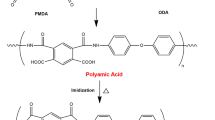
Abstract
The review addresses methods for modification of phenolic resins via introducing reactive compounds into synthesis and the reactions of hydroxyl phenolic and methylol groups which allow the preparation of adhesives with improved performance characteristics.


Similar content being viewed by others
REFERENCES
D. A. Aronovich and A. P. Petrova, “Modern phenolic adhesives. Part I. Influence of modifying additives,” Polymer Science, Series D 16, 777–792 (2023).
C. P. R. Nair, “Non-conventional phenolic resins—an overview on recent advances,” J. Sci. Ind. Res. 61, 17–33 (2002).
C. P. R. Nair, “Advances in addition-cure phenolic resins,” Prog. Polym. Sci. 29, 401–498 (2004).
L. Pilato, “Resin Chemistry,” in Phenolic Resins: A Century of Progress (Springer, New York, 2010).https://doi.org/10.1007/978-3-642-04714-5_4.
A. Puzari, “Novolac Resin: Novel Functional Materials,” in Chemistry of Phenolic Compounds: State of the Art, Ed. by J. B. Baruah (Nova Science Publish., 2010).
K. Tang, A. Zhang, T. Ge, et al., “Research progress on modification of phenolic resin,” Mater. Today Commun. 2020, 101879 (2020).
A. P. Aliyeva, “Composite materials based on phenol formaldehyde resins,” Prom. Proizvod. Ispolzov. Elastomerov, No. 1, 34–43 (2021).
Y. Xu, L.Guo, H. Zhang, et al., “Research status, industrial application demand and prospects of phenolic resin,” RSC Adv. 9, 28924–28935 (2019).
W. Qiao, S. Li, G. Guo, et al., “Synthesis and characterization of phenol-formaldehyde resin using enzymatic hydrolysis lignin,” J. Ind. Eng. Chem. 21, 1417–1422 (2015).
Y. Zhang, N. Li, Z. Chen, et al., “Synthesis of high-water-resistance lignin-phenol resin adhesive with furfural as a crosslinking agent,” Polymers 12, 2805 (2020).
P. Jia, F. Song, Q. Li, et al., “Recent development of cardanol based polymer materials—A review,” J. Renewable Mater. 7, 601–619 (2019).
H. Kumar, S. K. Tripathi, S. Mistry, and G. Bajpai, “Synthesis, characterization and application of coatings based on epoxy novolac and liquid rubber blend,” E-J. Chem. 6, 1253–1259 (2009).
A. K. Tiwari, H. Kumar, R. Bajpai, and S. K. Tripathi, “Preparation of blends of epoxidised novolac resin and carboxylic terminated polybutadiene (CTPB) liquid rubber and evaluation of their physico-chemical characteristic,” J. Chem. Pharm. Res. 2, 172–178 (2010).
V. D. Ramos, H. M. Costa, V. L. P. Soares, and S. V. R. Nascimento, “Modification of epoxy resin: A comparison of different types of elastomer,” Polym. Test. 24, 387–394 (2005).
C. Gouri, R. Ramaswamy, and K. Ninan, “Studies on the adhesive properties of solid elastomer-modified novolac epoxy resin,” Int. J. Adhes. Adhes. 20, 305–314 (2000).
C. Gouri, “Elastomer modification of epoxy based film adhesives: adhesive and mechanical properties,” J. Adhes. Sci. Technol. 16, 1569–1583 (2002).
C. M. Bhuvaneswari, S. S. Kale, G. Gouda, P. Jayapal, and K. Tamilmani, “Elastomers and Adhesives for Aerospace Applications,” in Aerospace Materials and Material Technologies (Springer, Singapore, 2017).
M. S. Trizno and E. V. Moskalev, Adhesives and Binding (Khimiya, Leningrad, 1980) [in Russian].
E. M. Rodionova, B. A. Zaitsev, and M. S. Trizno, “Modification of epoxy-novolac block copolymers with Rolivsan,” Zh. Prikl. Khim. 65, 2295–2299 (1992).
A. Salimi, H. Omidian, and M. J. Zohuriaan-Mehr, “Mechanical and thermal behavior of modified epoxy-novolak film adhesives,” J. Adhes. Sci. Technol. 17, 1847–1861 (2003).
Technical Data Sheet Loctite R EA 9497 TM. http://tds-loctite-ea-9497-ru-2014.pdf.
O. Chailee, T. Parnklang, P. Mora, et al., “Epoxy-based composite adhesives with improved lap shear strengths at high temperatures for steel-steel bonded joints,” J. Appl. Polym. Sci. 137, 49371 (2020).
