Abstract
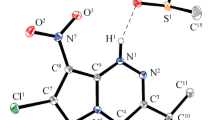
Reactivity of 7-R-3-tert-butyl-4-oxo-4,6-dihydropyrazolo[5,1-c][1,2,4]triazine-8-carboxylic acids (R = NH2, N3, H) and products of their decarboxylation towards different electrophilic agents (chloroacetone, Boc2O/NaN3, DMF/POCl3, TMSBr, N-halosuccinimides, and HNO2) was studied. A selective synthesis of new C(7),C(8)-functionalized and N(1)-tert-butyloxycarbonyl-substituted 4-oxopyrazolo[5,1-c][1,2,4]triazines was performed. The structures of the synthesized compounds were confirmed by IR and NMR spectroscopy, high resolution mass spectrometry and single crystal X-ray diffraction analysis.
Similar content being viewed by others
References
R. Ranjbar-Karimi, A. Darehkordi, F. Bahadornia, A. Poorfreidoni, J. Heterocycl. Chem., 2018, 55, 2516; DOI: https://doi.org/10.1002/jhet.3283.
S. Bátori, D. Csányi, D. Takács, O. Egyed, Z. Riedl, G. Hajós, Tetrahedron, 2019, 75, 180; DOI: https://doi.org/10.1016/j.tet.2018.11.070.
S. M. H. Sanad, A. E. M. Mekky, J. Heterocycl. Chem., 2018, 55, 836; DOI: https://doi.org/10.1002/jhet.3107.
A. N. Lzmest’ev, D. A. Vasileva, E. K. Melnikova, N. G. Kolotyrkina, I. A. Borisova, A. N. Kravchenko, G. A. Gazieva, New J. Chem., 2019, 43, 1038; DOI: https://doi.org/10.1039/C8NJ05058A.
G. M. Ziarani, M. Mostofi, N. Lashgari, M. Mahdavi, Heterocycles, 2018, 96, 1869; DOI: https://doi.org/10.3987/REV-18-893.
E. K. Voinkov, E. N. Ulomskiy, V. L. Rusinov, R. A. Drokin, V. V. Fedotov, E. B. Gorbunov, Mendeleev Commun., 2017, 27, 285; DOI: https://doi.org/10.1016/j.mencom.2017.05.023.
S. M. Ivanov, L. M. Mironovich, L. A. Rodinovskaya, A. M. Shestopalov, Russ. Chem. Bull., 2018, 67, 1482; DOI: https://doi.org/10.1007/s11172-018-2243-z.
V. L. Rusinov, V. N. Charushin, O. N. Chupakhin, Russ. Chem. Bull., 2018, 67, 573; DOI: https://doi.org/10.1007/s11172-018-2113-8.
M. A. Ibrahim, R. M. Abdel-Rahman, A. M. Abdel-Halim, S. S. Ibrahim, H. A. Allimony, ARKIVOC, 2008, Part xvi, 202; DOI: https://doi.org/10.3998/ark.5550190.0009.g19.
M. S. Karthikeyan, M. Mahalinga, P. Karegoundar, B. Poojary, B. S. Holla, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 3231; DOI: https://doi.org/10.1080/10426500902979917.
S. K. Pandey, A. Singh, A. Singh, Nizamuddin, Eur. J. Med. Chem., 2009, 44, 1188; DOI: https://doi.org/10.1016/j.ejmech.2008.05.033.
W. A. El-Sayed, I. F. Nassar, A. A.-H. Abdel-Rahman, J. Heterocycl. Chem., 2011, 48, 135; DOI: https://doi.org/10.1002/jhet.522.
S. M. Ivanov, L. M. Mironovich, N. G. Kolotyrkina, A. M. Shestopalov, Russ. Chem. Bull., 2019, 68, 614; DOI: https://doi.org/10.1007/s11172-019-2464-9.
