modus operandi were typical also for du Bois-Raymond’s followers, H. von Helmholtz [4], J. Bernstein [5], L. Hermann [6] and many others and, in some sense, persists till our days. The application of the most advanced physical techniques in medicine and bioresearch, which has resulted into the huge contemporary progress of these disciplines, simultaneously generates new demands, extremely stimulating for building new instruments.
The studies of animal electricity have represented not the only research direction into the neuroscience. There were also several path-breaking discoveries concerning the anatomy, morphology and histology of nervous tissues supported especially by the development of optical microscopy and photography. After the clear microscopic identification of nervous network − reticulum, its typical structural patterns, such as axons and dendrites were discovered. As the whole nervous system seemed to be a continuous network penetrating the whole body, the influential reticular doctrine, which considered the nervous system as a single morphological entity, was established. Many important discoveries in the second half of nineteen century were enabled by C. Golgi’s invention of the new histological method for the tissue impregnation [7]. Microscopic specimens made by this technique have excellent contrast and revealed the tiniest details of the bio-structures. In this way important organelles (e. g. Golgi’s apparatus) and structural patterns inside the cells were discovered [8]. Taking full advantage of Golgi’s method, S. Ramón y Cajal proved by means of careful extensive anatomical investigations that the nervous tissue is not continuous, but that it consists of many individual cells interconnected via special coupling devices, known now as the synapses [9]. Incidentally, at almost the same time von Waldeyer–Hartz suggested for a specialized cell forming a building block of neural network a term neuron [10]. In addition, based on the studies into the morphology of neural networks, Ramón y Cajal was also able to formulate general rules governing the nerve ramifications and the one-way, dynamically polarized, directions of nerve signalling [11]. Finally, he established the neural doctrine denying the above mentioned reticular one. This doctrine now provides a base for all modern views of neural networks.
Another, probably the most important part of the contemporary physiology, is biochemistry [12]. The advanced microanalytic and spectroscopic methods enabled one to identify chemical reactions and their products taking part in life processes with incredible sensitivity. In the frame of this discipline, the long-lasting imbroglio, namely, whether the signal transmission in nervous system is of electrical or chemical nature was solved [13] among others. Whereas the question of signal transfer via the neurons was decided in favour of the electric theory, the processes taking place within the gap between two terminations of individual neurons, for which C. Sherrington coined the term synapse [14], were, by J. Eccless [15], convincingly proved to be of chemical nature. At present, hundreds of specialized complex chemical compounds called neurotransmitters are identified. These enable the signal transfer over the synaptic gap between two neurons [16]. In the meantime, however, the transport of the electric signals through the nervous filaments and individual neurons has been very intensively studied, too. Monumental experimental and theoretical research of E. Adrian [17], B. Katz, A. L. Hodgkin, A. F. Huxley [18, 19], I. Tasaki [20], W. Rall [21] and many others, culminated at 1950’s to the formulation of consistent model, accounting for the electric signal transfer through the neurons, which till now has serve as a textbook standard [22] in medical faculties all over the world (Hodgkin–Huxley paradigm). During the last 15 years a concurring model, the so-called soliton model, of T. Heimburg et al. [23] has appeared. Accordingly, the nervous signal is primary not of the electric but of the mechanical (acoustic) nature and the observed electric effects are only secondary manifestations, due to the electromechanical coupling within the cell membrane. The more detail comparison of both these leading models is given in Sect. 6.
The very aim of this paper is to support macroscopic approach to the processes taking place in biological neural networks, entirely in the gist of “physicalism”. Of course, we are aware of the importance of detailed knowledge of biochemical reactions controlling life processes on molecular level. It is, however, the matter of non-disputable fact that all these complex biochemical reactions are inevitably governed by very general physical laws belonging to the domains of thermodynamics, electromagnetism, or quantum mechanics. What is thus missing, is the unifying picture based directly on these physical theories, the conceptual basis of which is reliable and relatively simple. In this work, we develop a simple physical model of neural stimulation based on experimental facts. There is a hope that it can contribute to more general models of life processes which might be simple and objective, too.
The present paper is organized as follows: we begin with a brief description of the electric properties of extracellular and intracellular somatic fluids. Then basic physical properties and constitution of lipid bilayers and real cell membranes are overviewed. In the next section a thought experiment elucidating some not generally known aspects of charging of cellular structures is discussed. The following section is devoted to the very fact, with far-reaching consequences, that the cations contained in somatic fluids behave at physiological conditions as quantum particles. In the next section leading theories of the action potential (AP) are compared and the empirical facts to be explained are surveyed. Then the section representing the core of the paper, in which our minimal model of AP is presented, follows. Our conclusions are then briefly summarized in the last section.
2 Electrical properties of somatic fluids
Somatic fluids, which are substantial components of any living organism, are composed of aqueous solutions of various inorganic salts, dissolved gases and of rich blend of organic molecules. Morphologically we can distinguish intracellular and extracellular fluids (ECF). The most significant components, which are in fact controlling the overall electrical properties of somatic fluids, are aqueous solutions of NaCl and KCl, that is electrolytes. For typical warm-blooded animals the mean concentrations of sodium and potassium cations at T = 310 K (~37 °C) in extracellular fluid are [Na+] = 150 mol m−3, [K+] = 5.5 mol m−3, whereas in the intracellular fluid – cytoplasm, [Na+] = 15 mol m−3, [K+] = 150 mol m−3 [24]. There are, however, some important exceptions, where ion concentrations in cytoplasm differ from these figures appreciably, e.g. endolymph in inner ear or fluid in capsules in lateral line of fishes [25], see Table 1.
Interestingly enough, the composition of the extracellular fluid resembles to that of Global Ocean Sea water in Precambrian era where the first animals started to develop. This is probably because for these organisms the sea water played the role of an extracellular system and the intracellular one had to accommodate to this situation. For further development the retaining of the same composition of somatic fluids was thus very “economic”. The traces of primeval Global Ocean water are preserved to our days in the form of juvenile waters. For example, in mineral water “Vincentka” from the spa Luhačovice, Czech Republic, we encounter [Na+] = 106 mol m−3, [K+] = 3.4 mol m−3, so that the cations ratio is ≈ 31.2, i.e., within the physiological limits is the same as that in the extracellular fluid, ≈ 27.3. These facts thus support the idea that the waters of Global Ocean, being the common cradle of all living creatures over the world, is preserved in our bodies in the form of a life-giving extracellular fluid.
As it has been shown in the literature [26] already many times, for the specification of electrical properties of somatic fluids the two-component model of aqueous electrolyte is adequate and sufficient. The influence of other components, even though they may be of decisive importance for the chemical and biological behaviour of the fluid, can be neglected. Among phenomenological parameters used for the description of effects of external electric fields on the electrolyte there are crucial quantities such as conductivity γ, dielectric constant ε, cavity potential φ and screening length λ. These quantities can, in principle, depend on the concentration of dissolved salts, their degree of dissociation or on corresponding cation concentrations [X+]. Since the concentrations of dissolved salts are small, we can classify these electrolytes as weak, so that the ingredients are fully dissociated. The difference of cavity potentials φN measured between two points in one-component inhomogeneous electrolyte or between two homogeneous electrolytes in electric contact (contact potential), describes the Nernst equation [27]
where z is ionic valence and k the Boltzmann constant. In the case of monovalent sodium and potassium cations for which is z = + 1, we obtain for normal body temperature T = 310 K (~ 37 °C) contact potential differences between cytoplasm and extracellular fluid induced by Na+ and K+ cations, − 6.15 × 10−2 V and + 8.83 × 10−2 V, respectively. The sum of these two Nernst’s potentials, equal to ~ + 2.68 × 10−2 V, has a simple physical meaning. It measures the energy (in eV) necessary for exchange of a pair of Na+ and K+ ions between both somatic fluids. Notice that just this process, the transfer of identifiable cations between somatic fluids, is the core of electric stimulation (Obviously, the indistinguishable chlorine anions Cl−, being common part of both somatic fluids, are for such a purpose useless). For the stimulation to be a causal and not a random process, the energy per cation must necessarily fall out of the (kT/e)-band, the half of which defines thermal excitation threshold (≈ 13.5 mV). It is a remarkable empirical fact that the numerical value of this quantity at physiological conditions amounts ~ + 2.67 × 10−2 V which is almost the same as the difference of Nernst’s potentials mentioned above. Such a coincidence is hardly accidental. Taking into account the formula (1) above, we immediately obtain for validity of such a coincidence a condition ln ([Na+ cyt.]/[K+ ECF]) = 1, which preserves unchanged the pre-factor in (1). Actually, we have ln(15/5.5) = 1.003, so that the cation concentrations in both somatic fluids are tuned with appreciable accuracy. This is probably rather a result of the long-lasting evolution of living organisms aiming to distinguish between causal and random excitations than of simple accidence.
