Abstract
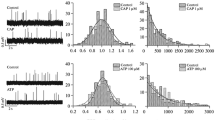
In this work we studied the influence of phospholipase A2 vurtoxin and its homologue lacking enzymatic activity (Vur-S49), isolated from the venom of steppe viper Vipera ursinii renardi, on the amplitude and temporal parameters of spontaneous and evoked endplate currents (EPCs) in the neuromuscular junction of frog Rana ridibunda. The experiments showed that both vurtoxin and Vur-S49 reduce the EPC quantal content. The amplitude and time course of spontaneous (one-quantal) signals remained unchanged, suggesting that these proteins do not block nicotinic acetylcholine receptors (nAChRs) on the postsynaptic membrane. Depressing effect in the presence of enzymatically inactive Vur-S49 suggested that the decrease in the EPC quantal content in the presence of these proteins cannot be explained exclusively by phospholipolytic activity manifested by vurtoxin. On the basis of our previous data we suggested an interaction of the proteins studied with presynaptic α7 nAChRs. Selective antagonist of α7 nicotinic receptors methyllycaconitine (MLA) reduced the EPC quantal content as well. Depressing action of MLA on the evoked secretion of acetylcholine implies the involvement of the presynaptic α7 nAChRs in the regulation of the evoked quantal secretion in the frog neuromuscular junction. However, in the presence of MLA the effects of vurtoxin and Vur-S49 on the EPC quantal content in the nerve-muscle preparation remained unchanged. The data obtained suggest that presynaptic effects of the proteins studied are not directed at α7 nAChRs but could be mediated by interaction with some other synaptic targets.



Similar content being viewed by others
REFERENCES
Tsetlin V.I., Hucho F. 2004. Snake and snail toxins acting on nicotinic acetylcholine receptors: Fundamental aspects and medical applications. FEBS Lett. 557, 9–13.
Kasheverov I.E., Utkin Y.N., Tsetlin V.I. 2009. Naturally occurring and synthetic peptides acting on nicotinic acetylcholine receptors. Curr. Pharm. Des. 15, 2430–2452.
Tsetlin V.I. 2015. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 36, 109–123.
McArdle J.J., Lentz T.L., Witzemann V., Schwarz H., Weinstein S.A., Schmidt J.J. 1999. Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 289, 543–550.
Utkin Y.N., Weise C., Kasheverov I.E., Andreeva T.V., Kryukova E.V., Zhmak M.N., Starkov V.G., Anh H.N., Bertrand D., Ramerstorfer J., Sieghart W., Thompson A.J., Lummis S.C.R., Tsetlin V.I. 2012. Azemiopsin from Azemiops feae viper venom, a novel polypeptide ligand of nicotinic acetylcholine receptor. J. Biol. Chem. 287, 27 079–27 086.
Vulfius C.A., Starkov V.G., Andreeva T.V., Tsetlin V.I., Utkin Y.N. 2015. New antagonosts of nicotinic cholinoreceptors – proteins from venom of Viperidae snakes. DAN (Rus.). 461, 604–607.
Montecucco C., Rossetto O., Caccin P., Rigoni M., Carli L., Morbiato L., Muraro L., Paoli M. 2009. Different mechanisms of inhibition of nerve terminals by botulinum toxin and snake presynaptic neurotoxins. Toxicon. 54, 561–564.
Warrell D.A. 1989. Snake venoms in science and clinical medicine. 1. Russell’s viper: Biology, venom and treatment of bites. Trans. Roy. Soc. Trop. Med. Hyg. 3, 732–740.
Rossetto O., Morbiato L., Caccin P., Rigoni M., Montecucco C. 2006. Presynaptic enzymatic neurotoxins. J. Neurochem. 97, 1534–1545.
Pungerčar J., Križaj I. 2007. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon. 50, 871–892.
Vardjan N., Mattiazzi M., Rowan E.G., Križaj I., Petrovič U., Petan T. 2013. Neurotoxic phospholipase A2 toxicity model: An insight from mammalian cells. Commun. Integr. Biol. doi https://doi.org/10.4161/cib.23600
Kini R.M., Evans H.J. 1989. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 27, 613–635.
