Abstract
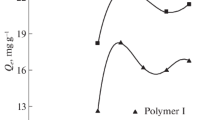
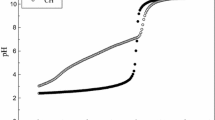
The results of studying the processes of sorption of copper(II) ions from aqueous solutions by unmodified and modified chitosan granules are presented. As a result of the surface immobilization of nickel 2-ethylimidazolate in the presence of a surfactant, the maximum sorption capacity of the sorbent based on chitosan increases to 19.5 mol/kg. In this case, there is a reduction in the time to reach adsorption equilibrium in the “sorbent–CuSO4 solution” system up to 60 min and an increase in the degree of extraction of copper(II) ions up to 99.9%. IR spectra, microphotographs, and the elemental composition of initial and modified chitosan granules were obtained.






Similar content being viewed by others
REFERENCES
Chen, Y., Bai, X., and Ye, Z., Nanomaterials, 2020, vol. 10, no. 8, p. 1481.
Azimi, A., Azari, A., Rezakazemi, M., et al., ChemBioEng Rev., 2017, vol. 4, no. 1, p. 37.
Xu, S., Lv, Y., Zeng, X., and Cao, D., Chem. Eng. J., 2017, vol. 323, p. 502.
Rasheed, T., Chemosphere, 2020, vol. 259, p. 127369.
Karadaş, C. and Kara, D., Food Chem., 2017, vol. 220, p. 242.
Velasco-Garduño, O., Martínez, M.E., Gimeno, M., et al., Environ. Sci. Pollut. Res., 2020, vol. 27, p. 28527.
Rehman, M., Liu, L., Wang, Q., et al., Environ. Sci. Pollut. Res., 2019, vol. 26, p. 18003.
Li, X., Wang, B., Cao, Y., Zhao, S., et al., ACS Sustainable Chem. Eng., 2019, vol. 7, p. 4548.
Manos, G. and Dunne, L., Nanomaterials, 2018, vol. 8, no. 10, p. 818.
Huang, Y., Zeng, X., Guo, L., et al., Sep. Purif. Technol., 2018, vol. 194, p. 462.
Li, M., Ren, G., Wang, F., et al., Inorg. Chem. Front., 2019, vol. 6, no. 5, p. 1129.
Chakraborty, R., Asthana, A., Singh, A.K., et al., Int. J. Environ. Anal. Chem., 2020, vol. 100, p. 1.
Arora, R., Mater. Today: Proc., 2019, vol. 18, p. 4745.
Wang, K., Tao, X., Xu, J., et al., Chem. Lett., 2016, vol. 45, no. 12, p. 1365.
Liu, L., Yang, W., Gu, D., et al., Front. Chem., 2019, vol. 7, p. 607.
Sugashini, S. and Gopalakrishnan, S., Res. J. Chem. Sci., 2012, vol. 2, no. 6, p. 55.
Huang, R., Yang, B., and Liu, Q., J. Appl. Polym. Sci., 2013, vol. 129, no. 2, p. 908.
Boamah, P.O., Huang, Y., Hua, M., et al., Carbohydr. Polym., 2015, vol. 122, p. 255.
Fernandes Queiroz, M., Melo, K., Sabry, D., et al., Mar. Drugs, 2015, vol. 13, p. 141.
Zhang, Y., Jia, Y., Li, M., et al., Sci. Rep., 2018, vol. 8, no. 1, p. 1.
Funding
This work was funded by the Ministry of Science and Higher Education of the Russian Federation (project no. FZZW–2020–0010).The study was carried out using the resources of the Center for Shared Use of Scientific Equipment of the ISUCT (with the support of the Ministry of Science and Higher Education of Russia, grant no. 075-15-2021-671).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fufaeva, V.A., Nikiforova, T.E. Extraction of Copper Ions by Chitosan-Based Sorbents Modified with Nickel 2-Ethylimidazolate. Prot Met Phys Chem Surf 58, 262–268 (2022). https://doi.org/10.1134/S2070205122020058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205122020058