Abstract
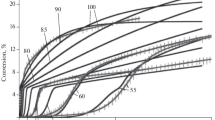
The reaction of dispersed aluminum with water under the exposure of an A-IX-2 aluminum-containing explosive compound to hydrocavitation has been discussed. A kinetic model of the process has been developed; the chemical reaction rate constant has been determined under experimental conditions. It has been shown that the reaction occurs in an autocatalytic mode and can lead to the complete conversion of aluminum to aluminum hydroxide. A method to stabilize dispersed aluminum during the hydrocavitational extraction of a conversion explosive by using a phosphate buffer has been developed. Experiments have shown that the phosphate buffer has a stabilizing effect and leads to an improvement of the characteristics of the resulting industrial explosive composition.
Similar content being viewed by others
Change history
27 February 2018
Add section:
27 February 2018
Add section:
References
B. V. Matseevich, V. P. Glinskii, and E. M. Sviridov, in Integrated Disposal of Conventional Types of Ammunition, Proceedings of the International Conference (RARAN, Krasnoarmeisk, 2007), p. 60.
Powders, Solid Fuels and Explosives (Min-vo Oborony SSSR, Moscow, 2005), p. 202 [in Russian].
D. I. Dement’eva, Introduction to Technology of Energy Saturated Materials (Altaisk. Gos. Tekh. Univ. im. I. I. Polzunova, Biisk, 2009) [in Russian].
GOST (State Standard) No. 5494-95, Aluminium Powder, Technical Conditions, 5th ed. (Mezhgos. Sovet Standartiz., Metrol. Sertifikats., Minsk, 2000).
Technical Conditions No. 075 118 19-108-97 (KNIIM, Krasnoarmeisk, 1997).
V. P. Glinskii, N. K. Shalygin, O. F. Mardasov, et al., in Actual Problems of Utilization of Missiles and Ammunition, Proceedings of the 8th International Conference (RARAN, Krasnoarmeisk, 2011), p. 96.
K. M. Kolmakov, V. K. Kolmakov, and G. V. Kozlov, in Proceedings of the International Symposium on Reliability and Quality (Penz. Gos. Univ., Pensa, 2010), Vol. 2, p. 286.
Yu. P. Perelygin, Vodoochistka, No. 7, 29 (2014).
V. N. Tikhonov, Analytical Chemistry of Aluminum, Ser. Analytical Chemistry of Elements (Nauka, Moscow, 1971) [in Russian].
B. Delmon, Introduction à la cinétique hétérogène (Technip, Paris, 1969).
R. A. Lidin, L. L. Andreeva, and V. A. Molochko, Constants of Inorganic Substances, The Handbook (Drofa, Moscow, 2006) [in Russian].
V. A. Rabinovich and Z. Ya. Khavin, Concise Chemical Handbook (Khimiya, Leningrad, 1991) [in Russian].
Yu. Yu. Lur’e, Handbook of Analytical Chemistry, 6th ed. (Khimiya, Moscow, 1989).
V. M. Talanov and G. M. Zhitnyi, Ion Equilibria in Aqueous Solutions (Akad. Estestvoznaniya, Moscow, 2007) [in Russian].
Technical Conditions No. 5729-090-00284530-00 (Plast-Rifei, Moscow, 2000).
Technical Conditions No. 5729-091-00284530-00 (Plast-Rifei, Moscow, 2000).
Chemist's Handbook, Ed. by B. P. Nikol’skii, 3rd ed. (Khimiya, Moscow, 1971), Vol. 1 [in Russian].
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements (Butterworth, Oxford, 1997), Vol. 1.
H. C. Grigoryan, E. F. Akimova, and T. A. Vagramyan, Phosphatization, The School-Book (Globus, Moscow, 2008) [in Russian].
I. I. Khain, Theory and Practice of Metal Phosphatization (Khimiya, Leningrad, 1973) [in Russian].
N. I. Koshkin and M. G. Shirkevich, Handbook on Elementary Physics, 9th ed. (Nauka, Moscow, 1982) [in Russian].
GOST (State Standard) No. 4545-88 (Izd-vo Standartov, Moscow, 1988).
GOST (State Standard) No. R 22.2.07-94 (Izd-vo Standartov, Moscow, 2001).
GOST (State Standard) No. 9.707-81 (Izd-vo Standartov, Moscow, 1990).
K. M. Kolmakov, A. L. Romanovskii, and G. V. Kozlov, RF Patent No. 2528726, Byull. Izobret. No. 26 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.M. Kolmakov, A.E. Rozen, A.V. Roshchin, E.O. Panin, A.M. Podval’nyi, 2017, published in Khimicheskaya Fizika, 2017, Vol. 36, No. 8, pp. 68–74.
Rights and permissions
About this article
Cite this article
Kolmakov, K.M., Rozen, A.E., Roshchin, A.V. et al. A kinetic model of the reaction of dispersed aluminum with water under exposure to hydrocavitation and stabilization of the final product. Russ. J. Phys. Chem. B 11, 684–690 (2017). https://doi.org/10.1134/S1990793117040170
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117040170