The important macroscopic parameter controlling the response of somatic fluids to external electric fields is the background free charge density ρ0 corresponding to the total concentration of distinguishable cations, i.e.
where [X+Z] is overall cation concentration and NA the Avogadro constant. For monovalent cations is again z = + 1. From Table 1, it is obvious, that the background free charge density is almost the same in both somatic fluids. Putting then for cytoplasm [X+] = 165 mol/m3 we obtain an estimate ρ0 ≈ 1.59 × 107 C/m3. The knowledge of the background free charge density enables one to compute, among others, the Debye–Hückel screening length λ [28], a parameter characterising the damping of external electric field in the environment containing movable charge carriers. The corresponding formula reads
where ε is dielectric constant of environment, which is, as a rule, in aqueous solutions equal to that of water, ε = 81. Considering then all relevant figures above, we immediately obtain for the screening length an estimate λ = 1.1 × 10−9 m which is under the physiological conditions valid practically for both somatic fluids.
It should be noticed that the ECF is not a single agent fully determining the overall electrical properties of the extracellular tissue. This tissue contains, namely, a huge number of solid and gel components having fibrous or flake-like structure. Since, for example, the oriented collagen fibres revealing an appreciable piezoelectric effect [29] are responsible for electromechanical activity of the extracellular tissue, the presence of organic flakes increases its tortuosity and Maxwell–Wagner polarisation. As a result, a typical tissue reveals a giant frequency dependent relative permittivity ε ≈ 104—105 at kilohertz range (see e.g. [30]). Whereas the piezoelectricity may play an important role in mechanically induced phenomena such as injury healing [31], the giant frequency dependent permittivity is decisive for the penetration of electromagnetic radiation into living tissues and for ephaptic coupling within the grey matter. On the other hand, the traditional empirical observation, that in vitro experiments with nerve fibres immersed into a simple electrolyte substituting the extracellular tissue closely reproduce the corresponding in vivo behaviour, supports the idea that for such a type of experiments the approximation based exclusively on somatic fluids is a good one. That is why, in this paper we have restricted our considerations to the case, where the cytoplasm and exoplasm are represented by the reservoirs of proper somatic fluids.
3 Constitution and physical properties of cell membrane
Other basic components of any living organism are membranes [26]. The bio-membranes consist prevailingly of lipids (phospholipids) which are amphiphilic compounds composed of polar hydrophilic head molecule group with attached pair of fatty acid molecules forming non-polar hydrophobic chains [32]. In the case where the phospholipids are placed in the water, or generally into the aqueous solution, they spontaneously form relatively stable bilayer with the hydrophilic heads oriented to the water and with the hydrophobic chains pointing to the interior of the bilayer. While the head molecule groups are, as a rule, hydrated, creating together with adjacent layer of water an integral part of membrane, the hydrophobic chains inside the bilayer interact by van der Waals forces, determining the mechanical strength of the system.
The present knowledge of real bio-membranes is in a large part based on studies of model lipid bilayers [32]. Pure artificial lipid bilayers, consisting only of one sort of lipids, reveal interesting thermodynamic properties. Their significant feature is that they melt at certain melting temperature TM which is typically slightly below ~37 °C. Above melting temperature the bilayers are in the state of two-dimensional disordered liquid (fluid), while below this temperature they solidify into ordered gel phase. In disordered liquid state the molecular packing is quite irregular and individual lipid molecules can freely laterally move across the whole surface of the bilayer. In addition, the chains of fatty acid are thermally randomized, so that the van der Waals attraction between neighbouring molecules is weakened. In contrast, the molecular packing of ordered gel phase is almost regular (hexagonal) and tight, with parallelly well oriented hydrophobic chains, what leads to the enhanced layer cohesion. It is evident that such a large structural difference must be macroscopically associated with appreciable enthalpy and entropy changes. Indeed, thermal analysis of artificial lipid bilayers systematically reveals sharp specific heat capacity peak at melting point TM [33].
Besides numerous analogies existing between model lipid bilayers and bio-membranes encountered in living organisms, they also differ in many aspects, which are not omissible. For example, any living cell membrane, cytolemma, controlling the matter exchange between environment and interior of the cell’s soma, must inevitably contain many specific molecules ensuring such specialized life-preserving functions. Their addition, however, must naturally change the structural, mechanical, and electrical properties of the membrane. Among macromolecules frequently embedded in the bio-membranes the most important are sterols (e.g. cholesterol) and various proteins. They can be incorporated either into one lipid leaflet or they can go through the whole structure as the transmembrane molecules. Incorporation of further molecules into lipid bilayer brings about important structural consequences. The functionalized lipid bilayer is no more either in ordered gel state or disordered liquid state, but both these phases coexist and in a certain relatively wide temperature range around TM, are forming a special third phase, called ordered liquid phase. This phase has the following peculiar properties. The fatty acid chains of lipid molecules remain perfectly ordered even above original TM, because the admixture of foreign macromolecules diminishes the free space in the core of layer. At temperatures below TM, however, the same foreign macromolecules, due to their incommensurability with lipid molecules, prevent the development of perfectly regular crystal-like structure of ordered gel phase. As a result, the structures of ordered liquid phases above and below TM are indistinguishable and the membranes are more rigid than those in disordered liquid phase, but without impeding the molecular migration across the membrane, intrinsic to ordered gel system. The real membranes, however, are obviously not homogeneous. More different phases can thus simultaneously coexist in a typical bio-membrane under physiological conditions. Indeed, lipid molecules of the same kind tend, according to general rules of thermodynamics, to segregate and create domains of pure lipid bilayers and the domains, having extension ~10–200 nm, called membrane rafts, which are enriched with cholesterol and proteins. Naturally, domains of pure lipid bilayers can undergo phase transitions regardless of other parts of the membrane. Therefore, even though the phase transition is absent in the majority of the membrane, the entropy change in the vicinity of TM is still experimentally observable [34].
Another fundamental property of bio-membranes is that in living cells the cytolemma must be inevitably asymmetrical, i.e., that its inner and outer leaflets are different, particularly, the inner leaflet is more fluid than the outer one. The main reason for such an asymmetry is the fact that the cytolemma separates domains of different biochemical composition and different physical properties, namely, cytoplasm and extracellular fluid. In addition, there is another reason, purely geometrical, represented by the difference of curvatures of inner and outer layer. Cytolemma must let in nutrition and signalling macromolecules and let out the waste of life processes. Besides, it also must exchange various ions to keep homeostatic composition of the cytoplasm. It is thus under the physiological conditions permanently in flux, ensuring many essentially selective one-way processes. Therefore, the loss of the cell membrane asymmetry would be very harmful, likely resulting in cell’s death.
Among the physical properties of bio-membranes and their precursor, lipid bilayers, electrical parameters play significant role. As we have seen, the main component of bio-membranes are lipid bilayers having a non-conductive hydrophobic core of thickness a ≈ 2–3 nm and polarized interface layers of hydrated hydrophilic lipid head groups. The electrical properties of such a system are determined mainly by the hydrophobic core, whereas the hydrated layers behave rather as parts of somatic fluids. The dielectric constant εA and the conductivity γA of typical bio-membrane can be thus identified with the parameters which are characteristic for the hydrophobic core of lipid bilayer, i.e. εA ≈ 2 and γA ≈ 10−13 S/m [35].