K. R. Akhmadieva, R. R. Mukhametov, E. V. Dolgova, and Yu. I. Merkulova, “Heat-resistant film adhesive for structural purposes,” Nov. Materialoved. Nauka Tekh., No. 2, 57–63 (2016).
L. Huang, C. Wang, and Y. Lu, “Thermal and moisture adsorption properties of cyanate ester modified epoxy resin and fiber-glass composites,” J. Reinf. Plast. Compos. 27, 725–738 (2008).
R. Pal, S. Sudhi, and R. Raghavan, “Fabrication and evaluation of structural film adhesive using oxazolidinone modified novolac epoxy resin,” J. Appl. Polym. Sci. 136, 47520 (2019). https://doi.org/10.1002/app.47520
Safety Data Sheet. http://msds-loctite-ea-3478-2020.pdf.
A. M. Atta, M. I. Abdou, A.-A. A. Elsayed, and M. E. Ragab, “New bisphenol novolac epoxy resins for marine primer steel coating applications,” Prog. Org. Coat. 63, 372–376 (2008).
R. Mirski, D. Dziurka, and J. Łęcka, “Propertries of alcohol-modified PF resin used in the production of wood-based materials,” Electron. J. Pol. Agric. Univ. 8, No. 22 (2005).
M. H. Choi, H. Y. Byun, and I. J. Chung, “The effect of chain length of flexible diacid on morphology and mechanical property of modified phenolic resin,” Polymer 43, 4437–4444 (2002).
P. S. Parameswaran, PhD Thesis (Cochin Univ. Sci. Technol., India, 2009).
M. N. Amiraslanova, A. M. Mustafaev, R. A. Rustamov, et al., “Synthesis of nitrogen-containing phenol-formaldehyde oligomers grafted with vegetable oils,” Plast. Massy, Nos. 3–4, 28–31 (2017).
M. N. Amiraslanova, A. M. Mustafaev, M. D. Ibragimova, et al., “Study of the physical and mechanical properties of protective coatings based on nitrogen-containing monoalkyl (C8–C12) phenol-formaldehyde oligomers grafted with soybean oil,” Plast. Massy, Nos. 11–12, 47–49 (2018).
L. Guo, L. Wang, and J. Li, “Study on modification of phenol formaldehyde resin adhesive with ionic liquid /,” in Proceedings of the 2nd International Conference on Electronic and Mechanical Engineering and Information Technology, 2012, pp. 1910–1913. https://www.atlantis-press.com/article/3640.pdf
Sh.-ch. Qi, G. Han, H.-r. Wang, et al., “Synthesis and characterization of carborane bisphenol resol phenolic resins with ultrahigh char yield,” Chin. J. Polym. Sci. 33, 1606–1617 (2015). https://doi.org/10.1007/s10118-015-1712-1
P. M. Valetskii and A. P. Petrova, “Polymer adhesives based on carborane-containing compounds,” Trudy VIAM (2004). https://viam.ru/sites/default/files/scipub/2004/2004-204186.pdf.
J. Gao, Y. Liu, and F. Wang, “Structure and properties of boron-containing bisphenol-a formaldehyde resin,” Eur. Polym. J. 37, 207–210 (2001).
Y. Zhu, Q. Zhang, X. Meng, et al., “Adhesive joint properties of advanced carbon/ceramic composite and tungsten–copper alloy for the hybrid rocket nozzle,” Int. J. Adhes. Adhes. 102, 102670 (2020).
J. Gao, X. Su, and L. Xia, “Synthesis and structure characterization of boron-nitrogen containing phenol formaldehyde resin,” Int. J. Polym. Mater. 54, 949–961 (2005). https://doi.org/10.1080/009140390504762
S. Wang, X. Jing, Y. Wang, and J. Si, “Synthesis and characterization of novel phenolic resins containing aryl-boron backbone and their utilization in polymeric composites with improved thermal and mechanical properties,” Polym. Adv. Technol. 25, 152–159 (2014). https://doi.org/10.1002/pat.3216
J. Gao, X. Li, W. Wu, and H. Lin, “Octa(aminophenyl) polyhedral oligomeric silsesquioxane/boron-containing phenol-formaldehyde resin nanocomposites: synthesis, cured, and thermal properties,” Polym. Compos. 32, 829–836 (2011). https://doi.org/10.1002/pc.21105
C. P. R. Nair, D. Mathew, and K. N. Ninan, “Imido-phenolictriazine network polymers derived from maleimide-functional novolac,” Eur. Polym. J. 37, 315–321 (2001).
C. P. R. Nair, R. L. Bindu, and K. N. Ninan, “Phenolic resins bearing maleimide functions; synthesis and characterization,’ J. Polym. Sci., Part A: Polym. Chem. 38, 641–652 (2000).
C. Gouri and R. Ramaswamy, “Adhesive and thermal characteristics of maleimide-functional novolac resins,” J. Appl. Polym. Sci. 73, 695–105 (1999).
