Abstract
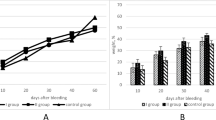
The widespread occurrence of anemia and the presence of side-effects of existing iron-containing drugs require the search for new drugs. In an experiment on male rats of the Wistar strain, posthemorrhagic anemia is simulated by collecting blood from the tail vein in an amount of 1.5% of body weight. Intramuscular administration of iron–molybdenum polyoxometallates in an amount of 1.5 mg/kg to rats with anemia results in a faster restoration of the content of red blood cells and hemoglobin, the hematocrit value in the blood, the concentration of iron in the blood plasma, and the content of erythrocyte precursors in the bone marrow, which recover one to seven days earlier than the parameters measured in a control group of untreated animals.
Similar content being viewed by others
REFERENCES
P. A. Vorobiev, Anemic Syndrome in Clinical Practice (Newdiamed, Moscow, 2001), p. 16 [in Russian].
B. de Benoist, E. McLean, I. Egli, and M. Cogswell, Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia (World Health Organization, Genewa, 2008).
S. R. Pasricha and H. Drakesmith, “Iron deficiency anemia: problems in diagnosis and prevention at the population level,” Hematol. Oncol. Clin. North Am. 30, 309 (2016).
V. Kumar, H. Haridas, P. Hunsigi, et al., “Evaluation of dental and bone age in iron-deficient anemic children of South India,” J. Int. Soc. Prev. Commun. Dent. 6, 430 (2016).
I. R. Demuth, A. Martin, and A. Weissenborn, “Iron supplementation during pregnancy—a cross-sectional study undertaken in four german states,” BMC Pregnancy Childbirth 18, 491 (2018).
M. Levi, M. Rosselli, M. Simonetti, et al., “Epidemiology of iron deficiency anaemia in four European countries: a population-based study in primary care,” Eur. J. Haematol. 97, 583 (2016).
Anemia in Children: Diagnosis, Differential Diagnosis, Treatment, Ed. A. G. Rumyantsev and Yu. N. Tokarev, 2nd ed. (MAX Press, Moscow, 2004) [in Russian].
V. A. Rodionov and M. S. Agandeyeva, “The prevalence of anemia in children of the city of Cheboksary,” Vestn. Chuvash. Univ., No. 3, 491 (2013).
M. Nairz, I. Theurl, D. Wolf, and G. Weiss, “Iron deficiency or anemia of inflammation?: differential diagnosis and mechanisms of anemia of inflammation,” Wien. Med. Wochenschr. 166 (13–14), 411 (2016).
Hematology Manual, Ed. by A. I. Vorob’ev (Newdiamed, Moscow, 2005), Vol. 3 [in Russian].
M. Hertl, Padiatrische Differentialdiagnose (Georg Thieme, Stuttgart, New York, 1986), Vol. 2.
G. Weiss, T. Ganz, and L. T. Goodnough, “Anemia of inflammation,” Blood 133, 40 (2019).
A. C. Ross, “Impact of chronic and acute inflammation on extra- and intracellular iron homeostasis,” Am. J. Clin. Nutr. 106, 1581 (2017).
J. Wang and K. Pantopoulos, “Regulation of cellular iron metabolism,” Biochem. J. 434, 365 (2011).
A. G. Rumyantsev, I. N. Zakharova, and V. M. Chernov, “Prevalence of iron deficiency,” Med. Sovet, No. 6, 62 (2015).
I. S. Tarasova, “Iron deficiency anemia in children and adolescents,” Vopr. Sovrem. Periatr. 10 (2), 40 (2011).
K. P. Flores, S. E. Blohowiak, J. J. Winzerling, et al., “The impact of erythropoietin and iron status on brain myelination in the newborn rat,” J. Neuros. Res. 96, 1586 (2018).
T. W. Bastian, W. C. von Hohenberg, D. J. Mickelson, et al., “Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism, and dendrite complexity,” Dev. Neurosci. 38, 264 (2016).
S. E. Juul, R. J. Derman, and M. Auerbach, “Perinatal iron deficiency: implications for mothers and infants,” Neonatology 115, 269 (2019).
L. M. Winchester, J. Powell, S. Lovestone, and A. J. Nevado-Holgado, “Red blood cell indices and anaemia as causative factors for cognitive function deficits and for Alzheimer’s disease,” Genome Med. 10, 51 (2018).
C. S. Lam, W. Doehner, and J. Comin-Colet, “Iron deficiency in chronic heart failure: case-based practical guidance,” ESC Heart Fail 5, 764 (2018).
G. Tourniaire, C. Milesi, J. Baleine, et al., “Anemia, a new severity factor in young infants with acute viral bronchiolitis?,” Arch Pediatr. 25, 189 (2018).