4 Charging of the cellular structure—thought experiment
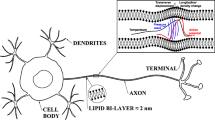
Let us make an instructive thought experiment with an idealized model of a living cell and its response to the electric stimulation (see Fig. 1). The cell is considered to be a prolonged cylindrical enclosure of inner diameter d filled with the electrolyte of composition corresponding to cytoplasm and the boundary consisting of impermeable membrane, cytolemma, of thickness a and relative permittivity εA. The whole cell is then surrounded by the electrolyte, the composition of which is identical with that of somatic fluid in extracellular tissue (ECF). Since the electrolytes inside and outside the cell are different and the membrane itself is assumed to be permanently polarized, a cavity potential difference between ECF and interior of the cell must exist. This potential difference brings about in the vicinity of the membrane charged screening Gouy’s layer [36] of thickness corresponding to the Debye-Hückel screening length λ = 1.1 × 10−9 m, see Eq. (3). Simultaneously, a similar layer consisting of counterions is built-up on the outer boundary of the cell.
If we introduce into the soma of the cell a capillary point sonde S filled with aqueous solution of KCl and biased with respect to the resting potential of ECF, the sonde will start to inject into the cytoplasm potassium cations. How is such a temporary point disturbance relaxed by the cell? At the beginning, the ions injected into the close vicinity of the sonde tip generate radial electric field, crossing the nearest parts of the cytolemma. This field immediately initiates the local reconstruction of Gouy’s layer adjacent to the cytolemma which continues till the external bias VB is fully compensated by the increase of the local cavity potential drop. The extra charge carriers supplied from the bulk of disturbed cytoplasm into Gouy’s layer then cancel on its inner boundary the normal component of electric field, what enables injected cations to proceed to the more distant parts of the cell. This process also known as a sphondyloid (= flow tube) formation [37, 38] which electrically decouples charge carriers inside the conductor from the surrounding, is in fact sine qua non for electric transport via conductors at all. The reconstruction of space charge Gouy’s layer adjacent to the cytolemma is controlled by the diffusion of ions toward the cell boundary. Such a transport is described by diffusion correction term to the Ohm-Kirchhoff’s law with microscopic diffusion constant given by a formula [39]
where γ and ε are conductivity and dielectric constant of cytoplasm, respectively, and λ is the screening length (3), measuring the length to be, in average, passed by charge carriers diffusing to establish new Gouy’s layer.
It is then important to know the amount of charge which must be supplied into Gouy’s layer to increase the cavity voltage drop about VB. Let ρE be the mean density of extra charge deposited within Gouy’s layer of thickness λ, then the product ρEλ has obviously the meaning of surface charge per unit area. This charge accommodated on the inner side of the membrane having per unit area capacitance ≈ εε0/a should induce a desired voltage drop VB. As a result, we obtain a simple relationship
This formula provides for bias voltage VB ≈ 75 mV, which is the typical amplitude of AP of warm-blooded animals, an estimate ρE ≈ 6.05 × 105 C/m3. If we compare it with the equilibrium background free charge density ρ0 ≈ 1.59 × 107 C/m3, we see that the charge transferred during the electric activation of the cell to its boundary performs only a small fraction ≈ 3.8% of free charge, which is locally in disposal. The electric stimulation can be thus classified as a “tiny effect”.
From the macroscopic point of view the spreading of the boundary of the cytolemma domain, where the sphondyloid is already formed, is of primary interest. The main idea of the cable diffusion theory of action potential may be briefly presented as follows. Building-up of the sphondyloid structure in the narrow strip of width Δx encircling the cell, requires supplying into this zone extra charge carriers, the amount of which is directly proportional to the product of cytoplasm conductivity γ and the cell cross section πd2/4. On the other hand, the spreading of the boundary is indirectly proportional to the capacitance to be charged, which is obviously given by a term εAε0 πd Δx/a. Combining these expressions, we immediately obtain a macroscopic diffusion constant controlling the kinetics of charging of the surface of the cell
This formula, reflecting macroscopic features of the spreading of the electric stimulation throughout the tube-like living cells, is in full agreement e.g. with the modified cable theory of AP in nervous filaments [40]. Accordingly, the traveling of quasi-wave across the surface of the cell have a character of diffusion and not of movement with constant speed.
The final state of the cell in our thought experiment may be thus described as follows. The whole surface of the cell is charged by ions which diffused from the bulk of the cytoplasm toward the cytolemma, the transmembrane potential difference is everywhere increased about the bias voltage VB and the supply of potassium cations from the sonde is stopped. Such a state is stable with the proviso that cytolemma is an ideal insulator. For real membranes where the rest membrane conductivity γA ≈ 10−13 S/m [35], the Maxwell relaxation time ~εε0/γA, possibly controlling the discharge of the cell, is of the order of hundreds of seconds (~ 180 s). Since in real living systems relaxations times are typically in milliseconds, it is quite clear that the electric stimulation of real cells cannot work without some additional devices, controlling exchange of ions through the membrane.
The fact that the spreading of electric stimulation in living organisms is controlled by the diffusion and not by regular wave-like electric impulses is not generally accepted. Therefore, we add the following notice. For a diffusion process, its speed (velocity) is not well-defined quantity; instead, the diffusion constant is used as a characteristic parameter. However, the instant speed u of diffusion front, satisfying classical relation
can be defined. Obviously, the observed instant speed is thus dependent on the distance x from the point of initial disturbance. This important fact, supported also by the experimental evidence [41], explains inconsistency of numerous time-of-flight determinations of “conduction velocity” of nervous signals performed by different authors using arbitrary lengths of nerve filaments. From this point of view the common textbook concept, conduction velocity (speed), cannot be regarded as a good characteristic of signalling in living structures.
It is further apparent from formula (6) that the traveling of quasi-wave across the surface of the cell is “quicker” if the inner diameter of the cell and the thickness of the cytolemma are larger. Both these conclusions are for the case of nervous cells confirmed experimentally many times [42]. It is worth noticing that the increase of cytolemma thickness a is extremely effective mean for increasing the constant DE. For example, the so called myelinization, i.e. the thickening of nerve walls due to specialized Schwann’s cells wrapped around the nerve, shorten the reaction times of e.g. efferent nerves many times, even if their diameter d is preserved. In this connection it is worth stressing that the “speeding up” of signal transfer via myelinated nerves can be accounted solely for by thickening of cytolemma, without reference to other models, as to e.g. very popular saltatory theory [43]. This theory, which is a standard item of medical syllabus, was inspired by the experimental observation of equalizing currents flowing during neuron cell excitation in the extracellular tissue between gaps in myelin coverage (Ranvier nodes). These were identified, in the gist of philosophical proposition “Causa equat effectum”, with the actual and quickest carriers of nervous signal. Of course, the equalizing currents flowing in the vicinity of the nervous cell through the conductive ECF inevitably do exist, being consequences of potential differences appearing between different points on the outer surface of the cell during the construction of the sphondyloid (see above). On the other hand, how to causally explain the active will of the current to jump from the one Ranvier node to the next one, in order to induce there AP necessary for information transfer? The existence of such currents which is essential e.g. for ephaptic coupling between neighbouring cells [44] is, however, for “speeding-up” of electric signals themselves unsubstantial, what makes the saltatory theory somewhat misleading.
As we have already seen, during electric stimulation of a cell two diffusion processes are active: one microscopic controlled by diffusion constant DΩ and the other one macroscopic controlled by diffusion constant DE. Both these processes operate simultaneously and are causally correlated. Whereas the microscopic diffusion transfers ions from the bulk of the cytoplasm toward the membrane, which is then charged, the movement of the front of charged domain across the membrane has a character of macroscopic diffusion. These two processes differ in prevailing directions of diffusion, these directions are orthogonal, and in addition in numerical values of diffusion constants. Taking the figures given above, γ = 1.44 S/m, ε = 81 and λ = 1.1 × 10−9 m, we obtain, from Eq. (4), an estimate DΩ ≈ 2.43 × 10−9 m2/s. Remarkably, since the values of γ, ε and λ are almost the same for all animals over the world, this constant is also practically universal. In contrast, macroscopic diffusion constant DE being dependent on geometrical dimensions of the cell can change appreciably. Its numerical value is, as a rule, about many orders of magnitude larger than that of DΩ. For example, if we take for the thickness a = 2 × 10−9 m, for the typical cell diameter d = 2 × 10−5 m and for εA = 2 we obtain from Eq. (6) an estimate DE ≈ 8.1 × 10−4 m2/s. Covering the cell with myelin layer of thickness a = 2 × 10−6 m and dielectric constant εA = 7 [35], the diffusion constant will be much larger, DE ≈ 2.3 × 10−1 m2/s.