C. Gouri, C. P. R. Nair, and R. Ramaswamy, “Effect of elastomer modification on the adhesive characteristics of maleimide-functional phenolic resins,” J. Appl. Polym. Sci. 74, 2321–2332 (1999).
S. A. Moroz, S. G. Gorbachev, and O. V. Chekina, “Structure and properties of allylphenol-formaldehyde resins,” Plast. Massy, No. 8, 34–35 (1987).
C. Gouri, C. P. R. Nair, and R. Ramaswamy, “Adhesive characteristics of alder-ene adduct of diallyl bisphenol A novolac and bisphenol A bismaleimide,” High Performance Polymers 12, 497–514 (2000).
Y. Yan, X. Shi, J. Liu, et al., “Thermosetting resin system based on novolak and bismaleimide for resin-transfer molding,” J. Appl. Polym. Sci. 83, 1651–1657 (2002).
C. Gouri, C. P. R. Nair, and R. Ramaswamy, “High-temperature adhesives based on alder-ene reaction of diallyl bisphenol A novolac and bismaleimide: effect of BMI structure and novolac molar mass,” Polym. Polym. Compos. 11, 311–320 (2003). https://doi.org/10.1177/096739110301100406
A. Gu, G. Liang. and L. Lan, “Modification of polyaralkyl[-phenolic resin and its copolymer with bismaleimide,” J. Appl. Polym. Sci. 59, 975–979 (1996).
J. Hao, W. Rumin, F. Shameel, and Zh. Shuirong. “Properties and curing behavior of reactive blended allyl novolak with bismaleimide using dicumyl peroxide as a novel curing agent,” J. Appl. Polym. Sci. 132 (2015).
A. P. Petrova and G.V. Malysheva, Glue, Adhesive Binder and Adhesive Prepregs: Tutorial (VIAM, Moscow, 2017) [in Russian].
A. P. Petrova and V. M. Buznik, “Performance of adhesives and materials based on them in conditions close to the coastal conditions of the Arctic,” in Proceedings of the Scientific and Technical Conference on Adhesive Materials (VIAM, Moscow, 2016), pp. 13–21.
B. K. Kandola, L. Krishnan, D. Deli, et al., “Fire and mechanical properties of a novel free-radically cured phenolicresin based on a methacrylate-functional novolac and of its blends with an unsaturated polyester resin,” RSC Adv., No. 43, 1–32 (2015).
C. P. R. Nair, V. Dhanya, and C. Gouri, “Dual cure propargyl novolac-epoxy resins: synthesis and properties,” Polym. Polym. Compos. 12, 43–53 (2004).
M. I. Shatirova and T. M. Naibova, “Synthesis of glycidyl and thioglycidyl ethers of the diacetylene series and their use as a modifier of phenol-formaldehyde oligomers,” Izv.Vyssh. Ucheb. Zaved. Ser. Khim. 62, 61–69 (2019).
K. Tang, A. Zhang, T. Ge, et al., “Research progress on modification of phenolic resin,” Mater. Today Commun. 101879 (2020).
S. Li, Y. Han, F. Chen, et al., “The effect of structure on thermal stability and anti-oxidation mechanism of silicone modified phenolic resin,” Polym. Degrad. Stab. 124, 68–76 (2016). https://doi.org/10.1016/j.polymdegradstab.2015
C. Wang and Y. Huang, “Study on preparation thermosetting phenolic resin modified with silicone and its adhesive properties,” Acta Mater. Compos. 21, 50–54 (2004).
S. Li, H. Li, Z. Li, et al., “Polysiloxane modified phenolic resin with co-continuous structure,” Polymers 120, 217–222 (2017).
S. Li, F. Chen, Y. Han, et al., “Enhanced compatibility and morphology evolution of the hybrids involving phenolic resin and silicone intermediate,” Mater. Chem. Phys. 165, 25–33 (2015). https://doi.org/10.1016/j.matchemphys.2015.07
J. Yun, L. Chen, X. Zhang, et al., “Synthesis and structure evolution of phenolic resin silicone hybrid composites with improved thermal stability,” J. Mater. Sci. 53, 14185–14203 (2018). https://doi.org/10.1007/s10853-018-2384-3
CN Patent No. CN104151556 (2014).
B. Li, C. He, M. Cao, et al., “Highly branched phenolic resin-grafted silicone rubber copolymer for high efficiency ablation thermal protection coating,” J. Appl. Polym. Sci. 137, 48353 (2020).
Y. Du, Y. Xia, Z. Luo, et al., “An addition-curable hybrid phenolic resin containing silicon and boron with improved thermal stability,” Polym. Degrad. Stab. 189, 109599 (2021).