P. Nielsen, R. Kongi, and R. Fischer, “Efficacy of an iron retard preparation in patients with iron deficiency anemia,” MMW Fortschr. Med. 158 (6), 17 (2016).
V. N. Chernov and I. S. Tarasova, “What drug should be chosen for the treatment of iron deficiency anemia in children—salt or hydroxide-based polymaltose iron complex?,” Pediatriya 91 (5), 90 (2012).
P. Geisser, “The pharmacology and safety profile of ferric carboxymaltose (Ferinject(R)): structure/reactivity relationships of iron preparations,” Port. J. Nephrol. Hypert. 23 (1), 11 (2009).
S. V. Moiseev, “Iron carboxymaltozat (Ferinzhekt)—a new intravenous drug for the treatment of iron deficiency anemia,” Klin. Farmakol. Ter. 21 (2), 2 (2012).
I. G. Danilova, I. F. Gette, S. Yu. Medvedeva, et al., “Influence of iron-molybdenum nanocluster polyoxometalates on the apoptosis of blood leukocytes and the level of heat-shock proteins in the cells of thymus and spleen in rats,” Nanotechnol. Russ. 11, 653 (2016).
A. Müller, E. Krickemeyer, H. Bögge, et al., “Organizational forms of matter: an inorganic superfullerene and keplerate based on molybdenum oxide,” Angew. Chem. Int. Ed. 37, 3360 (1998).
A. Müller, S. Sarkar, S. Q. Nazir Shah, et al., “Archimedian synthesis and magic numbers: ‘sizing’ giant molybdenum—oxide based molecular spheres of the keplerate type,” Angew. Chem., Int. Ed. Engl. 38, 3238 (1999).
A. A. Ostrousko, M. O. Tonkushina, V. Yu. Korotaev, et al., “Stability of the Mo72Fe30 polyoxometalate buckyball in solution,” Russ. J. Inorg. Chem. 57, 1210 (2012).
A. A. Ostroushko and M. O. Tonkushina, “Destruction of molybdenum nanocluster polyoxometallates in aqueous solutions,” Russ. J. Phys. Chem. A 89, 443 (2015).
A. A. Ostroushko, I. F. Gette, I. G. Danilova, et al., “Studies on the possibility of introducing iron-molybdenum buckyballs into an organism by electrophoresis,” Nanotechnol. Russ. 9, 586 (2014).
A. A. Ostroushko, I. F. Gette, S. Yu. Medvedeva, et al., “Study of acute and subacute action of iron-molybdenum nanocluster polyoxometallates,” Nanotechnol. Russ. 8, 672 (2013).
A. A. Ostroushko, I. F. Gette, S. Yu. Medvedeva, et al., “Safety assessment of iron-molybdenum nanocluster polyoxometalates intended for targeted drug delivery,” Vestn. Ural. Med. Akad. Nauki 34 (2), 107 (2011).
A. A. Ostroushko, I. G. Danilova, I. F. Gette, et al., “Study of safety of molybdenum and iron-molybdenum nanocluster polyoxometalates intended for targeter delivery of drugs,” J. Biomater. Nanobiotechnol., No. 2, 557 (2011).
I. F. Gette, I. G. Danilova, and A. A. Ostroushko, “The content of histone proteins in blood lymphocytes and the manifestation of the inflammatory process,” Ross. Immunol. Zh. 1, 444 (2015).
I. G. Danilova, I. F. Gette, S. Yu. Medvedeva, et al., “Changing the content of histone proteins and heat-shock proteins in the blood and liver of rats after the single and repeated administration of nanocluster iron-molybdenum polyoxometallates,” Nanotechnol. Russ. 10, 820 (2015).
B. G. Yushkov, V. G. Klimin, and M. V. Severin, The Blood System and Extreme Effects on the Body (Ural. Otdel. RAS, Yekaterinburg, 1999) [in Russian].
Funding
This study was conducted while implementing the State Assignment of the Ministry of Science and Higher Education of the Russian Federation (project nos. 4.6653.2017/8.9 and AAAA-A18-118020590107-0). The data are protected by patent no. 267 1077 of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Kadkin
Rights and permissions
About this article
Cite this article
Ostroushko, A.A., Gette, I.F., Brilliant, S.A. et al. APPLICATION OF NANOCLUSTER IRON–MOLYBDENE POLYOXOMETALATES FOR CORRECTION OF EXPERIMENTAL POSTHEMORRHAGIC ANEMIA. Nanotechnol Russia 14, 159–164 (2019). https://doi.org/10.1134/S1995078019020101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078019020101