The fact that the microscopic and macroscopic diffusions are simultaneous and closely cooperating processes has some far-reaching consequences. If we construct dimensionless ratio of both diffusion constants, we obtain a useful scaling parameter which can be decomposed either into two dimensionless monomials or, alternatively, into two dimensional monomials, the first one of which is universal and the second one is characteristic for geometry and electric constitution of the cell membrane. Indeed, from (4) and (6) we obtain
Other similar relationships can be constructed. These enable one to follow the general trends and changes encountered in living organisms during their ontogeny or phylogeny [45], or to quantify the characteristic differences among species.
5 Quantum diffusion of ions
Epistemologically, the numerical value of the phenomenological diffusion constant DΩ is of a special concern. As we have seen above, this constant, being practically universal, has a numerical value ≈ 2.43 × 10−9 m2/s. Let us then examine the mechanical action ζ corresponding to the diffusion of principal cations through the somatic fluid. The mechanical action can be defined as a product of diffusion constant and particle mass
Applying this formula to sodium and potassium cations, the masses of which are M(Na+) = 3.82 × 10−26 kg, M(K+) = 6.49 × 10−26 kg, respectively, we obtain the following estimates of corresponding actions
At first glance it is apparent that both these values are in the rank of Planck’s quantum of action, = \(\hbar\) 1.05 × 10−34 Js. This simple truth has, however, far-reaching consequences. According to the fundamental quantum criterion [46], the constant DΩ and cation mass M(X+) have to play, in this case, the role of quantum–mechanical conjugate variables. For the correct description of the diffusion process the recourse to the quantum theory is inevitable. Particularly, for conjugate variables the Heisenberg uncertainty relation
should take place. Inspecting the estimates (10) we immediately see that (11) is actually quantitatively valid, so that we have to do with genuine quantum effect. For the quantum–mechanical treatment of diffusion process, it is further advantageous to use the remarkable fact that the Schrödinger equations are isomorphous with the generalized diffusion equations. These equations can be mapped one onto another using, in diffusion equations, formally the Fürth Ansatz ~i\(\hbar\)/2M [47] instead of the diffusion constant. The purpose of imaginary unit in this term is to label and decouple quantities related to the configuration and momentum space. If one is satisfied, however, with quantum description of the diffusion in configuration space only, it is sufficient to introduce just one real diffusion constant DQ [48]
which can be then inserted into the classical diffusion equation instead of the phenomenological constant DΩ.
How can the quantum diffusion be then interpreted? What is the physical mechanism behind? As we are convinced, the most straightforward interpretation is provided by stochastic electrodynamics (SED) [49], a working theory alternative to quantum mechanics, which is conceptually based on the existence of zero-point electromagnetic fluctuations of vacuum (ZPF). These fluctuations are assumed to be omnipresent, universal, and independent of temperature. Their characteristic property is, that the action per mode is the lowest possible one, i.e. \(\hbar\)/2. If thus the action bounded to any mechanical or electromagnetic system is lowered to the value comparable with this lowest unsurmountable limit, its interaction with ZPF became observable as the quantum behaviour. For example, in the case of quantum diffusion, a particle, due to the random interaction with the background ZPF radiation, reveals the so-called quantum jiggling (“zitterbewegung”) [48], a stochastic movement of the particle without participation of molecular collisions. Consequently, such a type of diffusion, where DΩ → DQ, has much lower energy dissipation rate and entropy production then classical thermal molecular diffusion.
In this connection it should be pointed out, that the sodium and potassium ions are not the only ions fulfilling the quantum criterion (11), so that the quantum diffusion may play decisive role in much wider class of transport phenomena in living creatures then one usually expects. Indeed, practically all chemical reactions taking place between environment and living organism or among functional parts inside the organism, the concentrations of reactants or products are not the only parameters determining the reaction rates. It is mainly because of the presence of numerous membranes and tissues of appreciable tortuosity which strongly limits the transport of reactants. The chemical reaction rates are then actually controlled by diffusion processes which correspond to the so-called Nernst–Brunner kinetics [50]. In this limit, the intuitive traditional view on chemical kinetics as the natural tendency to reach the state of equilibrium by the shortest possible way is violated, and the adequate mathematical description in this situation provides Turing’s reaction–diffusion model [51]. Moreover, the exchange of reactant molecules is appreciably slowed down. Such an intrinsic “slowness” of diffusion kinetics, is, however, crucial for dumping of sudden spurious excitation events, preventing the chaotic behaviour of the system. In addition, the chemical reactions submitted to the quantum version of the Nernst–Brunner kinetics have a tendency to create periodic space–time patterns [52, 53], often interpreted as the manifestations of self-organisation or even as the precursors of life processes [54].
From the diffusion constant DQ, the time constant τ characteristic for redistribution of charge carriers within Gouy’s layers during the sphondyloid construction can be further computed. Using the Einstein–von Smoluchowski’s square root diffusion formula [55], we can immediately write
For sodium cation this relation yields a value τ ≈ 4.4 × 10−10 s which is evidently much shorter than the typical integral response of bio-systems to stimulation (~ 0.1 ms). The quantum kinetics of ions in somatic fluids is thus, despite of its intrinsic “slowness”, not a limiting factor for processing of stimulations in living systems.
All these quantitative coincidences lead to the conclusion, that in theoretical considerations concerning the kinetics of cation exchange in somatic fluids the quantum diffusion constant DQ can be freely substituted for phenomenological macroscopic diffusion constant DΩ. This also reflects the remarkable fact that the physiological processes taking place in all living creatures over the world are probably controlled by ZPF, the fundamental constituent of the Universe.
6 Comparison of leading theories of action potential
It is a matter of fact that the sensitivity to electric stimulation is proper to any living cell. However, the electrophysiology focuses mainly on neurons, cells constituting the nervous tissue, which are specialized for information transfer in organisms. These cells are distinct among the other animal cell types, because of their unique shapes, sinuous and elongated structure, sometimes of enormous size. The peculiar manifestation of their activity is the so-called action potential (AP), the specifically formed electric pulse, apt to transmit the controlling signals. The typical shape of AP as depicted at Fig. 2 reveals a rather sharp peak having an amplitude of order of ~100 mV and width of ~1 ms, followed by a shallow minimum of much longer duration. Interestingly enough, such a form of AP is at physiological conditions almost universal, with only small differences encountered between various species.
Typical shape of the action potential (AP), adapted according to [39]. The threshold voltage ≈ kT/2e (= 13.4 mV) above resting potential, which is necessary for the onset of signal spreading through the nerve, is simultaneously sufficient to supress the spurious stimulations due to the thermal noise
As we have already mentioned above (in Introduction), there are two main competing theories of signal propagation via neurons [56, 57]: 1. The standard, exclusively electric Hodgkin–Huxley quantitative model. It reproduces a wide range of associated experimental data; 2. The alternative theory of AP, proposed by T. Heimburg and his co-workers, which is known as the soliton model (theory). It explains the signal propagation through the nerve as a purely mechanical (acoustic) effect, whereas the accompanying electric AP is only a by-product of electromechanical properties of cell membrane.