A. M. Kawamoto, L. C. Pardini, M. F. Diniz, et al., “Synthesis of a boron modified phenolic resin,” J. Aerospace Technol Manage. 2, 169–182 (2010). https://doi.org/10.5028/jatm.2010.02027610
F. Wang, Z. Huang, G. Zhang, and Y. Li, “Preparation and thermal stability of heat-resistant phenolic resin system constructed by multiple heat-resistant compositions containing boron and silicon. High performance,” Polymers 29, 493–498 (2016).
M. Gao, W. Wu, and Y. Wang, “Phenolic foam modified with dicyandiamide as toughening agent,” J. Therm. Anal. Calorim. 124, 189–195 (2015). https://doi.org/10.1007/s10973-015-5156-1
V. M. Abbasov, M. N. Amiraslanova, N. R. Abdullaeva, et al., “Study of the structure and mechanism of synthesis of novolac phenol-formaldehyde oligomers modified with imidazolines based on natural petroleum acids and polyamines using IR spectroscopy,” Plast. Massy, Nos. 1–2, 18–21 (2019).
Abdullaeva N.R., M. N. Amiraslanova, L. I. Alieva, et al., “Study of physicochemical and thermal properties of phenol-formaldehyde oligomers modified with imidazolines,” Plast. Massy, Nos. 9–10, 7–10 (2018).
H. Li, J. Gu, D. Wang, et al., “Study on benzoxazine-based film adhesive and its adhesion properties with CFPR composites,” J. Adhes. Sci. Technol. 31, 1796–1806 (2017).
H. Li, L. Zhao, Y. Qiao, et al., “Toughening of Benzoxazine Structural Adhesives and Surface Films,” J. Adhes. Sci. and Technol, 1–15 (2022). https://doi.org/10.1080/01694243.2022.2041227
A. Kowalczyk, M. Tokarczyk, M. Weisbrodt, and K. Gziut, “Adhesive films based on benzoxazine resins and the photoreactive epoxyacrylate copolymer,” Materials 15, 1839 (2022). https://doi.org/10.3390/ma15051839
M. A. Espinosa, V. Cadiz, and M. Galia, “Synthesis and characterization of benzoxazine-based phenolic resins: crosslinking study,” J. Appl. Polym. Sci. 90, 470–481 (2003).
H. Kimura, A. Matsumoto, and K. Ohtsuka, “New type of phenolic resin: curing reaction of phenol-novolac based benzoxazine with bisoxazoline or epoxy resin using latent curing agent and the properties of the cured resin,” J. Appl. Polym. Sci. 112, 1762–1770 (2009).
D. Zhang, X.Liu, X. Bai, et al., “Synthesis, characterization and properties of phthalonitrile-etherified resole resin,” e-Polymers (2020). https://doi.org/10.1515/epoly-2020-0051
R. Mohammad-Rezaei, B. Massoumi, M. Abbasian, et al., “Electrically conductive adhesive based on novolac-grafted polyaniline: Synthesis and characterization,” J. Mater. Sci.: Mater. Electron. 30, 2821–2828 (2019).
K. Takemura and H. Nakao, “Development and applications of functional phenolic resins,” JFE Technical Report No. 27 (2022). (pdf jfe-steel.co.jp).
A. V. Petrov and V. B. Kovalev, “Preparation of new copolymers of polyphenolmethylenepolyphenylcarbamates by condensation of phenol and tert-butyl-N-phenylcarbamate with formaldehyde in the presence of N-toluenesulfonic acid,” in Proceedings of the International Scientific Conference on Innovative Technologies in Management, Education, and Industry (ASTINTEKh-2012), Astrakhan’, 2012, pp. 10–13.
L. N. Machulenko, S. A. Donetskaya, and M. I. Buzin, “Phenol-formaldehyde copolymers containing card groups,” Plast. Massy, Nos. 11–12, 10–16 (2019).
G. I. Konishi, T. Tajima, T. Kimura, et al., “Direct synthesis of functional novolacs and their polymer reactions,” Polym. J. 42, 443–449 (2010).
D. Lee, H. Hwang, J.-S. Kim, et al., “VATA: poly(vinyl alcohol) and tannic acid-based nontoxic underwater adhesive,” ACS Appl. Mater. Interfaces (2020). https://doi.org/10.1021/acsami.0c02037
B. Cheng, J. Yu, T. Arisawa, et al., “Ultrastrong underwater adhesion on diverse substrates using non-canonical phenolic groups,” Nature Commun. 13, 1892 (2022). https://doi.org/10.1038/s41467-022-29427-w
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Sh. Galyaltdinov
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aronovich, D.A., Petrova, A.P. Modern Phenolic Adhesives for Aviation and Engineering. Part 2. Chemical Modification. Polym. Sci. Ser. D 16, 840–854 (2023). https://doi.org/10.1134/S1995421223040044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995421223040044