Vulfius C.A., Kasheverov I.E., Starkov V.G., Osipov A.V., Andreeva T.V., Filkin S.Yu., Gorbacheva E.V., Astashev M.E., Tsetlin V.I., Utkin Y.N. 2014. Inhibition of nicotinic acetylcholine receptors, a novel facet in the pleiotropic activities of snake venom phospholipases A2. PLoS One. doi https://doi.org/10.1371/journal.pone.0115428
Rufini S., Cesaroni P., Desideri A., Farias R., Gubensek F., Gutiérrez. J.M., Luly P., Massoud R., Morero R., Pedersen J.Z. 1992. Calcium ion independent membrane leakage induced by phospholipase-like myotoxins. Biochemistry. 31, 12424–12430.
Smith T.G., Barker J.L., Smith B.M. & Colburn T.R. 1980. Voltage clamping with microelectrodes. J. Neurosci. Methods 3, 105–128.
Khaziev E., Samigullin D., Zhilyakov N., Fatikhov N., Bukharaeva E., Verkhratsky A., Nikolsky E. 2016. Acetylcholine-induced inhibition of presynaptic calcium signals and transmitter release in the frog neuromuscular junction. Front. Physiol. 7, 621. doi https://doi.org/10.3389/fphys.2016.00621
Kovyazina I.V., Tsentsevitskii A.N., Nikolskii E.E. 2016. Presynaptic nicotine cholinoreceptors modulate the conductance velocity in motor terminals at a high-frequency synaptic activity. DAN (Rus.). 468, 586–588.
Fedorin V.V., Balezina O.P. 2008. The involvement of N-cholinoreceptors of neuronal type in the regulation of the mediator release in mouse neuromuscular synapses. Neurokhimia (Rus.). 25, 99–104.
Gaidukov A.E., Bogacheva P.O., Tarasova E.O., Balezina O.P. 2014. Mechanism of the acetylcholine release suppression in mouse motor synapses. Acta naturae (Rus.). 6 (4), 117–122.
Petrov K.A., Girard E., Nikitashina A.D., Colasante C., Bernard V., Nurullin L., Leroy J., Samigullin D., Colak O., Nikolsky E., Plaud B., Krejci E. 2014. Schwann cells sense and control acetylcholine spillover at the neuromuscular junction by α7 nicotinic receptors and butyrylcholinesterase. J. Neurosci. 34, 11870–11883.
Séguéla P., Wadiche J., Dineley-Milller K., Dani J.A., Patrick J.W. 1993. Molecular cloning, functional properties, and distribution of rat brain α7: A nicotinic cationic channel highly permeable to calcium. J. Neurosci. 13, 596–604.
Castro N.G., Albuquerque E.X. 1995. The α-bungarotoxin-sensitive hippocampal nicotinic acetylcholine receptor has a high calcium permeability. Biophys. J. 68, 516–524.
Tsuneki H., Klink R., Léna C., Korn H., Changeux J.P. 2000. Calcium mobilization elicited by two types of nicotinic acetylcholine receptors in mouse substantia nigra pars compacta. Eur. J. Neurosci. 12, 2475–2485.
Rogers M., Sargent P.B. 2003. Rapid activation of presynaptic nicotinic acetylcholine receptors by nerve-released transmitter. Eur. J. Neurosci. 18, 2946–2956.
Shen J.X., Yakel J.L. 2009. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 30, 673–680.
ACKNOWLEDGMENTS
The work was supported by the Russian Foundation for Basic Research (project nos. 15-04-01843 and 17-04-00690) and by the subsidy in the frames of the Government Support for the Kazan (Privolzhskii) Federal university aimed at enhancing its competitiveness among world leading scientific and educational centers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by A. Dunina-Barkovskaya
Rights and permissions
About this article
Cite this article
Kovyazina, I.V., Kopylova, N.V., Utkin, Y.N. et al. Depression of the Evoked Quantal Acetylcholine Secretion in Frog Neuromuscular Junction by Phospholipases A2 from the Venom of Steppe Viper Vipera ursiniirenardi. Biochem. Moscow Suppl. Ser. A 13, 78–84 (2019). https://doi.org/10.1134/S1990747819010069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747819010069