The Hodgkin–Huxley model is based on many careful experiments made from 1930 to 1950s, which were accompanied with exceptionally creative theoretical work. Among experiments, worth of mentioning, are the evidence for electrical signal transmission in nerve and its persistence even if the nerve fibre is mechanically blocked [58], the first reliable record of AP from inside of nerve fibre [59], the demonstration that the flows of ions across the axolemma determine the shape of AP pulse [60] and that the resistivity of extracellular fluid influences the velocity of AP pulse [61]. The precursor of their theory was likely somewhat vague observation of the large increase (more than 30 times) of membrane conductance during the passage of AP by J. Bernstein [5], later conclusively confirmed by K. S. Cole and H. J. Curtis [62]. Since this temporal membrane breakdown involved the change of the sign of transmembrane potential (“overshoot”), the mechanism behind remained unclear.
For the establishment of the Hodgkin–Huxley model, voltage-clamp experiments with giant axons of the squid Loligo pealeii [63] were important. This technique enabling one to measure the ion currents across the membrane at a given constant transmembrane potential difference, inspired the idea of selective voltage-gated ionic channels. Authors showed further that the stepwise depolarization of the membrane by external bias voltage can trigger fast inward current carried mainly by Na+ ions, which is immediately followed by an opposite much slower outward current carried by K+ ions [64], explaining in this way the peculiar result of Cole–Curtis experiment. They were later able to demonstrate that the identical process took place also during the spreading of AP (“ionic hypothesis” [65]). The results of these studies then provided quantitative parameters for voltage-dependent conductance of selective protein-based channels. These parameters then entered the differential equations describing the shape and time evolution of AP. The electrical transport through the tubular structure immersed into the conductive fluid is mathematically described by the theory of submarine telegraph cable worked out by Kelvin and Heaviside [66, 67]. Hodgkin and Huxley making then somewhat arbitrary teleological assumption, i.e. that the AP pulse propagates with constant speed, reduced the original telegraph equation to the differential equation of the wave type, in which the empirical parameters of gated channels were substituted. The reduced equation, however, was not solvable analytically, so that there was the necessity to solve it numerically (The authors were the pioneers in this domain of science). The resulting quantitative model, being able to correctly fit a wide range of experimental data, was exceptionally successful, and became a governing paradigm in electrophysiology, which has persisted for more than 70 years. As an example of the continuation of the Hodgkin–Huxley approach to solving problems in bioelectricity by numerical simulations may serve the application of the “BioElectric Tissue Simulation Engine” (BETSE) [68], currently used for evaluation of electric interactions between large cellular clusters, which is of primary importance for the research into treatment of injuries, genetic defects, and cancer.
The main flaw of the Hodgkin–Huxley quantitative model is that it pays only a marginal attention to the energy dissipation and to the mechanical structural changes during the propagation of AP. Such an oversight of experimental observations might be justified by the fact, that these phenomena, for a long time already known, are extraordinary weak [69, 70] in comparison with dominating and much easily detectable electric effects. Indeed, experimental data [71, 72] and theoretical estimates [73] provides for heat released per unit area of membrane during the transit of AP signal values typically ~(5–20) × 10−5 J/m2 and for free energy bound to AP pulse value ~ 3 × 10−14 J. Analysing then the Hodgkin–Huxley mechanism of AP from the point of view of thermodynamics, it can be shown that it is very inefficient, because it dissipates, in a typical nerve fibre with resistive protein-based gated channels, two orders of magnitude more energy than what corresponds to the energy of signal itself [74, 75].
In 2005 T. Heimburg and A. D. Jackson [23] proposed an alternative thermodynamic theory of nerve pulse, generally known as a soliton theory, in which the AP is interpreted not as a primary effect but rather because of propagation of mechanical soliton wave through the membrane, having an intrinsic internal electromechanical coupling. The key point of this model is an assumption that the real membrane preserves most of essential thermodynamic properties of lipid bilayer, especially that the melting (gel-fluid) phase transition takes place only few degrees below normal body temperature. The volume and area compressibility of lipid bilayer reveals at the point of phase transition the non-linearity and frequency dispersion, i.e. the properties which are necessary for generation and propagation of solitary waves—solitons [76].
The soliton model correctly describes non-electric properties observed in experiments during AP propagation, such as the production of heat during lipid transition from fluid to gel phases and the reabsorption of heat as the system reversibly returns to its original state. It also nicely reproduces minute mechanical deformations encountered by the nerve excitation [74]. During the passage of soliton through the membrane, its thickness (in other words its specific capacitance) is changed. Since the membrane is polarized, a localized transmembrane voltage pulse, having properties of AP, appears simultaneously. In addition, the theory accounts for the effects of anaesthetics and of the pressure on nerve activity in a very natural way. The presence of anaesthetic in the membrane, namely, shifts the gel-fluid phase transition to the lower temperature, supressing in denser phase the generation of soliton. Summarizing, the soliton model, having a good predicative power, is explaining many puzzling phenomena.
On the other hand, in the frame of this model some hardly solvable problems have appeared. First, the proposed equation for acoustic waves in tubular structure is somewhat artificial. For example, in order to make the acoustic wave create a genuine soliton traveling with constant speed and without shape distortion throughout the whole nerve fibre, there was inserted a special ad hoc dispersion term [77]. Besides, the estimate for AP velocity in non-myelinated fibre is too high, ~ 175 m/s, whereas for myelinated fibres is paradoxically lower, ~ 80 m/s, being thus quite non-realistic for plausible explanation of myelination effect. Second, as the real axolemma contains many membrane rafts, i. e. the domains enriched with cholesterol and proteins, its mechanical properties must inevitably differ from those of ideal lipid bilayer. It is inhomogeneous, and in domains with ordered liquid phase (see Sect. 3) is more rigid and mechanically linear. Generation of stable solitary waves in such a system is not likely. Therefore, it can be concluded that the theory of solitons in nervous systems is not well-founded with sufficient rigor. The most serious objection against the soliton model is, however, the experimental evidence that electric AP precedes the acoustic signal by about ~250 μs and not vice versa [78]. That is why the AP cannot be the by-product of soliton deformation wave but just the observed minute displacements of axon membrane are direct consequence of passage of electric pulse.
It is apparent that both these theories account more-or-less successfully for various aspects of information transfer in living organisms. Moreover, the realms of phenomena covered by these models are partially disjunctive and both of these approaches have their own flaws. Therefore, there appears a natural question, namely: Can be both these theories reconciled? The answer is negative. Indeed, whereas the Hodgkin–Huxley model is based on dissipative processes within the resistive protein channels in the membrane, being thus strongly irreversible, the soliton theory of AP is nearly reversible or adiabatic, because the heat production during the signal transmission is immediately followed by re-absorption of equal quantity of heat. Evidently, such processes are thermodynamically incompatible.
Nevertheless, if we cannot combine the whole theories as building blocks, we can combine singular pieces of knowledge achieved in the frames of these theories. As an example of such an approach may serve an attempt published by A. El Hady and B. B. Machta [79].
7 An attempt at the scenario of action potential
In this section, we are presenting a sketch of a “physical” scenario of AP in nerve fibres, with endeavour to incorporate in it the most of non-disputable experimental facts. The crucial points and ingredients of the proposed scenario can be outlined as follows.
-
First it is assumed that the nervous signal is primarily of electric nature. This agrees with the observation that electric pulse precedes mechanical deformations of neuron membrane [78]. Such an assumption is thus direct consequence of principle of causality in the sense, “Post hoc ergo propter hoc”.
-
The initiation of AP, i.e. the sudden increase of local transmembrane potential at the membrane of entry synapse is assumed to be equivalent to the injection of K+ ions there. The spreading of such electric disturbance across the whole neuron membrane has then, in accordance with experiment [41] and with arguments given in Sect. 4, the character of diffusion, corresponding to the parabolic time dependence [39].
-
The voltage-gated ionic channels of otherwise non-specified kind must be involved for the realistic shaping of genuine AP (in contrast to our thought experiment in Sect. 4, where only a quasi-stationary diffusion of charging front is discussed). Experimentally the existence of such devices operating in the neuron membrane was conclusively proved by K. S. Cole and H. J. Curtis [62].
-
As we suggest below, the spontaneous operation of these voltage-gated ionic channels is likely due to the electro-mechanical coupling within the axon membrane, particularly to the reversible flexoelectric effect [80]. Moreover, it can be shown that these channels, having a character of simple temporal fissures, behave like partly selective channels for quantum ionic fluxes of Na+ and K+ ions. These selective channels are responsible for the formation of the main depolarization AP peak.