E. B. Gorbunov, E. N. Ulomsky, E. K. Voinkov, R. A. Drokin, D. N. Lyapustin, G. L. Rusinov, V. L. Rusinov, V. N. Charushin, O. N. Chupakhin, Synthesis, 2018, 50, 4889; DOI: https://doi.org/10.1055/s-0037-1610244.
S. M. Ivanov, L. M. Mironovich, L. A. Rodinovskaya, A. M. Shestopalov, Russ. Chem. Bull., 2018, 67, 1487; DOI: https://doi.org/10.1007/s11172-018-2244-y.
S. M. Ivanov, A. M. Shestopalov, J. Heterocycl. Chem., 2018, 55, 1966; DOI: https://doi.org/10.1002/jhet.3236.
I. V. Ledenyova, A. V. Falaleev, Kh. S. Shikhaliev, E. A. Ryzhkova, F. I. Zubkov, Russ. J. Gen. Chem., 2018, 88, 73; DOI: https://doi.org/10.1134/S1070363218010115.
S. M. Ivanov, J. K. Voronina, A. N. Fakhrutdinov, A. M. Shestopalov, J. Fluorine Chem., 2019, 220, 16; DOI: https://doi.org/10.1016/j.jfluchem.2019.02.004.
S. M. Ivanov, A. M. Shestopalov, J. Heterocycl. Chem., 2019; DOI: https://doi.org/10.1002/jhet.3615.
S. M. Ivanov, A. O. Dmitrienko, M. G. Medvedev, L. M. Mironovich, J. Organomet. Chem., 2019, 896, 168; DOI: https://doi.org/10.1016/j.jorganchem.2019.06.009.
L. M. Mironovich, M. V. Kostina, Chem. Heterocycl. Compd., 2012, 47, 1286; DOI: https://doi.org/10.1007/s10593-012-0904-7.
S. M. Ivanov, L. M. Mironovich, L. A. Rodinovskaya, A. M. Shestopalov, Tetrahedron Lett., 2017, 58, 1851; DOI: https://doi.org/10.1016/j.tetlet.2017.03.083.
H. Lebel, O. Leogane, Org. Lett., 2005, 7, 4107; DOI: https://doi.org/10.1021/ol051428b.
S. M. Ivanov, L. M. Mironovich, L. A. Rodinovskaya, A. M. Shestopalov, Russ. Chem. Bull., 2017, 66, 727; DOI: https://doi.org/10.1007/s11172-017-1801-0.
L. M. Mironovich, A. Y. Podol’nikova, Russ. J. Gen. Chem., 2014, 84, 2480; DOI: https://doi.org/10.1134/S1070363214120287.
L. M. Mironovich, A. Y. Podol’nikova, Russ. J. Org. Chem., 2016, 52, 453; DOI: https://doi.org/10.1134/S1070428016030283.
K. Jarowicki, P. Kocienski, J. Chem. Soc., Perkin Trans. 1, 2001, 2109; DOI: https://doi.org/10.1039/B103282H.
S. M. Ivanov, A. M. Shestopalov, L. M. Mironovich, L. A. Rodinovskaya, J. Heterocycl. Chem., 2017, 54, 2725; DOI: https://doi.org/10.1002/jhet.2874.
T. Yamaji, T. Saito, K. Hayamizu, M. Yanagisawa, O. Yamamoto, SDBSWeb: https://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, Japan), Version 2018.07.18, 2018.
C. M. Blair, P. D. Brass, D. M. Yost, J. Am. Chem. Soc., 1934, 56, 1916; DOI: https://doi.org/10.1021/ja01324a025.
G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1714–1722, September, 2019.
Rights and permissions
About this article
Cite this article
Ivanov, S.M., Lyssenko, K.A., Mironovich, L.M. et al. Decarboxylation and electrophilic substitution in 3-tert-butyl-4-oxopyrazolo[5,1-c][1,2,4]triazines. Russ Chem Bull 68, 1714–1722 (2019). https://doi.org/10.1007/s11172-019-2615-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-019-2615-z