-
Finally, the recovery phase of the AP pulse is controlled by the almost continuously operating selective protein-based channels of Hodgkin–Huxley type, strategically dispersed over the surface of nervous cell. Their main task is to keep, by means of Na+/K+ pumping [81], the internal homeostasis of nervous cell, what requires appreciable energy and oxygen costs [82].
To make this scenario a minimal model, it is necessary to discuss some of these points in more detail.
After the injection of K+ ions into the cytoplasm of the cell, the cytolemma is locally charged revealing a corresponding transmembrane potential drop there, as it was already explained in Sect. 4 (where the thought experiment with model of tubular cell was discussed). The progress of the boundary of the charged domain over the cytolemma is controlled by macroscopic diffusion constant DE, given by formula (6). The resulting long-lasting charging of the whole surface of the cell is, however, e.g. for effective electric signalling by nervous cells practically useless. Neuron must be, namely, prepared for further action in a short time after the pulse transmission. It is thus necessary, immediately after the passage of the boundary of charged domain, to discharge this part of the sphondyloid locally and effectively. The most effective way how to do it, is to interconnect temporarily Gouy’s layers existing on both sides of the cytolemma (axolemma) by an ionic channel. The evidence for such temporarily operating ionic channels provides just the experiment made by K. S. Cole, H. J. Curtis [62].
Since the results of this experiment are quite crucial for any theory of AP, it worth of a short description. In the trough made in insulating material, circulating sea water substituted extracellular somatic fluid. Non-myelinated giant nerve axon taken from squid Loligo pealeii was then inserted into this trough. The axon was provided with standard exciting and measuring electrodes on both its ends. Besides, electrodes for membrane transverse AC impedance measurements were added. The measuring signal of the AC bridge, operating at kHz frequencies, was kept well below ~kT/e, to not influence the shape of AP. While the membrane AC conductance increased during the passage of AP from its resting value of 10 S/m2 to 360 S/m2 the membrane capacitance decreased about less than < 2%. The onset of the AC resistance and capacitance changes coincided quite closely with the inflection point on the rising phase of AP curve (see Fig. 2). The experiment thus clearly indicates, that at voltages of ~ 45 mV above the resting potential, there exists the electrical connection between both sides of axolemma, accompanied by a small reduction of its chargeable area.
How can sudden appearance of such channels be plausibly accounted for? As we believe, a promising candidate for the mechanism of temporal creation of non-selective voltage-gated channels is the flexoelectric activity of the axolemma. Flexoelectricity, a mechano-electric phenomenon responsible for curvature-induced polarization of lipid bilayer-based bio-membranes, can be described as follows [80]. The main, purely geometrical characteristics of curved surfaces, is the mean curvature of a surface which is defined as
where r1 and r2 are principal radii at a given point of the surface and ν is the unit normal vector. Deviation of the membrane curvature from its relaxed equilibrium value measures the splay deformation of lipid chains, inducing, in the core of the lipid bilayer, the additional electric field E = E ν. The basic constitutive relation defining then the flexoelectric effect reads
where PS is value of dipole moment per unit area of the membrane in C/m and f is the flexoelectric coefficient measured in charge units, C (coulombs). In addition to direct flexoelectric effect, there exists also a converse thermodynamically complementary effect, which is due to the Maxwell’s reciprocal relations controlled by identical formula with the same coefficient f. Accordingly, the external electric field can change curvature and dimensions of the membrane. Applying formula (15) to the case of cylindrical tube-like membrane, the following working formula can be derived immediately
where R0 is the equilibrium axon radius (Notice, d = 2R0). For axolemma, some of the values used for lipid bilayers, which are ranging from f ~ 2 × 10−18 to 2 × 10−17 C [83], can be taken for flexoelectric coefficient. Similar value, f = 1.2 × 10−17 C, is also reported in [80] for converse flexoelectric coefficient of neurons from bovine brain measured at 500 Hz (i.e. period ~2 ms). Inserting then into (16) this figure together with other variables, the representative values of which were already given above, dR = 10−9 m [84], a = 2 × 10−9 m, εA = 2, R0 = 7 × 10−6 m, we obtain for corresponding change of depolarization potential an estimate dφ ≈ 48 mV, which is obviously comparable with the typical amplitude of AP (see Fig. 2). The flexoelectric effect can thus realistically account for observed swelling of axolemma during the passage of AP. The local area exposed to the action potential peak is also obviously changed in the same ratio as dR/R0. Lipid bilayer-based membranes, which behave nearly as the perfect soft matter [85], where the increase of the area is fully compensated by the thickening of the layer, have very low stretch modulus κS = 0.25 N/m, so that their critical rupture strain is only of order of ~ 1% [32]. Therefore, it is very likely that the deformation due to the transit of AP may cause temporary fissures in the membrane, in fact the non-selective ionic channels, through which the spontaneous diffusion flows of Na+ and K+ ions start (see Fig. 3). These ionic flows equalize the Nernst potentials existing between osmotically unbalanced electrolytes inside and outside of the axon. It is clear that the spontaneous appearance of such channels, triggering in both sides of axolemma the onset of reversion of ionic flows, should correspond to the inflection point on the AP curve.
The permeation of ions through the fissure (pore) made in the axolemma is facilitated by the fact, that the solvation self-energy of an ion inside the membrane with small εA, surrounded by aqueous environment with high dielectric constant εW = 81, is electrostatically lowered by an amount [86]
From this formula we immediately obtain for a = 2 × 10−9 m and εA = 2 an estimate ES ≈ 4 × 10−20 J = 0.249 eV ≈ 9 kT. Such a lowering of self-energy inside the pore, which thus represents a negative potential barrier, permits the ions to cross the membrane without an appreciable energy dissipation. Naturally, resulting cationic flow locally reverts the increase of AP, which then starts to decrease. If the AP decreases again approximately below the potential of the said inflection point, the fissures are closing, so that the potential decrease is slowed down.
Let us inspect the spontaneous redistribution of Na+ and K+ cations during the appearance of these channels in more detail. The concentration drops of the cations between ECF and cytoplasm are Δ[X+]. Using for diffusion constant DQ(X+) formula (12), the modified “quantum” Fick’s law [87, 88] may be written as
where J(X+) is the density of ion flux measured in mol/m2 s. For sodium and potassium ions we have then following concentration drops (see Table 1): Δ[Na+] = 150 − 15 = 135 mol/m3, Δ[K+] = 5.5 − 150 = − 144.5 mol/m3 and corresponding diffusion constants DQ(Na+) = 1.37 × 10−9 m2/s, DQ(K+) = − 8.09 × 10−10 m2/s. The resulting flux densities computed from (18) thus are
These two flux densities have opposite signs (plus sign corresponds to the direction from ECF to cytoplasm), i.e. the cations Na+ and K+ are simultaneously flowing in opposite directions through the non-selective channel. Since the Na+ flux density absolutely prevails over the K+ flux density, i.e.
any non-selective channel thus effectively behaves as a partially selective one. This fact nicely accounts for the fact that in the first phase of AP characterized by a sharp peak, strictly selective highly resistive protein-based channels are not necessary; the observed difference in fluxes of Na+ and K+ ions is a direct consequence of their different masses. Moreover, since the opening and closing of the channels is reversible and the selection of ions requires no extra energy costs, the processes in this phase of AP should be practically dissipationless. It makes a contrast with the process encountered in the recovery phase of AP. The decrease of signal amplitude is there eventually stopped and reverted very probably by almost continuously operating selective protein-based sodium/potassium adenosine triphosphatase (ATP) pumps (or “protein selective gated channels” known e. g. from Hodgkin–Huxley theory [81]). They operate till they reach on both sides of the membrane, equilibrium ion concentration values, establishing resting potential, which is characteristic for homeostasis. This process, which is common for practically all cells in the living organisms, is simultaneously the most energy- and oxygen- consuming phase of AP. In this connection it should be stressed that there is an essential difference between the channels realized by the fissures in the membrane and the protein-based channels of the Hodgkin–Huxley type. The simple fissures are only temporary and appears during every new passage of AP at different places, having for spontaneous quantum flow of ions almost negligible resistance. In contrast, the selective protein-based channels which are permanently localized within the membrane and are working against the ion concentration gradients on the costs of extra chemical energy provided by ATP, reveal a rather high effective resistance. These facts thus can account for the qualitative difference between energy dissipation during initial and recovery phases of AP.
8 Conclusions
In this paper we have made a brief critical, partially historical review of empirical facts and main theories concerning the propagation of nervous signals in biological neural networks. Finally, we have worked out a simple scenario and sketch of physical minimal model of progression of the action potential (AP) in nervous fibres. The key points of this scenario and model may be summarized as follows.
After the stimulation (i.e. electric charging) of a synapse, the electric disturbance starts to diffuse across the neuron membrane, inducing there the transmembrane potential difference, AP.
Due to the flexoelectric effect, the increase of transmembrane potential has two consequences, namely, the local increase of diameter of the nervous fibre and the appearance of temporary membrane fissures there.
The fissures then serve as channels for the spontaneous flows of Na+ and K+ ions striving to equalize the ion concentration differences existing between extracellular fluid and cytoplasm. Since the sodium and potassium cations are quantum particles of different masses, the fissures effectively behave as gated, partially selective, non-dissipative channels.
The decrease of transmembrane potential, due to the equalization of cation concentrations on both sides of membrane, then lead to the decrease of AP amplitude and finally to the shutting down of fissures.
The following recovery phase establishing the homeostasis conditions, is then controlled by the operation of dissipative protein-based channels with Na+/K+ ion pumps of Hodgkin–Huxley type.
In summary, our minimal model, being far from to be perfect or complete, reflects most of the non-disputable facts. It may thus provide a starting point for further development of the theory of stimulation in living creatures. In future, we would like to study the proposed model in more details, especially:
-
1.
The creation mechanism of membrane fissures using the approach based on the fluctuation–dissipation theorem.
-
2.
Modeling of ionic patterns likely arising in the vicinity of the cell membrane and ionic channels because of reaction–diffusion kinetics.
-
3.
The entropy production due to the quantum diffusion of cations passing through the temporary fissures in the membrane.
Data Availability Statement
No data associated with the manuscript.
References
A. Galvani, De viribus electricitatis in motu masculari commentarius. (Bononiae, 1791); Russian translation in: Izbrannye raboty o zhivotnom elektritschestve, ed. by E. E. Goldenberg, A. V. Lebedinskii (OGIZ, Leningrad, 1937)
E. du Bois-Raymond, Gesammelte Abhandlungen zur Allgemeinen Muskel- und Nervenphysik (Veit & Co, Leipzig, 1877)
I. Jahn, Die Anfänge der instrumentellen Elektrobiologie in den Briefen Humboldts an Emil du Bois-Raymond. Medizin Hist. J. 2, 135–156 (1967)
H. von Helmholtz: Messungen über die Fortpflanzungsgeschwindigkeit der Reizung in dem Nerven. Arch. Anat. Physiol. Wiss. Med. (1852) 199–216
J. Bernstein, Untersuchungen zur Thermodynamik der bioelektrischen Ströme. Pflügers Arch. Ges. Physiol. 92, 521–562 (1902)
L. Hermann, Zur Theorie der Erregungsleitung und der elektrischen Erregung. Pflügers Arch. Ges Physiol. 75, 574–590 (1899)
O. Torres-Fernández, The Golgi silver impregnation method. Biomedica 26, 498–508 (2006)
C. Golgi, Sur l’anatomie microscopique des organes centraux du systéme nerveux. Arch. Ital. Biol. 7, 15–47 (1886)
S. Ramón y Cajal, The structure and connections of neurons. (Nobel lecture, Dec. 12, 1906)
W. Waldeyer-Hartz, Über einige neuere Forschungen im Gebiete der Anatomie des Centralnerven Systems. Deutsche med. Wochenschrift 17, 1213–1218 (1891)
S.D. Barberies, Cajal’s law of dynamic polarization: mechanism and design. Philosophies 3, 11–26 (2018)
D.M. Vasudevan, S. Sreekumari, K. Vaidyanathan, Textbook of Biochemistry for Medical Students, 9th edn. (Jaypee Brothers Medical Publishers Ltd., New Delhi, 2019)
S.S. Maxwell, Is the conduction of the nerve impulse a chemical or a physical process? J. Biol. Chem. 3, 359–385 (1907)
C.S. Sherrington, The central nervous system. In: A Textbook of Physiology, 7th Ed. - M. Foster, Part III. (Mac Millan, London, 1897)
J.C. Eccles, From electrical to chemical transmission in central nervous system. Notes and Records of the Roy. Soc. London 30, 219–230 (1976)
E.R. Kandell, J.H. Schwartz, T.M. Jessell, S.A. Siegelbaum, A.J. Hudspeth (eds.), Principles of Neural Science, 5th edn. (McGraw-Hill, New York, 2013)
E.D. Adrian, The all-or-none principle in nerve. J. Physiol. 47, 460–474 (1914)
A.L. Hodgkin, A.F. Huxley, Resting and action potentials in single nerve fibres. J. Physiol. 104, 176–195 (1945)
A.L. Hodgkin, B. Katz, The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 108, 37–77 (1949)
I. Tasaki, T. Takeushi, Weitere Studien über den Aktionsstrom. Pflüg. Arch. ges. Physiol. 254, 764–782 (1942)
W. Rall: Core conductor theory and cable properties of neurons. In: The Nervous System, Cellular Biology of Neurons, Ed. E. R. Kandell (Am Physiol. Soc., Betheseda, 1977)
L. R. Squire, D. Berg, F. E. Bloom, S. du Lac, A. Ghosh, N. C. Spitzer: Fundamental Neuroscience, 4th ed. (Elsevier, Acad. Press, Amsterdam, 2012)
T. Heimburg, A.D. Jackson, On soliton propagation in bio-membranes and nerves. Proc. Natl. Acad. Sci. U.S.A. 102, 9790–9795 (2005)
R.K. Hobbie, B.J. Roth, Intermediate Physics for Medicine and Biology, 4th edn. (Springer, New York, 2007)
T.A. Ghanem, K.D. Breneman, R.D. Rabbitt, H.M. Brown, Ionic Composition of Endolymph and Perilymph in the Inner Ear of the Oyster Toadfish. Biol. Bull. 214, 83–90 (2008)
B. Hille, Ionic channels of excitable membranes (Cambridge University Press, Cambridge, 1992)
W. Nernst, Die elektromotorische Wirksamkeit der Ionen. Z. Physikalische Chemie 4, 129–181 (1889)
P. Debye, E. Hückel, Zur Theorie der Elektrolyte I. Phys. Z. 24, 185–206 (1923)
E. Fukada, I. Yasuda, Piezoelectric effects in collagen. Japan. J. Appl. Phys. 3, 117–121 (1964)
C. Gabriel, S. Gabriel, E. Corthout, The dielectric properties of biological tissues. Phys. Med. Biol. 41, 2231–2249 (1996)
M. Shamos, L. Lavine, Piezoelectricity as a Fundamental Property of Biological Tissues. Nature 213, 267–269 (1967)
R. Faller, UCD Biophysics 241: Membrane Biology (University of California, Davis, 2015)
T. Heimburg, A. Blicher, L. D. Mosgaard, K. Zecchi: Electromechanical properties of biomembranes and nerves. Journal of Physics, Conf. Ser. 558 (2014) 012018
T. Heimburg: The important consequences of reversible heat production in nerves and the adiabaticity of the action potential. (arXiv:2002.06031v2 [physics.bio-ph] 7 Aug 2020)
G. Gramse, A. Dols-Perez, M.A. Edwards, L. Fumagalli, G. Gomila, Nanoscale Measurement of the Dielectric Constant of Supported Lipid Bilayers in Aqueous Solutions with Electrostatic Force Microscopy. Biophys. J. 104, 1257–1262 (2013)
M. Gouy, Sur la constitution de la charge électrique à la surface d‘un électrolyte. J. Phys. Theor. Appl. 9, 457–468 (1910)
W.G.V. Rosser, What makes an electric current flow? Am. J. Phys. 31, 884–885 (1963)
A. Koch Torres Assis, J. A. Hernandes: Elektrischer Strom und Oberflächenladungen. (C. Roy Keys Inc., 2013, Montreal)
J.J. Mareš, P. Hubík, V. Špička, Diffusive propagation of nervous signals and their quantum control. Eur. Phys. J. Spec. Top. 227, 2329–2347 (2019)
J.J. Mareš, V. Špička, P. Hubík, Possible role of extracellular tissue in biological neural networks. Eur. Phys. J. Spec. Top. 230(2021), 1089–1098 (1906)
R. Milo, R. Phillips, Cell Biology by the Numbers (Garland Science, New York, 2016)
D. Purves et al. (eds.), Neuroscience, 2nd edn. (Sinauer Associates Inc, New York, 2001)
B. Frankenhaeuser, Saltatory conduction in myelinated nerve fibres. J. Physiol. 118, 107–112 (1952)
B. Katz, O.H. Schmitt, Electric interaction between two adjacent nerve fibres. J. Physiol. (London) 97, 471–488 (1940)
J.G. Needham, General Biology-A book of Outlines and Practical Studies for General Student. (Legare Street Press, 2022)
J.-M. Lévi-Leblond, F. Balibar, Quantics, rudiments of quantum physics (North-Holland, Amsterdam, 1990)
R. Fürth, Über einige Beziehungen zwischen klassischer Statistik und Quantenmechanik. Z. f. Phys. 81, 143–162 (1933)
J.J. Mareš, J. Šesták, J. Stávek, H. Ševčíková, J. Krištofik, P. Hubík, Do periodic chemical reactions reveal Fürth’s quantum diffusion limit? Physica E 29, 145–149 (2005)
L. de la Peña, A.M. Cetto, The Quantum Dice – An Introduction to Stochastic Electrodynamics (Kluwer, Academic Publishers, Dordrecht, 1996)
E. Brunner, Reaktiongeschwindigkeit in heterogenen Systemen. Z. Phys. Chem. 47, 56 (1904)
A. Turing, The chemical basis of morphogenesis. Phil. Trans. R. Soc. Lond. B 237, 37–72 (1952)
J.J. Mareš, J. Stávek, J. Šesták, Quantum aspects of self-organized periodic chemical reactions. J. Chem. Phys. 121, 1499–1503 (2004)
S. Kondo, T. Miura, Reaction–diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010)
F.F. Runge, R.E. Liesegang, B.P. Belousov, A.M. Zhabotinsky, in Selbsorganisation Chemischer Strukturen, ed. L. Kuhnert, U. Niedersen (Ostwald’s Klassiker, Verlag H. Deutsch, Frankfurt am Main, 1987)
A. Einstein, Investigations on the theory of the Brownian movement (Dover Publications Inc, New York, 1956)
R. Appali, S. Petersen, U. van Rienen, A comparison of Hodgkin-Huxley and soliton neural theories. Adv. Radio Sci. 8, 75–79 (2010)
M. Peyrard, How is information transmitted in a nerve? J. Biol. Phys. 46, 327–341 (2020)
A.L. Hodgkin, Evidence for electrical transmission in nerve. J. Physiol. 90, 183–210 (1937)
A.L. Hodgkin, A.F. Huxley, Action Potentials Recorded from Inside a Nerve Fibre. Nature 144, 710–711 (1939)
A.L. Hodgkin, Chance and design in electrophysiology: an informal account of certain experiments on nerve carried out between 1934 and 1952. J. Physiol. 263, 1–21 (1976)
A.L. Hodgkin, The relation between conduction velocity and the electrical resistance outside a nerve fibre. J. Physiol. 94, 560–570 (1939)
K.S. Cole, H.J. Curtis, Electric impedance of the squid giant axon during aktivity. J. Gen. Physiol. 22, 649–670 (1939)
A.L. Hodgkin, A.F. Huxley, A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
A.L. Hodgkin, R.D. Keynes, The potassium permeability of giant nerve fibre. J. Physiol. 128, 61–88 (1955)
M. Häusser, The Hodgkin – Huxley theory of the action potential. Nat. Neurosci. Suppl. 3, 1165 (2000)
W. Thomson: On the theory of electric telegraph. Proc. R. Soc. Lond. 7 (1854–1855) 382–399
O. Heaviside: Electromagnetic Theory, Vol.II. (The Electrician Co. Ltd., London, 1899; Reprint: Cambridge University Press, New York, 2011)
A. Pietak, M. Levin: Exploring Instructive Physiological Signaling with the Bioelectric Tissue Simulation Engine. Front. Bioeng. Biotechnol. 4 (2016) 55(26)
T.P. Feng, The heat production of nerve. Ergeb. Physiol. Biol. Chem. Exp. Pharmakol. 38, 73–132 (1936)
A.V. Hill, Heat Production of Muscle and Nerve. Nature 135, 721–724 (1935)
J.W. Howarth, J.M. Ritchie, D. Stagg, The initial heat production in garfish olfactory nerve fibres. Proc. R. Soc. Lond. B 205, 347–367 (1979)
K. Iwasa, I. Tasaki, R.C. Gibbons, Swelling of nerve fibres associated with action potentials. Science 210, 338–339 (1980)
A.C.L. de Lichtervelde, J.P. Souza, M.Z. Bazant, Heat of nervous conduction: a thermodynamic framework. Phys. Rev. E 101, 022406 (2020)
S. Andersen, A.D. Jackson, T. Heimburg, Towards a thermodynamic theory of nerve pulse propagation. Progr. Neurobiol. 88, 104–113 (2009)
T. Heimburg, A.D. Jackson, On the action potential as a propagating density pulse and the role of anesthetics. Biophys. Rev. Lett. 2, 57–78 (2007)
S. Manukure, T. Booker, A short overview of solitons and applications. Partial Differential Equations in Applied Mathematics 4, 100140 (2021)
P.C. Bressloff, Waves in Neural Media (Springer, Berlin, 2014)
B.C. Hill, E.D. Schubert, M.A. Nokes, R.P. Michelson, Laser Interferometer Measurement of Changes in Crayfish Axon Diameter Concurrent with Action Potential. Science 196, 426–428 (1977)
A. El Hady, B.B. Machta, Mechanical surface waves accompany action potential propagation. Nat. Commun. 6, 6697 (2015)
A.G. Petrov, Flexoelectricity of model and living membranes. Biochem. Biophys. Acta. 1561, 1–25 (2001)
J.C. Skou, The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochem. Biophys. Acta. 23, 394–401 (1957)
R. Vávra, J. Mělka, Nárys moderní elektrofysiologie (Melantrich Ltd., Prague, 1949)
L.P. Liu, P. Sharma, Flexoelectricity and thermal fluctuations of lipid bilayer membranes. Phys. Rev. E 87, 032715 (2013)
B.C. Hill, E.D. Schubert, M.A. Nokes, R.P. Michelson, Laser interferometer measurements of changes in crayfish axon diameter concurrent with action potential. Science 196, 426–428 (1977)
M. Mert-Terzi, M. Deserno, J.F. Nagle, Mechanical properties of lipid bilayers: a note on the Poisson ratio. Soft Matter 15, 9085–9092 (2019)
A. Parsegian, Energy of an Ion crossing a Low Dielectric Membrane. Nature 221, 844–846 (1969)
A. Fick, Ueber Diffussion. Ann. der Phys. 94, 59–86 (1855)
X. Bian, C. Kim, G.E. Karniadakis, 111 years of Brownian motion. Soft Matter 12, 6331–6346 (2016)
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mareš, J.J., Špička, V. & Hubík, P. On physical processes controlling nerve signalling. Eur. Phys. J. Spec. Top. 232, 3561–3576 (2023). https://doi.org/10.1140/epjs/s11734-023-01045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-01045-7